Sweet Selenium: Synthesis and Properties of Selenium-Containing Sugars and Derivatives
Abstract
1. Introduction
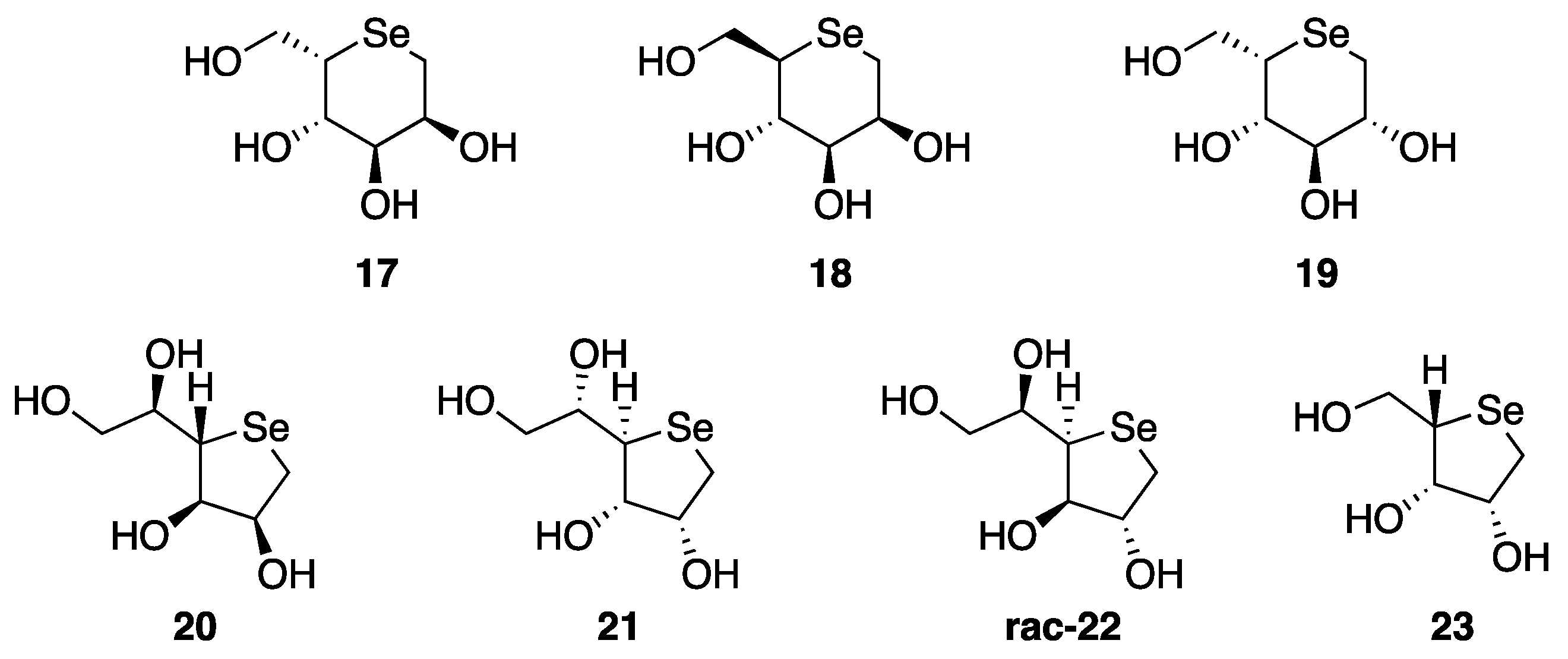
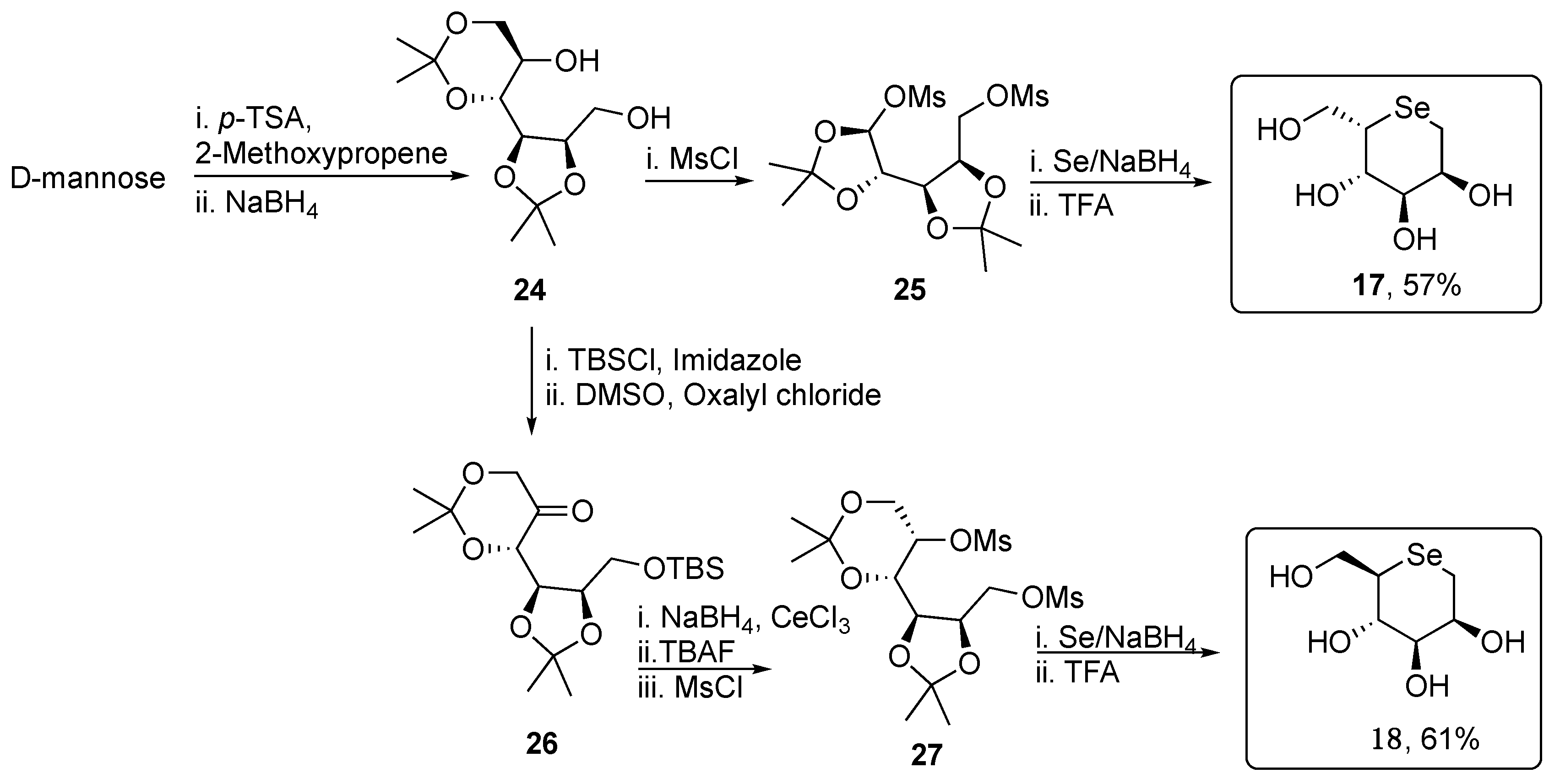
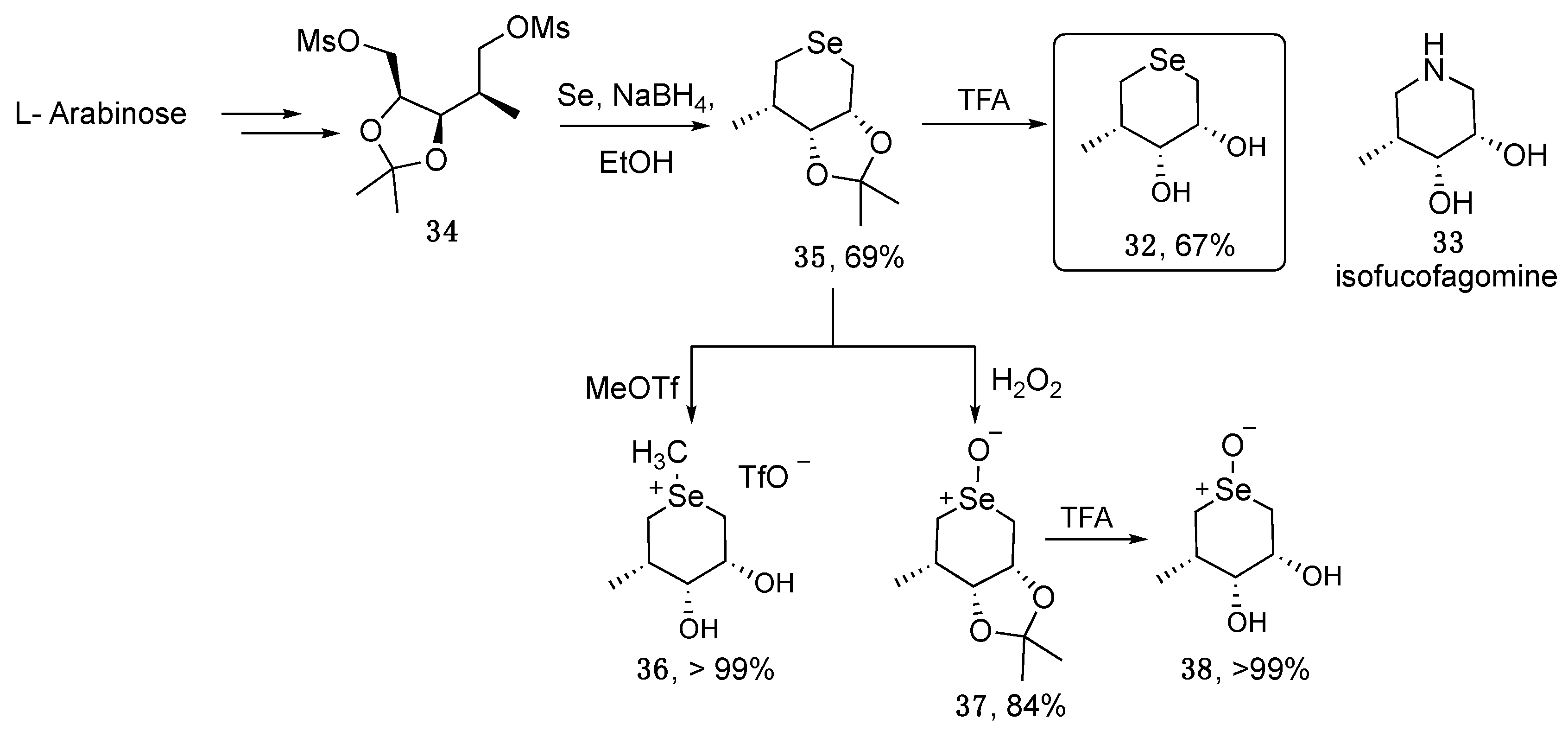
2. Selenopyranes and Selenofuranes
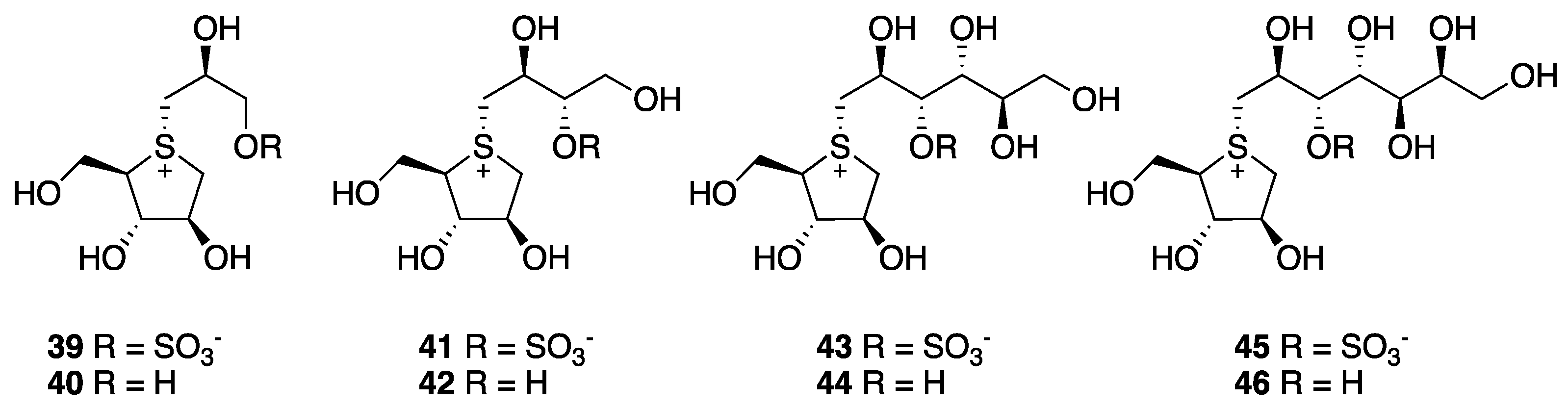
3. Selenonium Salts
- Modifications of the heterocyclic ring, and
- Modification on the polyhydroxylated chain.
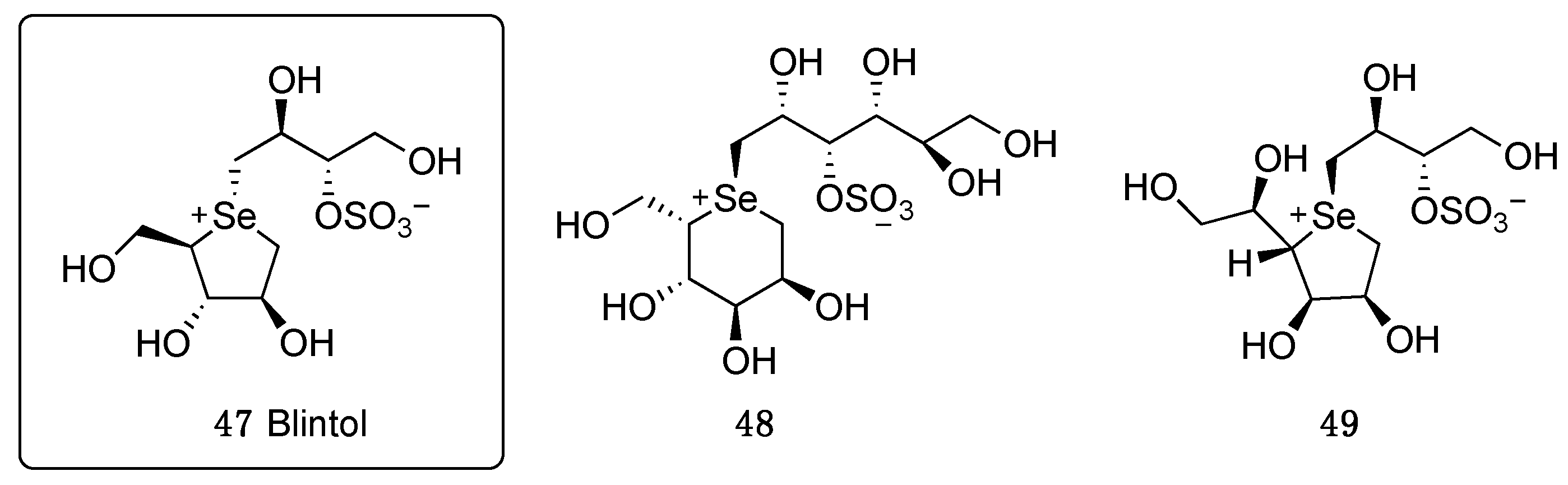
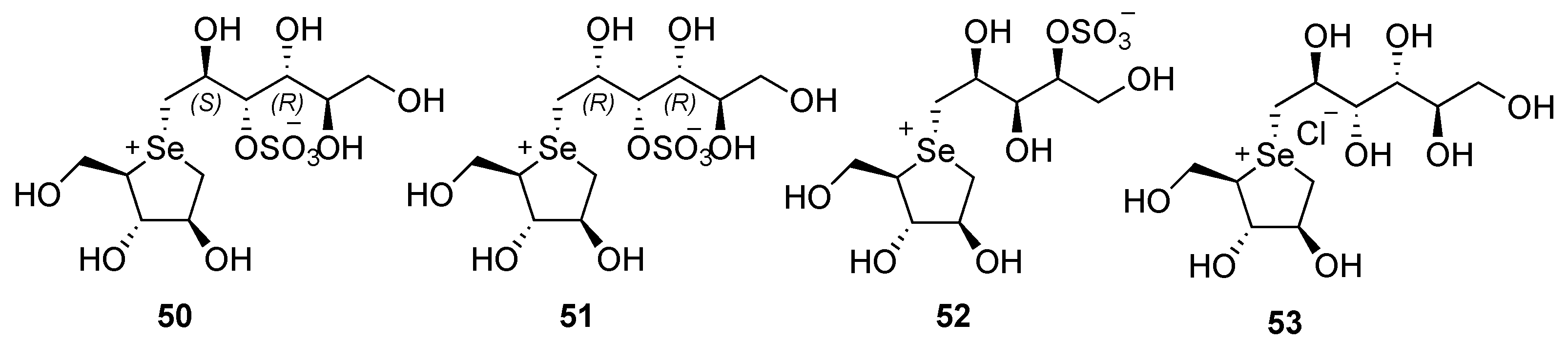
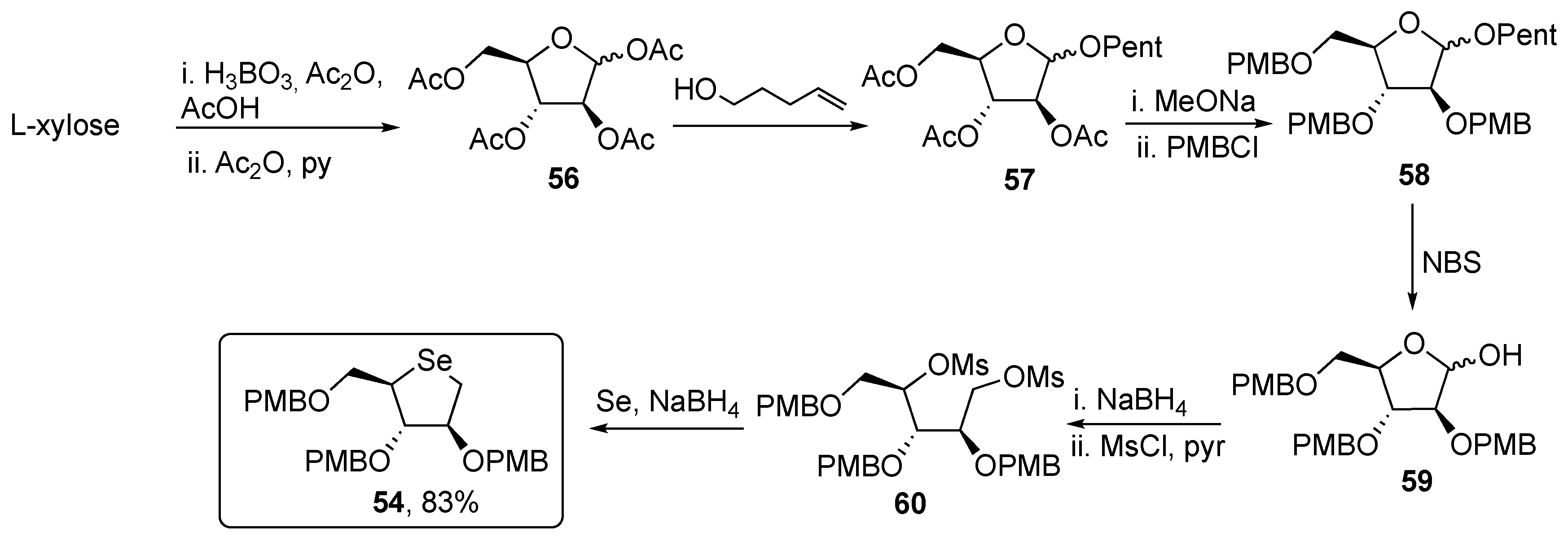
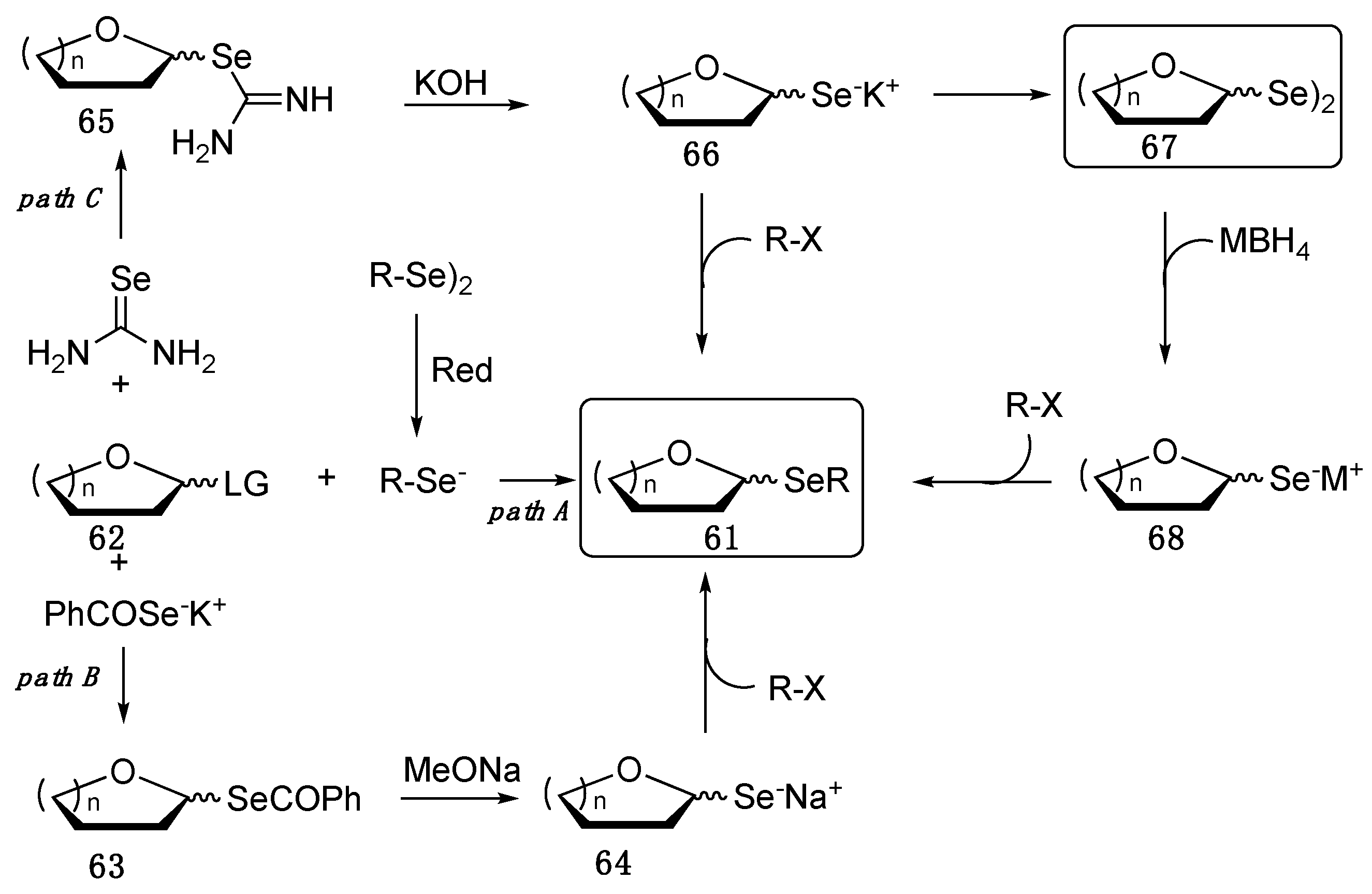
3.1. Modification of the Heterocyclic Ring
3.2. Modification on the Polyhydroxylated Chain
4. Selenoglycosides
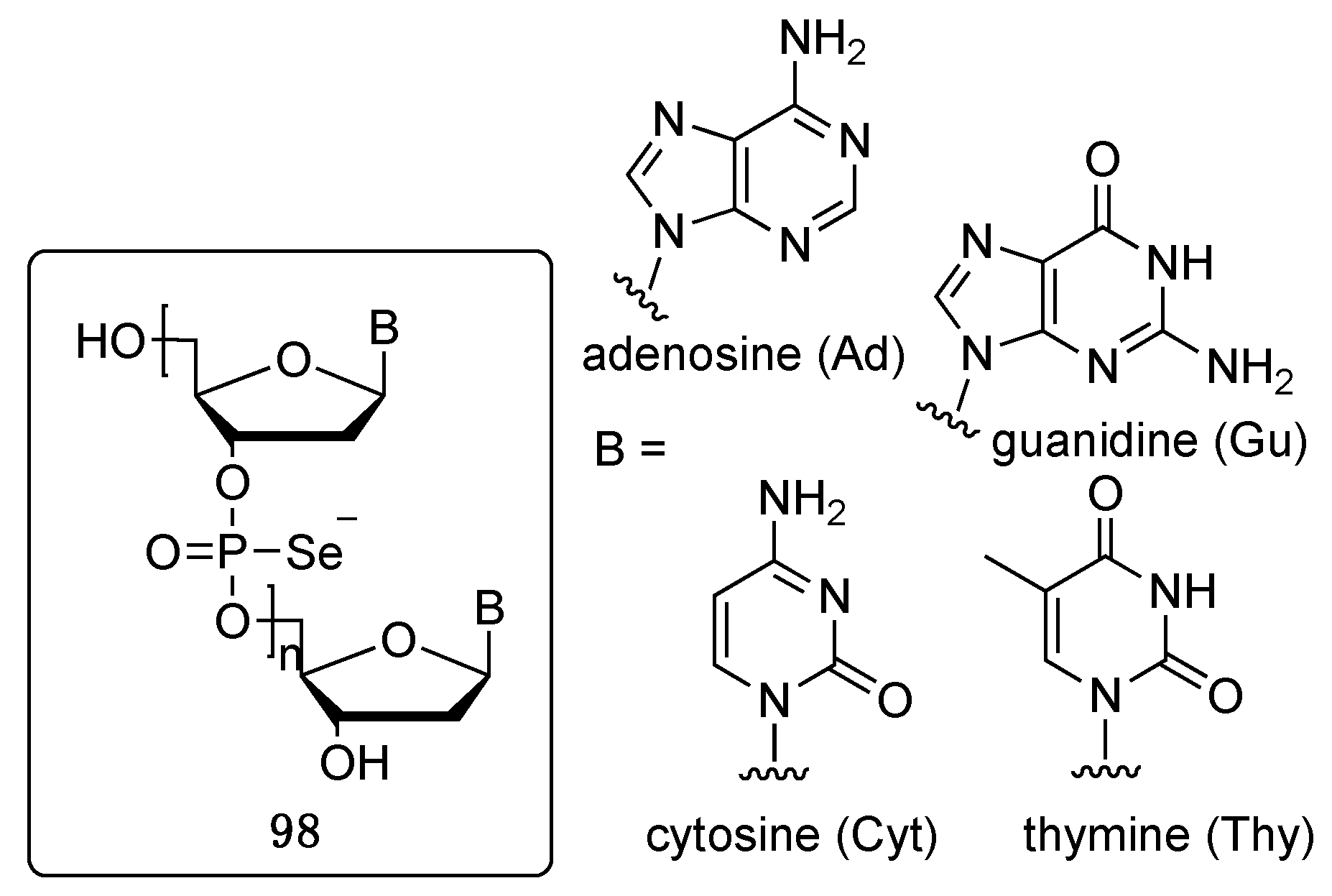
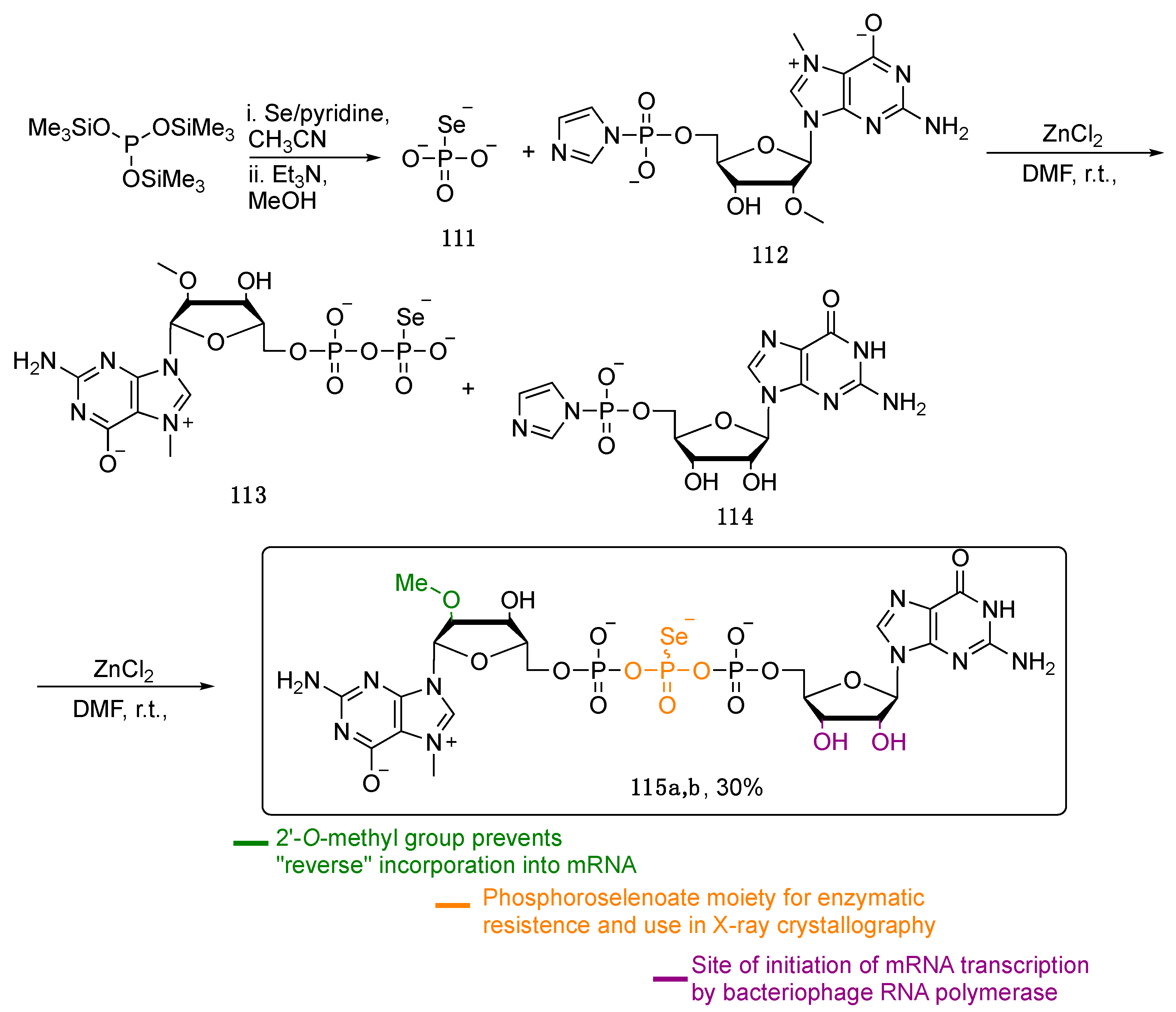
5. Phosphoroselenoate Oligonucleotides
6. Se-Nucleosides
6.1. Selenium in the Sugar Backbone
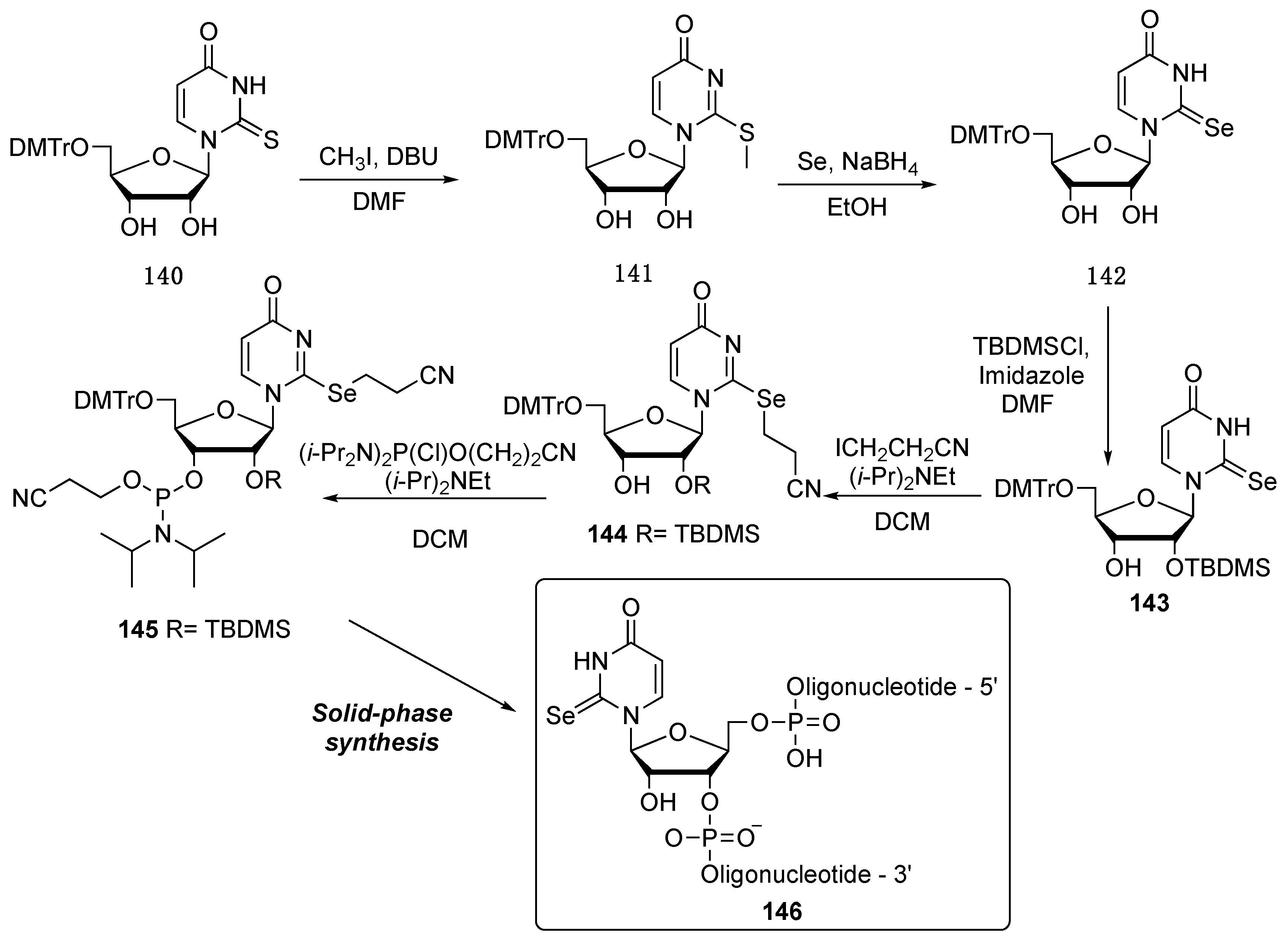
6.2. Selenium in the Nucleobase
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lenardão, E.J.; Santi, C.; Sancineto, L. New Frontiers in Organoselenium Compounds; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-92404-5. [Google Scholar]
- Santi, C.; Marini, F.; Lenardão, E.J. Synthetic Advances on Bioactive Selenium CompoundsChapter 2: Looking Beyond the Traditional Idea of Glutathione Peroxidase Mimics as Antioxidants. In Organoselenium Compounds in Biology and Medicine; Royal Society of Chemistry: Cambridge, UK, 2017; pp. 35–76. [Google Scholar]
- Spengler, G.; Kincses, A.; Mosolygó, T.; Marć, M.A.; Nové, M.; Gajdács, M.; Sanmartín, C.; McNeil, H.E.; Blair, J.M.A.; Domínguez-Álvarez, E. Domínguez-Álvarez Antiviral, Antimicrobial and Antibiofilm Activity of Selenoesters and Selenoanhydrides. Molecules 2019, 24, 4264. [Google Scholar] [CrossRef] [PubMed]
- Geoffrion, L.D.; Hesabizadeh, T.; Medina-Cruz, D.; Kusper, M.; Taylor, P.; Vernet-Crua, A.; Chen, J.; Ajo, A.; Webster, T.J.; Guisbiers, G. Naked Selenium Nanoparticles for Antibacterial and Anticancer Treatments. ACS Omega 2020, 5, 2660–2669. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Pinteala, M.; Maier, S.S.; Simionescu, B.C.; Milaneschi, A.; Abbas, G.; del Prete, S.; Capasso, C.; Capperucci, A.; Tanini, D.; et al. Evaluation of Thio- and Seleno-Acetamides Bearing Benzenesulfonamide as Inhibitor of Carbonic Anhydrases from Different Pathogenic Bacteria. Int. J. Mol. Sci. 2020, 21, 598. [Google Scholar] [CrossRef] [PubMed]
- Perfileva, A.I.; Nozhkina, O.A.; Graskova, I.A.; Dyakova, A.V.; Pavlova, A.G.; Aleksandrova, G.P.; Klimenkov, I.V.; Sukhov, B.G.; Trofimov, B.A. Selenium Nanocomposites Having Polysaccharid Matrices Stimulate Growth of Potato Plants in Vitro Infected with Ring Rot Pathogen. Dokl. Biol. Sci. 2019, 489, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, D.; Sancineto, L.; Fabro de Bem, A.; Tew, K.D.; Santi, C.; Radi, R.; Toquato, P.; Galli, F. Selenocompounds in Cancer Therapy: An Overview. In Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2017; Volume 136, pp. 259–302. ISBN 9780128120163. [Google Scholar]
- Müller, A.; Cadenas, E.; Graf, P.; Sies, H. A novel biologically active seleno-organic compound-1. Glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ 51 (Ebselen). Biochem. Pharmacol. 1984, 33, 3235–3239. [Google Scholar] [CrossRef]
- Krasowska, D.; Iraci, N.; Santi, C.; Drabowicz, J.; Cieslak, M.; Kaźmierczak-Barańska, J.; Palomba, M.; Królewska-Golińska, K.; Magiera, J.; Sancineto, L. Diselenides and Benzisoselenazolones as Antiproliferative Agents and Glutathione-S-Transferase Inhibitors. Molecules 2019, 24, 2914. [Google Scholar] [CrossRef]
- Domínguez-Álvarez, E.; Gajdács, M.; Spengler, G.; Palop, J.A.; Marć, M.A.; Kieć-Kononowicz, K.; Amaral, L.; Molnár, J.; Jacob, C.; Handzlik, J.; et al. Identification of selenocompounds with promising properties to reverse cancer multidrug resistance. Bioorg. Med. Chem. Lett. 2016, 26, 2821–2824. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.; Park, M.-S. Synthesis of new diorganodiselenides from organic halides: Their antiproliferative effects against human breast cancer MCF-7 cells. Arch. Pharm. Res. 2015, 38, 659–665. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Kaczor, K.B.; Antosiewicz, J.; Janecka, A.; Długosz, A.; Janecki, T.; Wojtczak, A.; Ścianowski, J. New Chiral Ebselen Analogues with Antioxidant and Cytotoxic Potential. Molecules 2017, 22, 492. [Google Scholar] [CrossRef]
- Sarma, B.K.; Mugesh, G. Antioxidant Activity of the Anti-Inflammatory Compound Ebselen: A Reversible Cyclization Pathway via Selenenic and Seleninic Acid Intermediates. Chem. A Eur. J. 2008, 14, 10603–10614. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Quinhones, E.B.; Jung, E.A.C.; Zeni, G.; Rocha, J.B.T. Anti-inflammatory and antinociceptive activity of diphenyl diselenide. Inflamm. Res. 2003, 52, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Pinto Brod, L.M.; Fronza, M.G.; Vargas, J.P.; Lüdtke, D.S.; Luchese, C.; Wilhelm, E.A.; Savegnago, L. Involvement of monoaminergic system in the antidepressant-like effect of (octylseleno)-xylofuranoside in the mouse tail suspension test. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 65, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Halliday, A.C.; Thomas, J.M.; Kuznetsova, O.V.; Baldwin, R.; Woon, E.C.Y.; Aley, P.K.; Antoniadou, I.; Sharp, T.; Vasudevan, S.R.; et al. A safe lithium mimetic for bipolar disorder. Nat. Commun. 2013, 4, 1332. [Google Scholar] [CrossRef] [PubMed]
- Sancineto, L.; Piccioni, M.; De Marco, S.; Pagiotti, R.; Nascimento, V.; Braga, A.L.; Santi, C.; Pietrella, D. Diphenyl diselenide derivatives inhibit microbial biofilm formation involved in wound infection. BMC Microbiol. 2016, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Chiou, J.; Wan, S.; Chan, K.-F.; So, P.-K.; He, D.; Chan, E.W.; Chan, T.; Wong, K.; Tao, J.; Chen, S. Ebselen as a potent covalent inhibitor of New Delhi metallo-β-lactamase (NDM-1). Chem. Commun. 2015, 51, 9543–9546. [Google Scholar] [CrossRef] [PubMed]
- Macegoniuk, K.; Grela, E.; Palus, J.; Rudzińska-Szostak, E.; Grabowiecka, A.; Biernat, M.; Berlicki, Ł. 1,2-Benzisoselenazol-3(2H)-one Derivatives As a New Class of Bacterial Urease Inhibitors. J. Med. Chem. 2016, 59, 8125–8133. [Google Scholar] [CrossRef]
- Sancineto, L.; Mariotti, A.; Bagnoli, L.; Marini, F.; Desantis, J.; Iraci, N.; Santi, C.; Pannecouque, C.; Tabarrini, O. Design and Synthesis of DiselenoBisBenzamides (DISeBAs) as Nucleocapsid Protein 7 (NCp7) Inhibitors with anti-HIV Activity. J. Med. Chem. 2015, 58, 9601–9614. [Google Scholar] [CrossRef]
- Sancineto, L.; Iraci, N.; Tabarrini, O.; Santi, C. NCp7: Targeting a multitasking protein for next-generation anti-HIV drug development part 1: Covalent inhibitors. Drug Discov. Today 2018, 23, 260–271. [Google Scholar] [CrossRef]
- Iraci, N.; Tabarrini, O.; Santi, C.; Sancineto, L. NCp7: Targeting a multitask protein for next-generation anti-HIV drug development part 2. Noncovalent inhibitors and nucleic acid binders. Drug Discov. Today 2018, 23, 687–695. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Lipinski, C.A. Poor Aqueous Solubility—An Industry Wide Problem in ADME Screening. Am. Pharm. Rev. 2002, 5, 82–85. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Davies, M.J.; Schiesser, C.H. 1,4-Anhydro-4-seleno-d-talitol (SeTal): A remarkable selenium-containing therapeutic molecule. New J. Chem. 2019, 43, 9759–9765. [Google Scholar] [CrossRef]
- Lucas, M.A.; Nguyen, O.T.K.; Schiesser, C.H.; Zheng, S.-L. Preparation of 5-Selenopentopyranose Sugars from Pentose Starting Materials by Samarium(II) Iodide or (Phenylseleno)formate Mediated Ring Closures. Tetrahedron 2000, 56, 3995–4000. [Google Scholar] [CrossRef]
- Storkey, C.; Davies, M.J.; White, J.M.; Schiesser, C.H. Synthesis and antioxidant capacity of 5-selenopyranose derivatives. Chem. Commun. 2011, 47, 9693. [Google Scholar] [CrossRef] [PubMed]
- Storkey, C.; Pattison, D.I.; White, J.M.; Schiesser, C.H.; Davies, M.J. Preventing Protein Oxidation with Sugars: Scavenging of Hypohalous Acids by 5-Selenopyranose and 4-Selenofuranose Derivatives. Chem. Res. Toxicol. 2012, 25, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Hawkins, C.L.; Pattison, D.I.; Rees, M.D. Mammalian Heme Peroxidases: From Molecular Mechanisms to Health Implications. Antioxid. Redox Signal. 2008, 10, 1199–1234. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J.; Kettle, A.J.; Rosen, H.; Winterbourn, C.C.; Nauseef, W.M. Myeloperoxidase: A front-line defender against phagocytosed microorganisms. J. Leukoc. Biol. 2013, 93, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Yan, L.-J. Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol. 2014, 2, 165–169. [Google Scholar] [CrossRef]
- Storkey, C.; Pattison, D.I.; Ignasiak, M.T.; Schiesser, C.H.; Davies, M.J. Kinetics of reaction of peroxynitrite with selenium- and sulfur-containing compounds: Absolute rate constants and assessment of biological significance. Free Radic. Biol. Med. 2015, 89, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.; Pattison, D.I.; Fu, S.; Schiesser, C.H.; Davies, M.J.; Hawkins, C.L. Catalytic oxidant scavenging by selenium-containing compounds: Reduction of selenoxides and N-chloramines by thiols and redox enzymes. Redox Biol. 2017, 12, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.H.; Leo, C.H.; O’Sullivan, K.; Alexander, S.-A.; Davies, M.J.; Schiesser, C.H.; Parry, L.J. 1,4-Anhydro-4-seleno-d-talitol (SeTal) protects endothelial function in the mouse aorta by scavenging superoxide radicals under conditions of acute oxidative stress. Biochem. Pharmacol. 2017, 128, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Kötzler, M.P.; Hancock, S.M.; Withers, S.G. Glycosidases: Functions, Families and Folds. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2014. [Google Scholar]
- Gerber-Lemaire, S.; Juillerat-Jeanneret, L. Glycosylation Pathways as Drug Targets for Cancer: Glycosidase Inhibitors. Mini-Rev. Med. Chem. 2006, 6, 1043–1052. [Google Scholar] [CrossRef]
- Merino-Montiel, P.; López, Ó.; Fernández-Bolaños, J.G. l-Isofucoselenofagomine and derivatives: Dual activities as antioxidants and as glycosidase inhibitors. Tetrahedron 2012, 68, 3591–3595. [Google Scholar] [CrossRef]
- Medagama, A.B. Salacia reticulata (Kothala himbutu) revisited; a missed opportunity to treat diabetes and obesity? Nutr. J. 2015, 14, 21. [Google Scholar] [CrossRef]
- Stohs, S.J.; Ray, S. Anti-diabetic and Anti-hyperlipidemic Effects and Safety of Salacia reticulata and Related Species. Phyther. Res. 2015, 29, 986–995. [Google Scholar] [CrossRef]
- Meigs, J.B. The Genetic Epidemiology of Type 2 Diabetes: Opportunities for Health Translation. Curr. Diab. Rep. 2019, 19, 62. [Google Scholar] [CrossRef]
- Morikawa, T.; Akaki, J.; Ninomiya, K.; Kinouchi, E.; Tanabe, G.; Pongpiriyadacha, Y.; Yoshikawa, M.; Muraoka, O. Salacinol and Related Analogs: New Leads for Type 2 Diabetes Therapeutic Candidates from the Thai Traditional Natural Medicine Salacia chinensis. Nutrients 2015, 7, 1480–1493. [Google Scholar] [CrossRef]
- Mohan, S.; Pinto, B.M. Towards the elusive structure of kotalanol, a naturally occurring glucosidase inhibitor. Nat. Prod. Rep. 2010, 27, 481. [Google Scholar] [CrossRef]
- Mohan, S.; Eskandari, R.; Pinto, B.M. Naturally Occurring Sulfonium-Ion Glucosidase Inhibitors and Their Derivatives: A Promising Class of Potential Antidiabetic Agents. Acc. Chem. Res. 2014, 47, 211–225. [Google Scholar] [CrossRef]
- Mohan, S.; Pinto, B.M. Sulfonium-ion glycosidase inhibitors isolated from Salacia species used in traditional medicine, and related compounds. Collect. Czechoslov. Chem. Commun. 2009, 74, 1117–1136. [Google Scholar] [CrossRef]
- Mohan, S.; Pinto, B.M. Zwitterionic glycosidase inhibitors: Salacinol and related analogues. Carbohydr. Res. 2007, 342, 1551–1580. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H. Pharmacology of α-glucosidase inhibition. Eur. J. Clin. Invest. 2010, 24, 3–10. [Google Scholar] [CrossRef]
- Eskandari, R.; Jones, K.; Rose, D.R.; Pinto, B.M. The effect of heteroatom substitution of sulfur for selenium in glucosidase inhibitors on intestinal α-glucosidase activities. Chem. Commun. 2011, 47, 9134. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.D.; Ghavami, A.; Jensen, M.T.; Svensson, B.; Pinto, B.M. Synthesis of Selenium Analogues of the Naturally Occurring Glycosidase Inhibitor Salacinol and Their Evaluation as Glycosidase Inhibitors. J. Am. Chem. Soc. 2002, 124, 8245–8250. [Google Scholar] [CrossRef]
- Liu, H.; Sim, L.; Rose, D.R.; Pinto, B.M. A New Class of Glucosidase Inhibitor: Analogues of the Naturally Occurring Glucosidase Inhibitor Salacinol with Different Ring Heteroatom Substituents and Acyclic Chain Extension. J. Org. Chem. 2006, 71, 3007–3013. [Google Scholar] [CrossRef]
- Liu, H.; Pinto, B.M. Synthesis of zwitterionic selenonium and sulfonium sulfates from d-mannose as potential glycosidase inhibitors. Can. J. Chem. 2006, 84, 497–505. [Google Scholar] [CrossRef]
- Liu, H.; Pinto, B.M. Design and synthesis of selenonium and sulfonium ions related to the naturally occurring glucosidase inhibitor salacinol. Can. J. Chem. 2006, 84, 1351–1362. [Google Scholar] [CrossRef]
- Nasi, R.; Sim, L.; Rose, D.R.; Pinto, B.M. New Chain-Extended Analogues of Salacinol and Blintol and Their Glycosidase Inhibitory Activities. Mapping the Active-Site Requirements of Human Maltase Glucoamylase. J. Org. Chem. 2007, 72, 180–186. [Google Scholar] [CrossRef]
- Nasi, R.; Sim, L.; Rose, D.R.; Pinto, B.M. Synthesis and glycosidase inhibitory activities of chain-modified analogues of the glycosidase inhibitors salacinol and blintol. Carbohydr. Res. 2007, 342, 1888–1894. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pinto, B.M. Efficient synthesis of the glucosidase inhibitor blintol, the selenium analogue of the naturally occurring glycosidase inhibitor salacinol. J. Org. Chem. 2005, 70, 753–755. [Google Scholar] [CrossRef] [PubMed]
- Bonner, W.A.; Robinson, A. The Preparation and Properties of Phenyl β-D-Selenoglucoside and its Tetraacetate. J. Am. Chem. Soc. 1950, 72, 354–356. [Google Scholar] [CrossRef]
- Whistler, R.L.; Witczak, Z.J. Carbohydrates Containing Selenium. Heterocycles 1982, 19, 1719. [Google Scholar] [CrossRef]
- Mehta, S.; Mario Pinto, B. Phenylselenoglycosides as novel, versatile glycosyl donors. Selective activation over thioglycosides. Tetrahedron Lett. 1991, 32, 4435–4438. [Google Scholar] [CrossRef]
- Mehta, S.; Pinto, B.M. Novel glycosidation methodology. The use of phenyl selenoglycosides as glycosyl donors and acceptors in oligosaccharide synthesis. J. Org. Chem. 1993, 58, 3269–3276. [Google Scholar] [CrossRef]
- Grice, P.; Ley, S.V.; Pietruszka, J.; Osborn, H.M.I.; Priepke, H.W.M.; Warriner, S.L. A New Strategy for Oligosaccharide Assembly Exploiting Cyclohexane-1,2-diacetal Methodology: An Efficient Synthesis of a High Mannose Type Nonasaccharide. Chem. A Eur. J. 2006, 3, 431–440. [Google Scholar] [CrossRef]
- van Well, R.M.; Kärkkäinen, T.S.; Kartha, K.P.R.; Field, R.A. Contrasting reactivity of thioglucoside and selenoglucoside donors towards promoters: Implications for glycosylation stereocontrol. Carbohydr. Res. 2006, 341, 1391–1397. [Google Scholar] [CrossRef]
- Buts, L.; Loris, R.; De Genst, E.; Oscarson, S.; Lahmann, M.; Messens, J.; Brosens, E.; Wyns, L.; De Greve, H.; Bouckaert, J. Solving the phase problem for carbohydrate-binding proteins using selenium derivatives of their ligands: A case study involving the bacterial F17-G adhesin. Acta Crystallogr. Sect. D Biol. Crystallogr. 2003, 59, 1012–1015. [Google Scholar] [CrossRef]
- Suzuki, T.; Makyio, H.; Ando, H.; Komura, N.; Menjo, M.; Yamada, Y.; Imamura, A.; Ishida, H.; Wakatsuki, S.; Kato, R.; et al. Expanded potential of seleno-carbohydrates as a molecular tool for X-ray structural determination of a carbohydrate–protein complex with single/multi-wavelength anomalous dispersion phasing. Bioorg. Med. Chem. 2014, 22, 2090–2101. [Google Scholar] [CrossRef]
- Saino, H.; Ago, H.; Ukita, Y.; Miyano, M. Seleno-detergent MAD phasing of leukotriene C 4 synthase in complex with dodecyl-β-D-selenomaltoside. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2011, 67, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Di Bussolo, V.; Fiasella, A.; Balzano, F.; Uccello Barretta, G.; Crotti, P. Stereoselective Synthesis of β-Phenylselenoglycosides from Glycals and Rationalization of the Selenoglycosylation Processes. J. Org. Chem. 2010, 75, 4284–4287. [Google Scholar] [CrossRef]
- Chambers, D.J.; Evans, G.R.; Fairbanks, A.J. Elimination reactions of glycosyl selenoxides. Tetrahedron 2004, 60, 8411–8419. [Google Scholar] [CrossRef]
- Valerio, S.; Iadonisi, A.; Adinolfi, M.; Ravidà, A. Novel Approaches for the Synthesis and Activation of Thio- and Selenoglycoside Donors. J. Org. Chem. 2007, 72, 6097–6106. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Misra, A.K. Indium(I) iodide mediated efficient synthesis of selenoglycosides. Tetrahedron Lett. 2006, 47, 2345–2348. [Google Scholar] [CrossRef]
- Mukherjee, C.; Tiwari, P.; Misra, A.K. Synthesis of thio- and selenoglycosides by cleavage of dichalconides in the presence of zinc/zinc chloride and reaction with glycosyl bromides. Tetrahedron Lett. 2006, 47, 441–445. [Google Scholar] [CrossRef]
- Witczak, Z.J.; Czernecki, S. Synthetic Applications of Selenium-Containing Sugars. In Advances in Carbohydrate Chemistry and Biochemistry; Elsevier: Amsterdam, The Netherlands, 1998; pp. 143–199. [Google Scholar]
- Frenzel, H.; Nuhn, P.; Wagner, G. Über Thiophenol- und Selenophenol-α-D-glucopyranoside. Arch. Pharm. (Weinh.) 1969, 302, 62–72. [Google Scholar] [CrossRef]
- Sancineto, L.; Palomba, M.; Bagnoli, L.; Marini, F.; Santi, C. Advances in Electrophilic Organochalcogen Reagents. Curr. Org. Chem. 2015, 20, 122–135. [Google Scholar] [CrossRef]
- Santi, C.; Tomassini, C.; Sancineto, L. Organic diselenides: Versatile reagents, precursors, and intriguing biologically active compounds. CHIMIA (Aarau) 2017, 71. [Google Scholar] [CrossRef]
- Braga, H.C.; Stefani, H.A.; Paixão, M.W.; Santos, F.W.; Lüdtke, D.S. Synthesis of 5′-seleno-xylofuranosides. Tetrahedron 2010, 66, 3441–3446. [Google Scholar] [CrossRef]
- Braga, H.C.; Wouters, A.D.; Zerillo, F.B.; Lüdtke, D.S. Synthesis of seleno-carbohydrates derived from d-galactose. Carbohydr. Res. 2010, 345, 2328–2333. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.A.; Illyés, T.Z.; Kövér, K.E.; Szilágyi, L. Convenient syntheses of 1,2-trans selenoglycosides using isoselenuronium salts as glycosylselenenyl transfer reagents. Carbohydr. Res. 2012, 360, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Komura, N.; Imamura, A.; Ando, H.; Ishida, H.; Kiso, M. A facile method for synthesizing selenoglycosides based on selenium-transfer to glycosyl imidate. Tetrahedron Lett. 2014, 55, 1920–1923. [Google Scholar] [CrossRef]
- Guan, Y.; Townsend, S.D. Metal-Free Synthesis of Unsymmetrical Organoselenides and Selenoglycosides. Org. Lett. 2017, 19, 5252–5255. [Google Scholar] [CrossRef]
- Knapp, S.; Darout, E. New Reactions of Selenocarboxylates. Org. Lett. 2005, 7, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Ando, H.; Ozeki, H.; Koketsu, M.; Ishihara, H. A Facile Method for β-Selenoglycoside Synthesis Using β-p-Methylbenzoyl Selenoglycoside as the Selenating Unit. Org. Lett. 2005, 7, 4653–4656. [Google Scholar] [CrossRef] [PubMed]
- Boutureira, O.; Bernardes, G.J.L.; Fernández-González, M.; Anthony, D.C.; Davis, B.G. Selenenylsulfide-Linked Homogeneous Glycopeptides and Glycoproteins: Synthesis of Human “Hepatic Se Metabolite A”. Angew. Chem. Int. Ed. 2012, 51, 1432–1436. [Google Scholar] [CrossRef]
- Gamblin, D.P.; Garnier, P.; van Kasteren, S.; Oldham, N.J.; Fairbanks, A.J.; Davis, B.G. Glyco-SeS: Selenenylsulfide-Mediated Protein Glycoconjugation—A New Strategy in Post-Translational Modification. Angew. Chem. Int. Ed. 2004, 43, 828–833. [Google Scholar] [CrossRef]
- Ahn, S.J.; Koketsu, M.; Ishihara, H.; Lee, S.M.; Ha, S.K.; Lee, K.H.; Kang, T.H.; Kima, S.Y. Regulation of Melanin Synthesis by Selenium-Containing Carbohydrates. Chem. Pharm. Bull. (Tokyo) 2006, 54, 281–286. [Google Scholar] [CrossRef]
- Yamago, S.; Yamada, T.; Hara, O.; Ito, H.; Mino, Y.; Yoshida, J. A New, Iterative Strategy of Oligosaccharide Synthesis Based on Highly Reactive β-Bromoglycosides Derived from Selenoglycosides. Org. Lett. 2001, 3, 3867–3870. [Google Scholar] [CrossRef]
- Yamago, S.; Yamada, T.; Ito, H.; Hara, O.; Mino, Y.; Yoshida, J. Combinatorial Synthesis of an Oligosaccharide Library by Using β-Bromoglycoside-Mediated Iterative Glycosylation of Selenoglycosides: Rapid Expansion of Molecular Diversity with Simple Building Blocks. Chem. A Eur. J. 2005, 11, 6159–6174. [Google Scholar] [CrossRef] [PubMed]
- Affeldt, R.F.; Braga, H.C.; Baldassari, L.L.; Lüdtke, D.S. Synthesis of selenium-linked neoglycoconjugates and pseudodisaccharides. Tetrahedron 2012, 68, 10470–10475. [Google Scholar] [CrossRef]
- Fournière, V.; Cumpstey, I. Synthesis of non-glycosidically linked selenoether pseudodisaccharides. Tetrahedron Lett. 2010, 51, 2127–2129. [Google Scholar] [CrossRef]
- Cumpstey, I.; Ramstadius, C.; Akhtar, T.; Goldstein, I.J.; Winter, H.C. Non-Glycosidically Linked Pseudodisaccharides: Thioethers, Sulfoxides, Sulfones, Ethers, Selenoethers, and Their Binding to Lectins. Eur. J. Org. Chem. 2010, 2010, 1951–1970. [Google Scholar] [CrossRef]
- Cumpstey, I. Neodisaccharide diglycosyl compounds: Ethers, thioethers and selenoethers. A survey of their synthesis and biological activity. Comptes Rendus Chim. 2011, 14, 274–285. [Google Scholar] [CrossRef]
- Stec, W.J.; Zon, G.; Egan, W. Automated solid-phase synthesis, separation, and stereochemistry of phosphorothioate analogs of oligodeoxyribonucleotides. J. Am. Chem. Soc. 1984, 106, 6077–6079. [Google Scholar] [CrossRef]
- Mori, K.; Boiziau, C.; Cazenave, C.; Matsukura, M.; Subasinghe, C.; Cohen, J.S.; Broder, S.; Toulmé, J.J.; Stein, C.A. Phosphoroselenoate oligodeoxynucleotides: Synthesis, physico-chemical characterization, anti-sense inhibitory properties and anti-HIV activity. Nucleic Acids Res. 1989, 17, 8207–8219. [Google Scholar] [CrossRef]
- Conlon, P.F.; Eguaogie, O.; Wilson, J.J.; Sweet, J.S.T.; Steinhoegl, J.; Englert, K.; Hancox, O.G.A.; Law, C.J.; Allman, S.A.; Tucker, J.H.R.; et al. Solid-phase synthesis and structural characterisation of phosphoroselenolate-modified DNA: A backbone analogue which does not impose conformational bias and facilitates SAD X-ray crystallography. Chem. Sci. 2019, 10, 10948–10957. [Google Scholar] [CrossRef]
- Eguaogie, O.; Conlon, P.F.; Vyle, J.S. Synthesis of nucleoside phosphoroselenolates via the efficient Michaelis–Arbuzov reaction of selenocyanates. Tetrahedron Lett. 2016, 57, 5000–5002. [Google Scholar] [CrossRef]
- Bartoszewicz, A.; Kalek, M.; Stawinski, J. The Case for the Intermediacy of Monomeric Metaphosphate Analogues during Oxidation of H-Phosphonothioate, H-Phosphonodithioate, and H-Phosphonoselenoate Monoesters: Mechanistic and Synthetic Studies. J. Org. Chem. 2008, 73, 5029–5038. [Google Scholar] [CrossRef]
- Kowalska, J.; Lukaszewicz, M.; Zuberek, J.; Darzynkiewicz, E.; Jemielity, J. Phosphoroselenoate Dinucleotides for Modification of mRNA 5′ End. ChemBioChem 2009, 10, 2469–2473. [Google Scholar] [CrossRef] [PubMed]
- Jeong, L.S.; Tosh, D.K.; Kim, H.O.; Wang, T.; Hou, X.; Yun, H.S.; Kwon, Y.; Lee, S.K.; Choi, J.; Zhao, L.X. First Synthesis of 4‘-Selenonucleosides Showing Unusual Southern Conformation. Org. Lett. 2008, 10, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Tosh, D.K.; Choi, W.J.; Kim, H.O.; Lee, Y.; Pal, S.; Hou, X.; Choi, J.; Choi, S.; Jeong, L.S. Stereoselective Synthesis and Conformational Study of Novel 2′,3′-Didehydro-2′,3′-dideoxy-4′-selenonucleosides. J. Org. Chem. 2008, 73, 4259–4262. [Google Scholar] [CrossRef] [PubMed]
- Jeong, L.S.; Choi, Y.N.; Tosh, D.K.; Choi, W.J.; Kim, H.O.; Choi, J. Design and synthesis of novel 2′,3′-dideoxy-4′-selenonucleosides as potential antiviral agents. Bioorg. Med. Chem. 2008, 16, 9891–9897. [Google Scholar] [CrossRef] [PubMed]
- Jeong, L.S.; Tosh, D.K.; Choi, W.J.; Lee, S.K.; Kang, Y.-J.; Choi, S.; Lee, J.H.; Lee, H.; Lee, H.W.; Kim, H.O. Discovery of a New Template for Anticancer Agents: 2′-deoxy-2′-fluoro-4′-selenoarabinofuranosyl-cytosine (2′-F-4′-Seleno-ara-C). J. Med. Chem. 2009, 52, 5303–5306. [Google Scholar] [CrossRef]
- Alexander, V.; Choi, W.J.; Chun, J.; Kim, H.O.; Jeon, J.H.; Tosh, D.K.; Lee, H.W.; Chandra, G.; Choi, J.; Jeong, L.S. A New DNA Building Block, 4′-Selenothymidine: Synthesis and Modification to 4′-Seleno-AZT as a Potential Anti-HIV Agent. Org. Lett. 2010, 12, 2242–2245. [Google Scholar] [CrossRef]
- Yu, J.; Kim, J.-H.; Lee, H.W.; Alexander, V.; Ahn, H.-C.; Choi, W.J.; Choi, J.; Jeong, L.S. New RNA Purine Building Blocks, 4′-Selenopurine Nucleosides: First Synthesis and Unusual Mixture of Sugar Puckerings. Chem. A Eur. J. 2013, 19, 5528–5532. [Google Scholar] [CrossRef]
- Inagaki, Y.; Minakawa, N.; Matsuda, A. Synthesis of 4′selenoribo nucleosides. Nucleic Acids Symp. Ser. 2007, 51, 139–140. [Google Scholar] [CrossRef]
- Jayakanthan, K.; Johnston, B.D.; Pinto, B.M. Stereoselective synthesis of 4′-selenonucleosides using the Pummerer glycosylation reaction. Carbohydr. Res. 2008, 343, 1790–1800. [Google Scholar] [CrossRef]
- Abdo, M.; Knapp, S. Biomimetic Seleninates and Selenonates. J. Am. Chem. Soc. 2008, 130, 9234–9235. [Google Scholar] [CrossRef]
- Jordheim, L.P.; Durantel, D.; Zoulim, F.; Dumontet, C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discov. 2013, 12, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jarhad, D.B.; Yu, J.; Lee, C.; Jeong, L.S. Asymmetric Synthesis of 2′-C-Methyl-4′-selenonucleosides as Anti-Hepatitis C Virus Agents. J. Org. Chem. 2019, 84, 14414–14426. [Google Scholar] [CrossRef] [PubMed]
- Bur, S.K.; Padwa, A. The Pummerer Reaction: Methodology and Strategy for the Synthesis of Heterocyclic Compounds. Chem. Rev. 2004, 104, 2401–2432. [Google Scholar] [CrossRef] [PubMed]
- Byun, W.S.; Jin, M.; Yu, J.; Kim, W.K.; Song, J.; Chung, H.-J.; Jeong, L.S.; Lee, S.K. A novel selenonucleoside suppresses tumor growth by targeting Skp2 degradation in paclitaxel-resistant prostate cancer. Biochem. Pharmacol. 2018, 158, 84–94. [Google Scholar] [CrossRef]
- Sahu, P.K.; Kim, G.; Yu, J.; Ahn, J.Y.; Song, J.; Choi, Y.; Jin, X.; Kim, J.-H.; Lee, S.K.; Park, S.; et al. Stereoselective Synthesis of 4′-Selenonucleosides via Seleno-Michael Reaction as Potent Antiviral Agents. Org. Lett. 2014, 16, 5796–5799. [Google Scholar] [CrossRef]
- Sun, H.; Sheng, J.; Hassan, A.E.A.; Jiang, S.; Gan, J.; Huang, Z. Novel RNA base pair with higher specificity using single selenium atom. Nucleic Acids Res. 2012, 40, 5171–5179. [Google Scholar] [CrossRef]
- Braga, A.L.; Severo Filho, W.A.; Schwab, R.S.; Rodrigues, O.E.D.; Dornelles, L.; Braga, H.C.; Lüdtke, D.S. Synthesis of selenium- and tellurium-containing nucleosides derived from uridine. Tetrahedron Lett. 2009, 50, 3005–3007. [Google Scholar] [CrossRef]
- Abdo, M.; Zhang, Y.; Schramm, V.L.; Knapp, S. Electrophilic Aromatic Selenylation: New OPRT Inhibitors. Org. Lett. 2010, 12, 2982–2985. [Google Scholar] [CrossRef]
- Salon, J.; Jiang, J.; Sheng, J.; Gerlits, O.O.; Huang, Z. Derivatization of DNAs with selenium at 6-position of guanine for function and crystal structure studies. Nucleic Acids Res. 2008, 36, 7009–7018. [Google Scholar] [CrossRef]
- Kaur, M.; Huang, Z. Synthesis and optical behaviors of 6-seleno-deoxyguanosine. Sci. China Chem. 2014, 57, 314–321. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.; Yan, W.; Zhang, C.; Yang, Z.; Neubauer, P.; Mikhailopulo, I.A.; Huang, Z. Biocatalytic synthesis of seleno-, thio- and chloro-nucleobase modified nucleosides by thermostable nucleoside phosphorylases. Catal. Commun. 2019, 121, 32–37. [Google Scholar] [CrossRef]






















© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangiavacchi, F.; Coelho Dias, I.F.; Di Lorenzo, I.; Grzes, P.; Palomba, M.; Rosati, O.; Bagnoli, L.; Marini, F.; Santi, C.; Lenardao, E.J.; et al. Sweet Selenium: Synthesis and Properties of Selenium-Containing Sugars and Derivatives. Pharmaceuticals 2020, 13, 211. https://doi.org/10.3390/ph13090211
Mangiavacchi F, Coelho Dias IF, Di Lorenzo I, Grzes P, Palomba M, Rosati O, Bagnoli L, Marini F, Santi C, Lenardao EJ, et al. Sweet Selenium: Synthesis and Properties of Selenium-Containing Sugars and Derivatives. Pharmaceuticals. 2020; 13(9):211. https://doi.org/10.3390/ph13090211
Chicago/Turabian StyleMangiavacchi, Francesca, Italo Franco Coelho Dias, Irene Di Lorenzo, Pawel Grzes, Martina Palomba, Ornelio Rosati, Luana Bagnoli, Francesca Marini, Claudio Santi, Eder Joao Lenardao, and et al. 2020. "Sweet Selenium: Synthesis and Properties of Selenium-Containing Sugars and Derivatives" Pharmaceuticals 13, no. 9: 211. https://doi.org/10.3390/ph13090211
APA StyleMangiavacchi, F., Coelho Dias, I. F., Di Lorenzo, I., Grzes, P., Palomba, M., Rosati, O., Bagnoli, L., Marini, F., Santi, C., Lenardao, E. J., & Sancineto, L. (2020). Sweet Selenium: Synthesis and Properties of Selenium-Containing Sugars and Derivatives. Pharmaceuticals, 13(9), 211. https://doi.org/10.3390/ph13090211









