Modulation of Amyloidogenic Peptide Aggregation by Photoactivatable CO-Releasing Ruthenium(II) Complexes
Abstract
1. Introduction
2. Results and Discussion
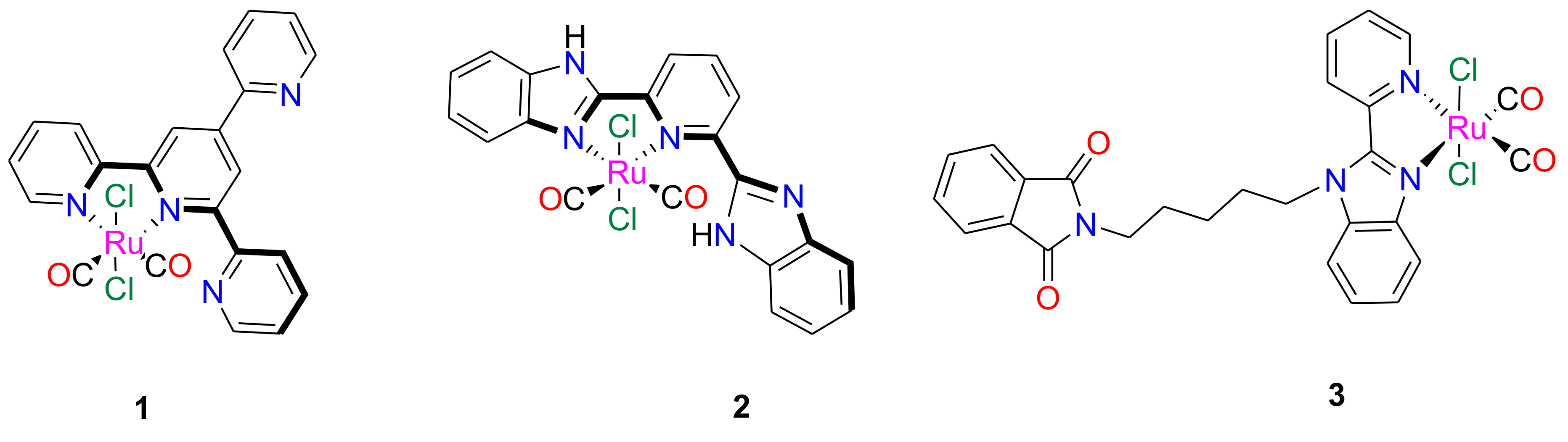
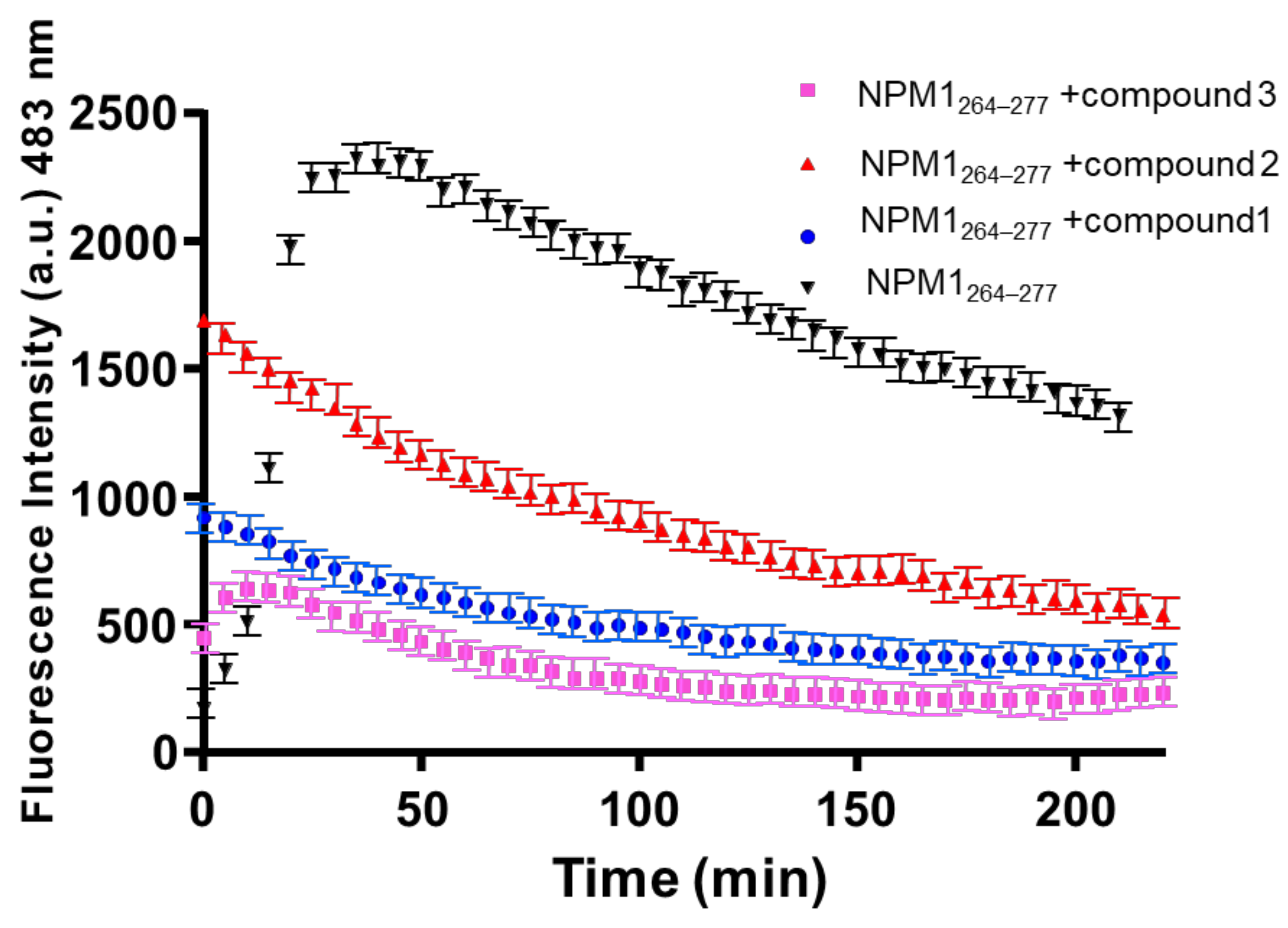
2.1. NPM1264–277 Aggregation Is Suppressed by the Presence of the Investigated Ru(II) Complexes
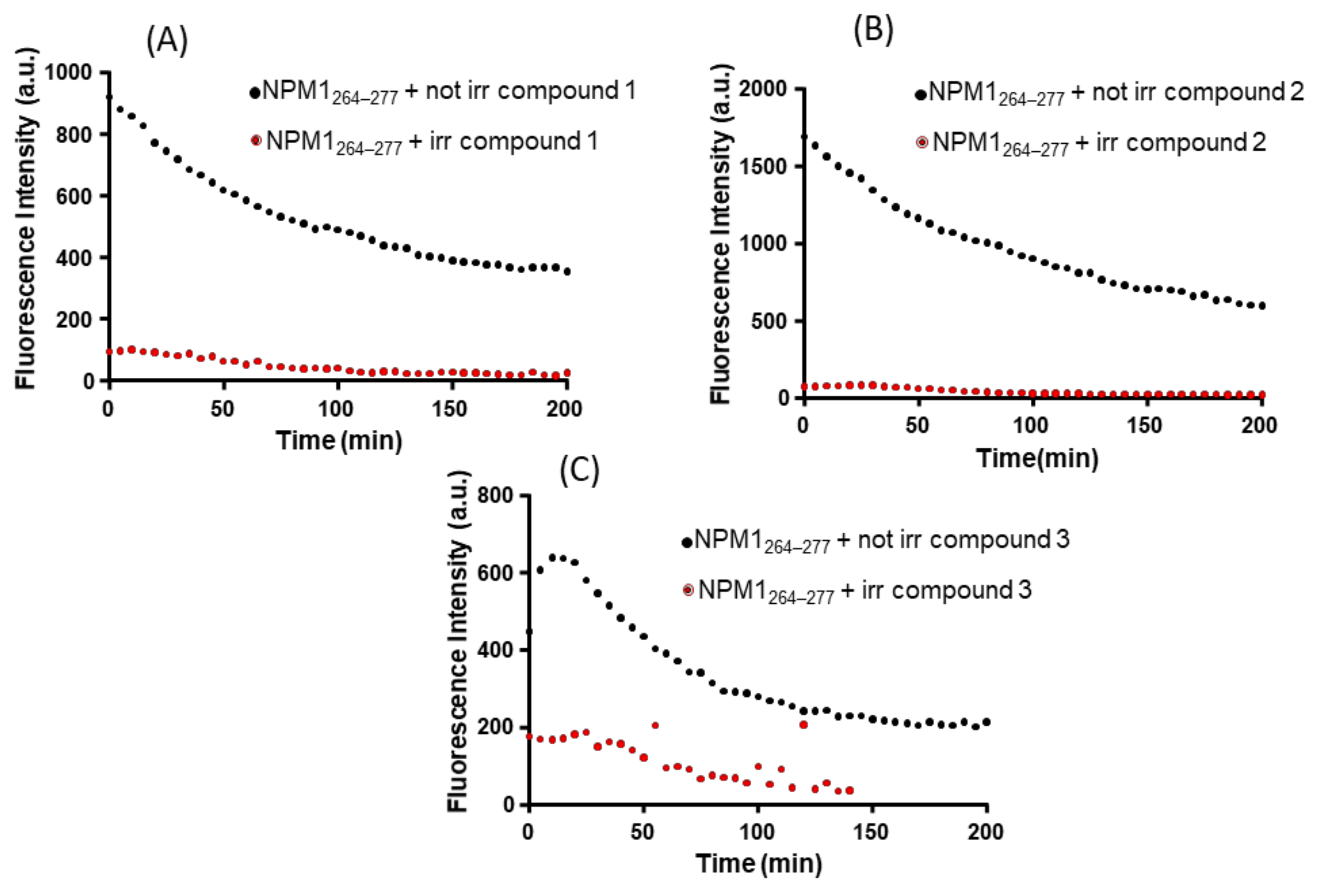
2.2. The Ligand Field of Ru(II) Complexes Changes in the Presence of NPM1264–277
2.3. ESI-MS Analysis of NPM1264–277 in the Presence of the Ru Compounds
3. Materials and Methods
3.1. Peptide and Metal Compound Synthesis
3.2. ThT Fluorescence
3.3. UV/Vis Spectroscopy
3.4. ESI-MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, K.; Zhao, Z.Z.; Bo, H.B.; Hao, X.J.; Wang, J.Q. Applications of Ruthenium Complex in Tumor Diagnosis and Therapy. Front. Pharmacol. 2018, 9, 1323. [Google Scholar] [CrossRef]
- Moreno, V.; Font-Bardia, M.; Calvet, T.; Lorenzo, J.; Aviles, F.X.; Garcia, M.H.; Morais, T.S.; Valente, A.; Robalo, M.P. DNA interaction and cytotoxicity studies of new ruthenium(II) cyclopentadienyl derivative complexes containing heteroaromatic ligands. J. Inorg. Biochem. 2011, 105, 241–249. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Li, G.; Zhang, P.; Jin, C.; Zeng, L.; Ji, L.; Chao, H. Ruthenium(II) polypyridyl complexes as mitochondria-targeted two-photon photodynamic anticancer agents. Biomaterials 2015, 56, 140–153. [Google Scholar] [CrossRef]
- Levina, A.; Mitra, A.; Lay, P.A. Recent developments in ruthenium anticancer drugs. Metallomics 2009, 1, 458–470. [Google Scholar] [CrossRef]
- Merlino, A. Interactions of proteins and ruthenium compounds of medicinal interest: A structural perspective. Coord. Chem. Rev. 2016, 326, 111–134. [Google Scholar] [CrossRef]
- Howerton, B.S.; Heidary, D.K.; Glazer, E.C. Strained ruthenium complexes are potent light-activated anticancer agents. J. Am. Chem. Soc. 2012, 134, 8324–8327. [Google Scholar] [CrossRef]
- Li, F.; Collins, J.G.; Keene, F.R. Ruthenium complexes as antimicrobial agents. Chem. Soc. Rev. 2015, 44, 2529–2542. [Google Scholar] [CrossRef]
- Shum, J.; Leung, P.K.; Lo, K.K. Luminescent Ruthenium(II) Polypyridine Complexes for a Wide Variety of Biomolecular and Cellular Applications. Inorg. Chem. 2019, 58, 2231–2247. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Amyloid formation by globular proteins under native conditions. Nat. Chem. Biol. 2009, 5, 15–22. [Google Scholar] [CrossRef]
- Hayne, D.J.; Lim, S.; Donnelly, P.S. Metal complexes designed to bind to amyloid-beta for the diagnosis and treatment of Alzheimer’s disease. Chem. Soc. Rev. 2014, 43, 6701–6715. [Google Scholar] [CrossRef] [PubMed]
- Spinello, A.; Bonsignore, R.; Barone, G.; Keppler, B.K.; Terenzi, A. Metal Ions and Metal Complexes in Alzheimer’s Disease. Curr. Pharm. Des. 2016, 22, 3996–4010. [Google Scholar] [CrossRef]
- Sava, G.; Capozzi, I.; Clerici, K.; Gagliardi, G.; Alessio, E.; Mestroni, G. Pharmacological control of lung metastases of solid tumours by a novel ruthenium complex. Clin. Exp. Metastasis 1998, 16, 371–379. [Google Scholar] [CrossRef]
- Jakupec, M.A.; Arion, V.B.; Kapitza, S.; Reisner, E.; Eichinger, A.; Pongratz, M.; Marian, B.; Graf von Keyserlingk, N.; Keppler, B.K. KP1019 (FFC14A) from bench to bedside: Preclinical and early clinical development--an overview. Int. J. Clin. Pharmacol. Ther. 2005, 43, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Messori, L.; Camarri, M.; Ferraro, T.; Gabbiani, C.; Franceschini, D. Promising in Vitro anti-Alzheimer Properties for a Ruthenium(III) Complex. ACS Med. Chem. Lett. 2013, 4, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Huffman, S.E.; Yawson, G.K.; Fisher, S.S.; Bothwell, P.J.; Platt, D.C.; Jones, M.A.; Hamaker, C.G.; Webb, M.I. Ruthenium(iii) complexes containing thiazole-based ligands that modulate amyloid-beta aggregation. Metallomics 2020, 12, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Valensin, D.; Anzini, P.; Gaggelli, E.; Gaggelli, N.; Tamasi, G.; Cini, R.; Gabbiani, C.; Michelucci, E.; Messori, L.; Kozlowski, H.; et al. fac-{Ru(CO)(3)}(2+) selectively targets the histidine residues of the beta-amyloid peptide 1-28. Implications for new Alzheimer’s disease treatments based on ruthenium complexes. Inorg. Chem. 2010, 49, 4720–4722. [Google Scholar] [CrossRef]
- Chan, S.L.; Lu, L.; Lam, T.L.; Yan, S.C.; Leung, C.H.; Ma, D.L. A Novel Tetradentate Ruthenium(II) Complex Containing Tris(2- Pyridylmethyl)amine (tpa) As An Inhibitor Of Beta-amyloid Fibrillation. Curr. Alzheimer. Res. 2015, 12, 434–438. [Google Scholar] [CrossRef]
- He, L.; Wang, X.; Zhao, C.; Wang, H.; Du, W. Ruthenium complexes as novel inhibitors of human islet amyloid polypeptide fibril formation. Metallomics 2013, 5, 1599–1603. [Google Scholar] [CrossRef]
- Gong, G.; Xu, J.; Huang, X.; Du, W. Influence of methionine-ruthenium complex on the fibril formation of human islet amyloid polypeptide. J. Biol. Inorg. Chem. 2019, 24, 179–189. [Google Scholar] [CrossRef]
- Gong, G.; Wang, W.; Du, W. Binuclear ruthenium complexes inhibit the fibril formation of human islet amyloid polypeptide. RSC Adv. 2017, 7, 18512–18522. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kundu, S.; Bhattacharyya, A.; Hartinger, C.G.; Dyson, P.J. The ruthenium(II)-arene compound RAPTA-C induces apoptosis in EAC cells through mitochondrial and p53-JNK pathways. J. Biol. Inorg. Chem. 2008, 13, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, A.E.; Walz, D.T.; Batista, V.; Mizraji, M.; Roisman, F.; Misher, A. Auranofin. New oral gold compound for treatment of rheumatoid arthritis. Ann. Rheum. Dis. 1976, 35, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, L.K.; Ortiz, D.; Dyson, P.J. Histidine Targeting Heterobimetallic Ruthenium(II)-Gold(I) Complexes. Inorg. Chem. 2019, 58, 2501–2513. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.L.; He, H.Z.; Leung, K.H.; Chan, D.S.; Leung, C.H. Bioactive luminescent transition-metal complexes for biomedical applications. Angew Chem. Int. Ed. Engl. 2013, 52, 7666–7682. [Google Scholar] [CrossRef]
- Cook, N.P.; Torres, V.; Jain, D.; Marti, A.A. Sensing amyloid-beta aggregation using luminescent dipyridophenazine ruthenium(II) complexes. J. Am. Chem. Soc. 2011, 133, 11121–11123. [Google Scholar] [CrossRef]
- Babu, E.; Muthu Mareeswaran, P.; Sathish, V.; Singaravadivel, S.; Rajagopal, S. Sensing and inhibition of amyloid-beta based on the simple luminescent aptamer-ruthenium complex system. Talanta 2015, 134, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.J.; Zhao, W.; Xie, M.; Li, X.; Sun, M.; He, J.; Wang, L.; Yu, L. Real-Time Monitoring of Self-Aggregation of beta-Amyloid by a Fluorescent Probe Based on Ruthenium Complex. Anal. Chem. 2020, 92, 2953–2960. [Google Scholar] [CrossRef]
- Kenny, R.G.; Marmion, C.J. Toward Multi-Targeted Platinum and Ruthenium Drugs-A New Paradigm in Cancer Drug Treatment Regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef]
- Pizarro, M.D.; Rodriguez, J.V.; Mamprin, M.E.; Fuller, B.J.; Mann, B.E.; Motterlini, R.; Guibert, E.E. Protective effects of a carbon monoxide-releasing molecule (CORM-3) during hepatic cold preservation. Cryobiology 2009, 58, 248–255. [Google Scholar] [CrossRef]
- Caterino, M.; Petruk, A.A.; Vergara, A.; Ferraro, G.; Marasco, D.; Doctorovich, F.; Estrin, D.A.; Merlino, A. Mapping the protein-binding sites for iridium(iii)-based CO-releasing molecules. Dalton Trans. 2016, 45, 12206–12214. [Google Scholar] [CrossRef] [PubMed]
- Pontillo, N.; Ferraro, G.; Messori, L.; Tamasi, G.; Merlino, A. Ru-Based CO releasing molecules with azole ligands: Interaction with proteins and the CO release mechanism disclosed by X-ray crystallography. Dalton Trans. 2017, 46, 9621–9629. [Google Scholar] [CrossRef] [PubMed]
- Kapetanaki, S.M.; Burton, M.J.; Basran, J.; Uragami, C.; Moody, P.C.E.; Mitcheson, J.S.; Schmid, R.; Davies, N.W.; Dorlet, P.; Vos, M.H.; et al. A mechanism for CO regulation of ion channels. Nat. Commun. 2018, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.M.; Shehab, O.R. {Ru(CO)x}-core terpyridine complexes: Lysozyme binding affinity, DNA and photoinduced carbon monoxide releasing properties. J. Photochem. Photobiol. Chem. 2018, 364, 406–414. [Google Scholar] [CrossRef]
- Bani-Hani, M.G.; Greenstein, D.; Mann, B.E.; Green, C.J.; Motterlini, R. A carbon monoxide-releasing molecule (CORM-3) attenuates lipopolysaccharide- and interferon-gamma-induced inflammation in microglia. Pharmacol. Rep. 2006, 58, 132–144. [Google Scholar] [PubMed]
- Schallner, N.; Schwemmers, S.; Schwer, C.I.; Froehlich, C.; Stoll, P.; Humar, M.; Pahl, H.L.; Hoetzel, A.; Loop, T.; Goebel, U. p38beta-regulated induction of the heat shock response by carbon monoxide releasing molecule CORM-2 mediates cytoprotection in lung cells in vitro. Eur. J. Pharmacol. 2011, 670, 58–66. [Google Scholar] [CrossRef]
- Motterlini, R.; Mann, B.E.; Foresti, R. Therapeutic applications of carbon monoxide-releasing molecules. Exp. Opin. Investig. Drugs 2005, 14, 1305–1318. [Google Scholar] [CrossRef]
- Ling, K.; Men, F.; Wang, W.C.; Zhou, Y.Q.; Zhang, H.W.; Ye, D.W. Carbon Monoxide and Its Controlled Release: Therapeutic Application, Detection, and Development of Carbon Monoxide Releasing Molecules (CORMs). J. Med. Chem. 2018, 61, 2611–2635. [Google Scholar] [CrossRef]
- Brouard, S.; Otterbein, L.E.; Anrather, J.; Tobiasch, E.; Bach, F.H.; Choi, A.M.; Soares, M.P. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J. Exp. Med. 2000, 192, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Tao Lu, H.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Zuckerbraun, B.S.; Haga, M.; Liu, F.; Song, R.; Usheva, A.; Stachulak, C.; Bodyak, N.; Smith, R.N.; Csizmadia, E.; et al. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat. Med. 2003, 9, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, N.; Dallas, M.; Al-Owais, M.; Griffiths, H.; Hooper, N.; Scragg, J.; Boyle, J.; Peers, C. Heme oxygenase-1 protects against Alzheimer’s amyloid-beta(1-42)-induced toxicity via carbon monoxide production. Cell Death Dis. 2014, 5, e1569. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, N.T.; Boyle, J.P.; Dallas, M.L.; Al-Owais, M.M.; Scragg, J.L.; Peers, C. Heme oxygenase-1 derived carbon monoxide suppresses Abeta1-42 toxicity in astrocytes. Cell Death Dis. 2017, 8, e2884. [Google Scholar] [CrossRef] [PubMed]
- De Simone, A.; Naldi, M.; Tedesco, D.; Milelli, A.; Bartolini, M.; Davani, L.; Widera, D.; Dallas, M.L.; Andrisano, V. Investigating in Vitro Amyloid Peptide 1-42 Aggregation: Impact of Higher Molecular Weight Stable Adducts. ACS Omega 2019, 4, 12308–12318. [Google Scholar] [CrossRef]
- Scognamiglio, P.L.; Di Natale, C.; Leone, M.; Poletto, M.; Vitagliano, L.; Tell, G.; Marasco, D. G-quadruplex DNA recognition by nucleophosmin: New insights from protein dissection. Biochim. Biophys. Acta 2014, 1840, 2050–2059. [Google Scholar] [CrossRef]
- Di Natale, C.; Scognamiglio, P.L.; Cascella, R.; Cecchi, C.; Russo, A.; Leone, M.; Penco, A.; Relini, A.; Federici, L.; Di Matteo, A.; et al. Nucleophosmin contains amyloidogenic regions that are able to form toxic aggregates under physiological conditions. FASEB J. 2015, 29, 3689–3701. [Google Scholar] [CrossRef]
- Russo, A.; Diaferia, C.; La Manna, S.; Giannini, C.; Sibillano, T.; Accardo, A.; Morelli, G.; Novellino, E.; Marasco, D. Insights into amyloid-like aggregation of H2 region of the C-terminal domain of nucleophosmin. Biochim. Biophys. Acta Proteins Proteom. 2017, 1865, 176–185. [Google Scholar] [CrossRef]
- La Manna, S.; Roviello, V.; Scognamiglio, P.L.; Diaferia, C.; Giannini, C.; Sibillano, T.; Morelli, G.; Novellino, E.; Marasco, D. Amyloid fibers deriving from the aromatic core of C-terminal domain of nucleophosmin 1. Int. J. Biol. Macromol. 2019, 122, 517–525. [Google Scholar] [CrossRef]
- Di Natale, C.; La Manna, S.; Malfitano, A.M.; Di Somma, S.; Florio, D.; Scognamiglio, P.L.; Novellino, E.; Netti, P.A.; Marasco, D. Structural insights into amyloid structures of the C-terminal region of nucleophosmin 1 in type A mutation of acute myeloid leukemia. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 637–644. [Google Scholar] [CrossRef]
- Scognamiglio, P.L.; Di Natale, C.; Leone, M.; Cascella, R.; Cecchi, C.; Lirussi, L.; Antoniali, G.; Riccardi, D.; Morelli, G.; Tell, G.; et al. Destabilisation, aggregation, toxicity and cytosolic mislocalisation of nucleophosmin regions associated with acute myeloid leukemia. Oncotarget 2016, 7, 59129–59143. [Google Scholar] [CrossRef]
- La Manna, S.; Scognamiglio, P.L.; Roviello, V.; Borbone, F.; Florio, D.; Di Natale, C.; Bigi, A.; Cecchi, C.; Cascella, R.; Giannini, C.; et al. The acute myeloid leukemia-associated Nucleophosmin 1 gene mutations dictate amyloidogenicity of the C-terminal domain. FEBS J. 2019, 286, 2311–2328. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.M.; Shehab, O.R. Photoactivatable CO-Releasing Properties of {Ru(CO)2}-Core Pyridylbenzimidazole Complexes and Reactivity towards Lysozyme. Eur. J. Inorg. Chem. 2017, 37, 4299–4310. [Google Scholar] [CrossRef]
- Mansour, A.M. RuII–Carbonyl photoCORMs with N,N -Benzimidazole Bidentate Ligands: Spectroscopic, Lysozyme Binding Affinity, and Biological Activity Evaluation. Eur. J. Inorg. Chem. 2018, 7, 852–860. [Google Scholar] [CrossRef]
- Florio, D.; Iacobucci, I.; Ferraro, G.; Mansour, A.M.; Morelli, G.; Monti, M.; Merlino, A.; Marasco, D. Role of the Metal Center in the Modulation of the Aggregation Process of Amyloid Model Systems by Square Planar Complexes Bearing 2-(2’-pyridyl)benzimidazole Ligands. Pharmaceuticals 2019, 12, 154. [Google Scholar] [CrossRef]
- Florio, D.; Malfitano, A.M.; Di Somma, S.; Mugge, C.; Weigand, W.; Ferraro, G.; Iacobucci, I.; Monti, M.; Morelli, G.; Merlino, A.; et al. Platinum(II) O,S Complexes Inhibit the Aggregation of Amyloid Model Systems. Int. J. Mol. Sci. 2019, 20, 829. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, M.L.; Grasso, G.; Ruvo, M.; Pedone, C.; Saporito, A.; Marasco, D.; Pignataro, B.; Cascio, C.; Copani, A.; Rizzarelli, E. Abeta(25-35) and its C- and/or N-blocked derivatives: Copper driven structural features and neurotoxicity. J. Neurosci. Res. 2007, 85, 623–633. [Google Scholar] [CrossRef]
- Doti, N.; Scognamiglio, P.L.; Madonna, S.; Scarponi, C.; Ruvo, M.; Perretta, G.; Albanesi, C.; Marasco, D. New mimetic peptides of the kinase-inhibitory region (KIR) of SOCS1 through focused peptide libraries. Biochem. J. 2012, 443, 231–240. [Google Scholar] [CrossRef]

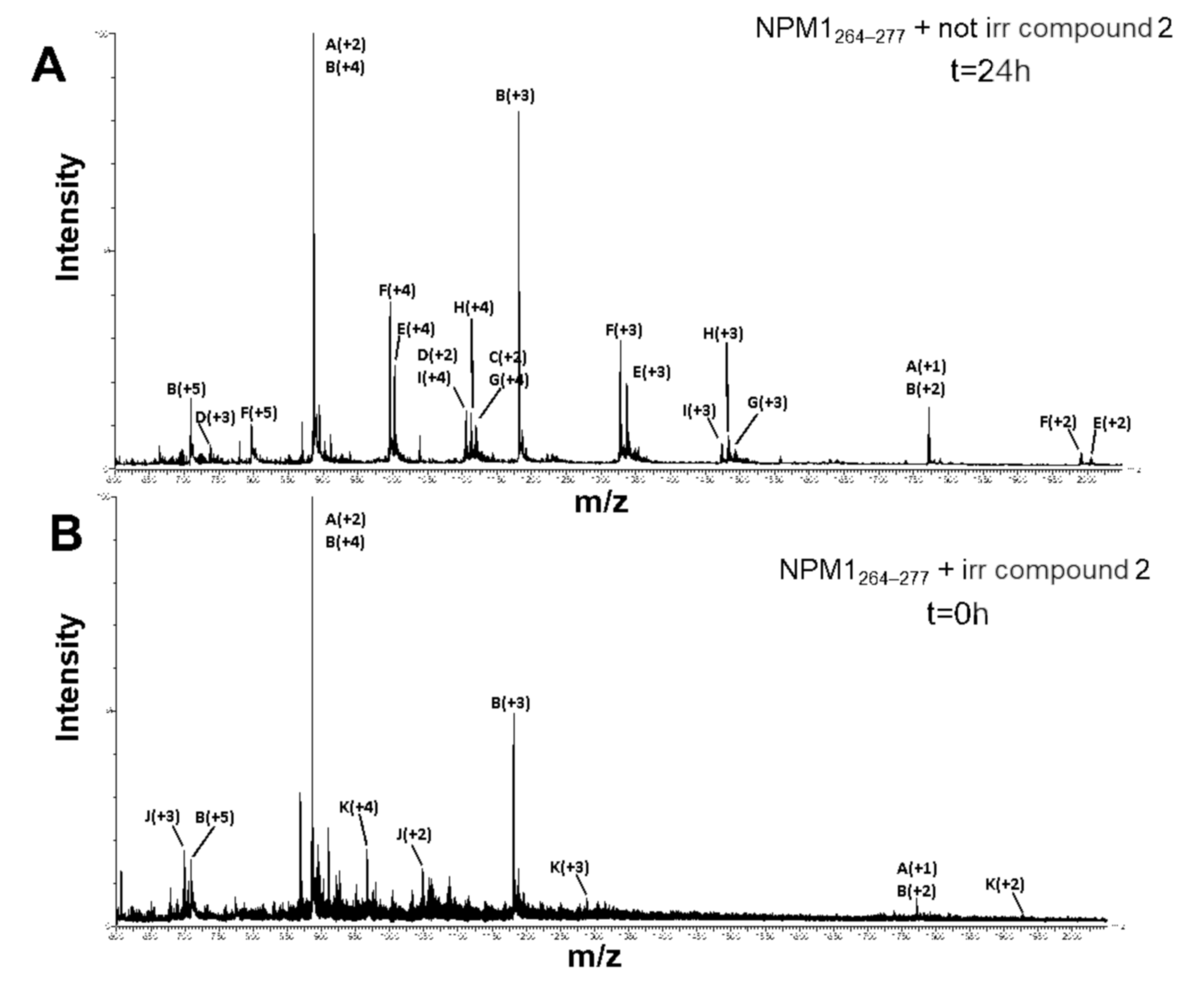
| m/z Signal | Charge | Experimental MW | Theoretical MW | Species |
|---|---|---|---|---|
| 886.47 1771.93 | A + 2 A + 1 | 1770.93 ± 0.01 | 1770.91 | Monomer (NPM1264–277(M)) |
| 708.83 885.97 1181.63 1771.93 | B + 5 B + 4 B + 3 B + 2 | 3541.19 ± 0.95 | 3539.82 | Dimer (NPM1264–277(D)) |
| 746.78 1118.72 | C + 3 C + 2 | 2236.37 ± 0.94 | 2234.27 | NPM1264–277(M) + 1 compound 2 – 2 HCl + 1 TFA |
| 736.65 1104.48 | D + 3 D + 2 | 2207.28 ± 0.48 | 2206.26 | NPM1264–277(M) + 1 compound 2 – 2 HCl – 1 CO + 1 TFA |
| 1002.23 1335.97 2004.43 | E + 4 E + 3 E + 2 | 4005.54 ± 0.93 | 4003.16 | NPM1264–277(D) + 1 compound 2 – 2 HCl + 1 TFA |
| 796.18 994.48 1326.63 1988.96 | F + 5 F + 4 F + 3 F + 2 | 3975.64 ± 1.08 | 3975.15 | NPM1264–277(D) + 1 compound 2 – 2 HCl – 1 CO + 1 TFA |
| 1118.47 1491.32 | G + 4 G + 3 | 4470.40 ± 0.53 | 4466.55 | NPM1264–277(D) + 2 compound 2 – 4 HCl + 2 TFA |
| 1111.71 1481.64 | H + 4 H + 3 | 4442.35 ± 0.46 | 4438.49 | NPM1264–277(D) + 2 compound 2 – 4 HCl – 1 CO + 2 TFA |
| 1104.23 1471.97 | I + 4 I + 3 | 4412.90 ± 0.01 | 4410.48 | NPM1264–277(D) + 2 compound 2 – 4 HCl – 2 CO + 2 TFA |
| m/z Signal | Charge | Experimental MW | Theoretical MW | Species |
|---|---|---|---|---|
| 886.48 1772.00 | A + 2 A + 1 | 1770.97 ± 0.02 | 1770.91 | Monomer (NPM1264–277(M)) |
| 709.39 885.99 1180.99 1771.49 | B + 5 B + 4 B + 3 B + 2 | 3540.68 ± 0.81 | 3539.82 | Dimer (NPM1264–277(D)) |
| 698.31 1046.99 2093.92 | J + 3 J + 2 J + 1 | 2092.28 ± 0.68 | 2093.23 | NPM1264–277(M) + 1 compound 2 – 2 HCl – 1 CO |
| 644.94 773.39 966.48 1288.99 1932.73 | K + 6 K + 5 K + 4 K + 3 K + 2 | 3862.96 ± 0.89 | 3862.13 | NPM1264–277(D) + 1 compound 2 – 2 HCl – 1 CO |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florio, D.; Cuomo, M.; Iacobucci, I.; Ferraro, G.; Mansour, A.M.; Monti, M.; Merlino, A.; Marasco, D. Modulation of Amyloidogenic Peptide Aggregation by Photoactivatable CO-Releasing Ruthenium(II) Complexes. Pharmaceuticals 2020, 13, 171. https://doi.org/10.3390/ph13080171
Florio D, Cuomo M, Iacobucci I, Ferraro G, Mansour AM, Monti M, Merlino A, Marasco D. Modulation of Amyloidogenic Peptide Aggregation by Photoactivatable CO-Releasing Ruthenium(II) Complexes. Pharmaceuticals. 2020; 13(8):171. https://doi.org/10.3390/ph13080171
Chicago/Turabian StyleFlorio, Daniele, Maria Cuomo, Ilaria Iacobucci, Giarita Ferraro, Ahmed M. Mansour, Maria Monti, Antonello Merlino, and Daniela Marasco. 2020. "Modulation of Amyloidogenic Peptide Aggregation by Photoactivatable CO-Releasing Ruthenium(II) Complexes" Pharmaceuticals 13, no. 8: 171. https://doi.org/10.3390/ph13080171
APA StyleFlorio, D., Cuomo, M., Iacobucci, I., Ferraro, G., Mansour, A. M., Monti, M., Merlino, A., & Marasco, D. (2020). Modulation of Amyloidogenic Peptide Aggregation by Photoactivatable CO-Releasing Ruthenium(II) Complexes. Pharmaceuticals, 13(8), 171. https://doi.org/10.3390/ph13080171










