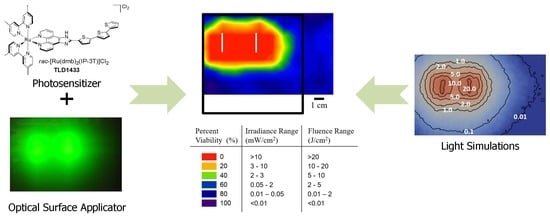
1. Introduction
There is no effective therapy for patients with non-small cell lung cancer (NSCLC) with pleural dissemination or malignant pleural mesothelioma [
1,
2,
3]. Intraoperative photodynamic therapy (IO-PDT) with surgery has been reported to improve overall survival in the treatment of NSCLC and malignant mesothelioma, when compared to historical data [
4,
5,
6]. The current IO-PDT regimen involves systemic administration of a photosensitizer (PS) approximately 24–48 h prior to surgery [
7]. During surgery, after removal of all macroscopic resectable cancer cells and before closing the surgical wound, a therapeutic laser light is delivered to activate the PS and ablate residual viable cancer cells that could remain in the cavity [
8]. The light delivery is accomplished with a handheld light source that the treating physician moves within the thoracic cavity to administer a prescribed threshold light dose (fluence, J/cm
2) [
1,
5,
7,
8]. The delivered light is measured with isotropic detectors that are secured within close-end catheters sutured in about eight anatomical sites that are at high risk for local recurrence due to retention of microscopic cancer cells within the thoracic cavity.
Here, we report on the potential use of a novel ruthenium (Ru)-based PS (TLD1433) in ablating lung cancer cells. TLD1433 is a Ru(II) polypyridyl complex that can be classified as a metal-organic dyad. It contains a metal center that facilitates efficient population of triplet excited states (either directly or indirectly) and an organic α-terthienyl group that affords a triplet intraligand charge transfer (
3ILCT) excited state with a prolonged lifetime and high sensitivity to oxygen. The
3ILCT states can be populated (i) indirectly via singlet metal-to-ligand charge transfer (
1MLCT) and
1ILCT excited states that are formed following absorption of green light, or (ii) directly with red light. The PDT effects obtained with green light are more potent than those with red light owing to the lower molar extinction coefficients of TLD1433 at the red wavelengths. The attenuated PDT effects at longer wavelengths can be improved by formulating TLD1433 with transferrin (Rutherrin), and this has been demonstrated using bladder cancer cells [
9]. The choice of wavelength will depend on the desired tissue penetration depth, with green light being preferred when the penetration depth must be kept minimal (to preserve healthy tissue) and red light when a larger margin of treatment is needed.
TLD1433 is currently being evaluated in a phase II clinical trial for treating high-risk non-muscle invasive bladder cancer (NMIBC) with PDT. The treatment uses green light to avoid damaging the underlying urothelial muscle tissue. In this indication, TLD1433 demonstrates high retention in bladder cancer cells when administered through instillation (in the bladder) 1 h prior to light delivery [
10,
11,
12]. The initial results suggest that this treatment is safe, while inducing effective tumor regression. We suggest that TLD1433-mediated IO-PDT can also be used to treat pleural malignancy. We propose to administer TLD1433 by instillation with sterile saline, in the thoracic cavity. Currently, instillation with sterile saline or intralipid is used to improve optical index matching when administering IO-PDT in the thoracic cavity [
7]. We expect that TLD1433 will also exhibit high retention in cancer cells compared to normal lung tissue.
In this paper we propose to activate TLD1433 with surface illumination using a recently developed optical surface applicator (OSA) [
13]. The OSA was designed specifically for efficient light delivery in IO-PDT in the thoracic cavity. So far, the OSA has not yet been validated with TLD1433. The purpose of the present study was to test whether the OSA could be used to activate TLD1433 for the destruction of lung cancer cells in vitro before moving to in vivo studies. The OSA’s novel construction allows precise adjustments of the light irradiance (mW/cm
2) and fluence that are key parameters for effective PDT [
14,
15]. The OSA includes optical fibers for laser light delivery, and dosimetry fibers for light measurements. A detailed description of the OSA can be found in Chamberlain et al. 2019 [
13]. Briefly, the OSA is made of a flexible silicon-based mesh of interconnected spheres 10-mm in diameter with parallel channels that enable placement of optical fibers. The fibers are at fixed distance of 5 mm from the mesh surface. This design is expected to reduce the time of light administration with improved control of light irradiance and fluence [
13]. Herein, we also present the first simulation of light irradiance and fluence propagation from the OSA.
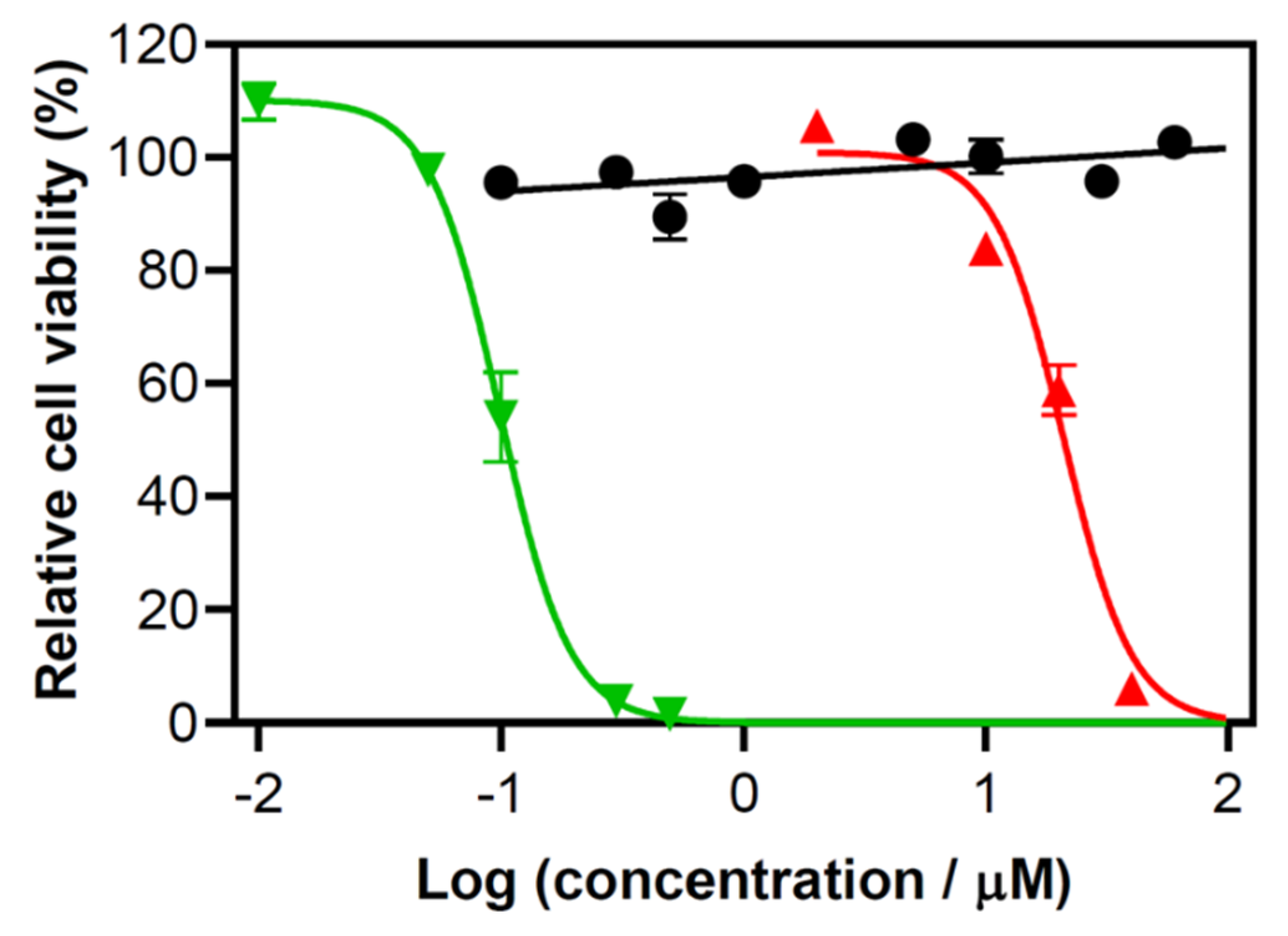
This paper is the first report that highlights the potential use of TLD1433-mediated PDT with the OSA in the treatment of human adenocarcinoma (A549) cells. We studied the response of A549 cells to TLD1433 with two clinically approved light wavelengths: 630 and 532 nm. The spatial distribution of cell viability was compared to the distribution of the simulated light irradiance and fluence, laying the foundation for a pretreatment planning for TLD1433-mediated IO-PDT with OSA for pleural malignancy.
3. Discussion
We evaluated the response of the aggressive human lung cancer A549 cell line to TLD1433-mediated PDT with both 532- and 630-nm light delivered from a laser fiber with a micro lens. The 532-nm light was found to be more potent than 630-nm light. It has been reported that TLD1433 is a reactive oxygen species generator, with a singlet oxygen quantum yield near unity [
9,
10]. The photophysical model of TLD1433 suggests that 532-nm light populates excited
1MLCT/
1ILCT states that decay to reactive
3ILCT states that sensitize singlet oxygen with high efficiency, whereas 630-nm light populates
3MLCT states that are spin forbidden and, once formed, generate singlet oxygen less effectively [
10]. The difference in photocytotoxicities elicited by these states at the two different wavelengths could be attributed to an increase in the production of
1O
2 or other reactive oxygen species (ROS), and thus a more effective and potent photoreaction at 532 nm in comparison to 630-nm light, although this is not yet confirmed. At 532 nm, we also observed a steeper increase in potency with irradiance in comparison to 630-nm light (
Figure 2). These data suggest that 532 nm is associated with a higher production of ROS in comparison to 630-nm light, when there is sufficient oxygen in vitro. More in vivo work is needed, and underway, to assess this speculation in vivo.
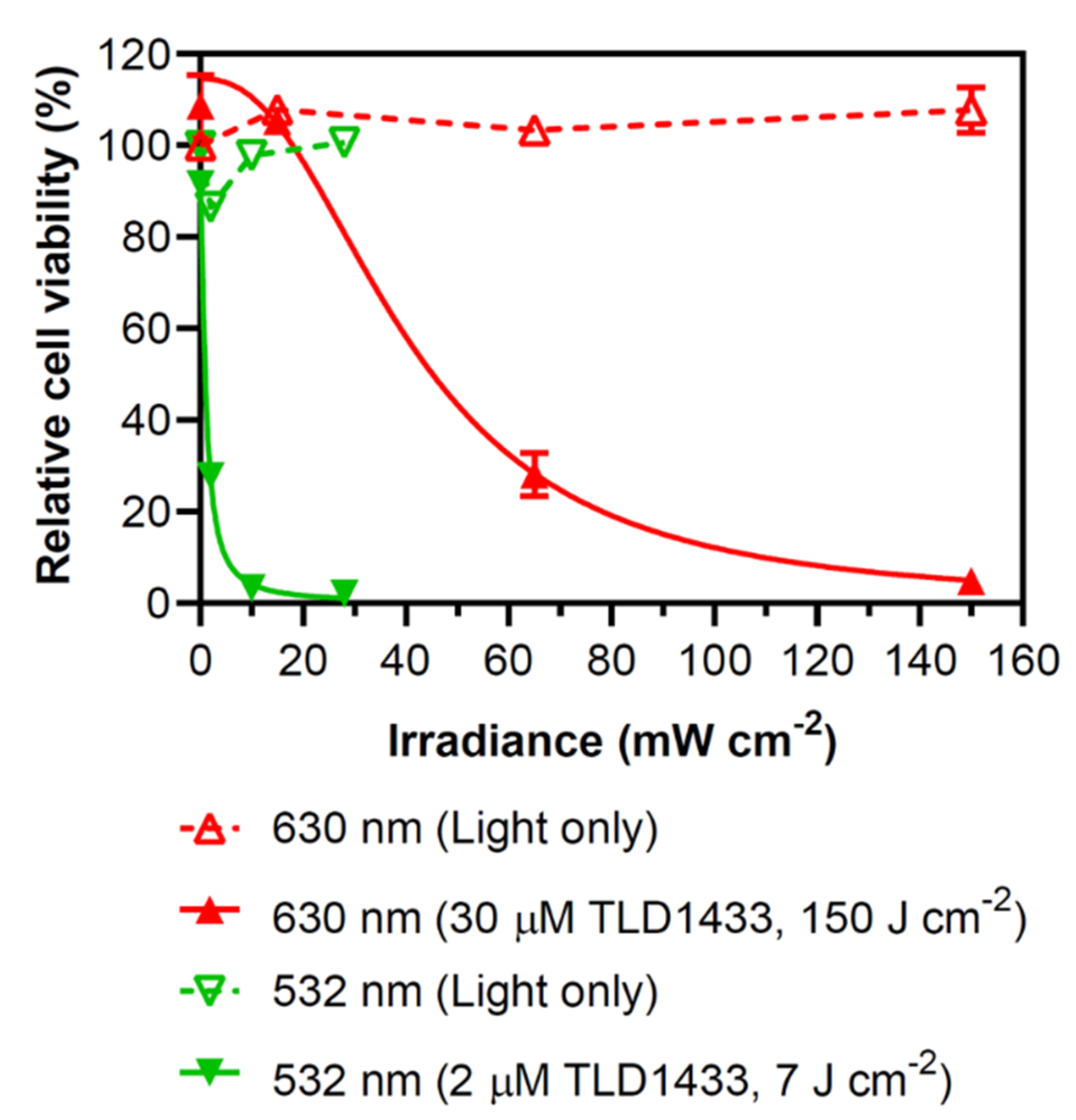
The 532-nm light, that was found to be potent against the A549 cells, also has a shallow (3–5 mm) tissue penetration depth and thus has the potential to inflict less collateral damage to underlying healthy tissue. We therefore suggest that this treatment is a good candidate for IO-PDT in the thoracic cavity, where underlying sensitive structures need to be protected. We then evaluated the A549 cell response to the OSA with 532-nm light at 3- and 5-mm depth, by using tissue phantoms to mimic depth of penetration. The OSA was found to be effective in delivering 532-nm light irradiance and fluence to induce cell death at 3 mm. The OSA was specifically developed for IO-PDT in the thoracic cavity [
13], and its construction makes it possible to develop a pretreatment plan to simulate the fluence and irradiance (
Figure 4 and
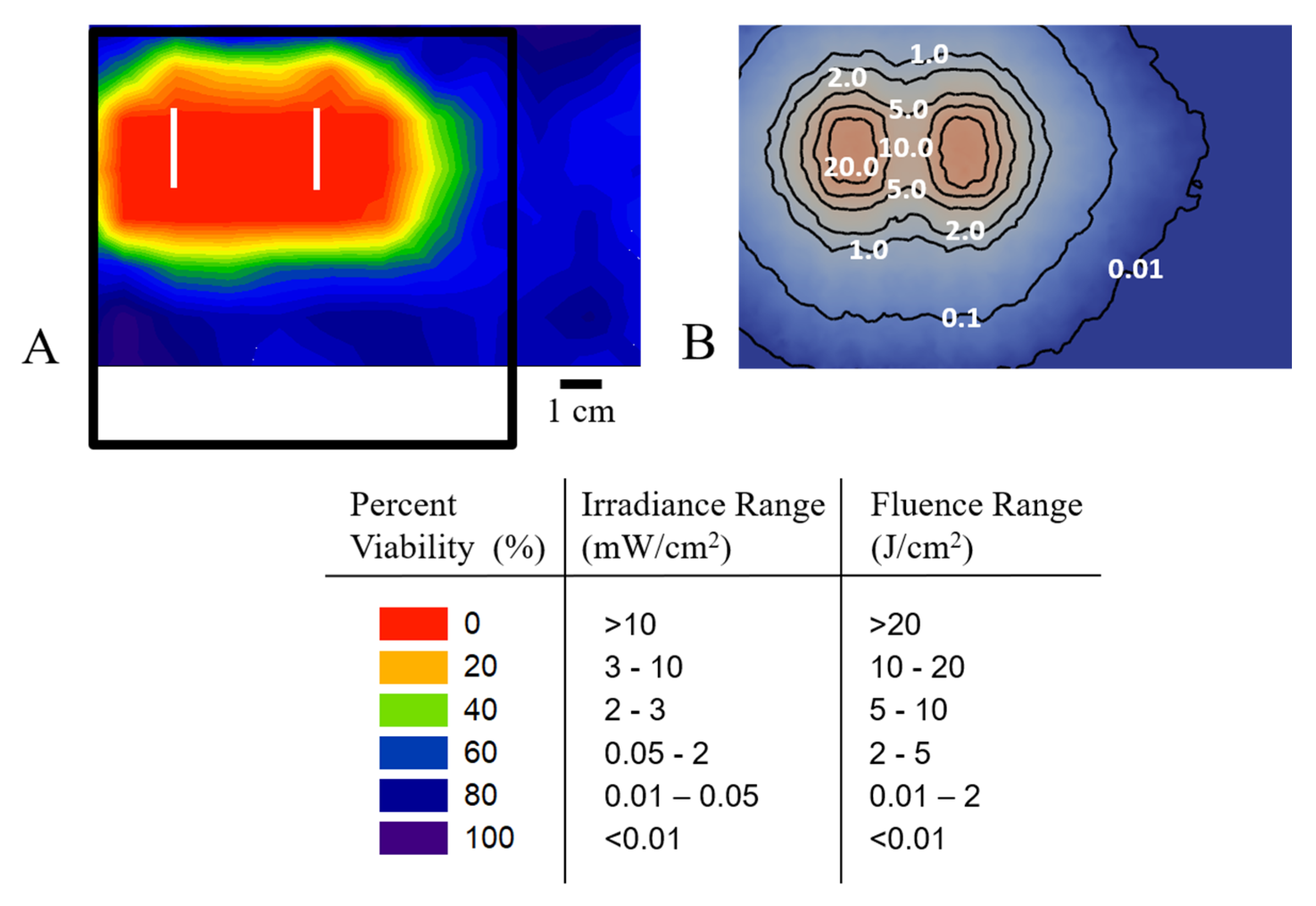
Figure 5). These Monte Carlo (MC) simulations were used to assess the relationship between the calculated irradiance and fluence distribution and cell viability. TLD1433-treated A549 cells growing in a 96-well plate were illuminated with the OSA and assessed for viability. Cell viability was determined over the OSA light field, both at the surface, and after passage through a tissue-mimicking phantom. The results (
Figure 4) revealed that the OSA delivered an effective light irradiance and fluence to a planned treatment area where a uniform cell death was measured in the illuminated region. The OSA enabled the delivery of precise and consistent light, confirmed by constructing viability maps at each phantom depth. Our simulations also suggest that the presence of tissue backscatter, which is often the case in areas such as the thoracic cavity, can affect light delivery. This result agrees with the dosimetry measurements performed in our previous studies [
13] and supports the notion that the OSA can be used for TLD1433-mediated IO-PDT.
In summary, this study suggests that TLD1433-mediated PDT with the OSA can be used to treat human adenocarcinoma (A549) cells. The use of 532 nm is more potent than 630 nm. The FullMonte is a promising platform to develop a pretreatment planning for TLD1433-mediated IO-PDT with OSA. More work is underway to test our treatment planning and tumor response to TLD1433-mediated IO-PDT in the thoracic cavity of animal models.
4. Materials and Methods
4.1. TLD1433
TLD1433 was prepared as previously described [
16,
17]. Its structure was confirmed by one- and two-dimensional nuclear magnetic resonance (NMR) spectroscopy and electrospray ionization mass spectrometry (ESI-MS). The purity was determined to be >95% using both high-pressure liquid chromatography (HPLC) and NMR [
17]. The UV-visible absorption spectrum was measured (data not shown) and agreed with previously reported optical properties [
18].
4.2. Cell Culture
Human adenocarcinoma cells (A549) were purchased from ATCC (ATCC, Manassas, VA, USA). The cells were cultured in Ham’s F-12K Nutrient Mixture, Kaighn’s Mod. All medium was supplemented with 10% fetal bovine serum (FBS) and 1% penicillin streptomycin. Cells were grown in a humidified incubator at 37 °C with 5% CO2. For plating, cells were washed with phosphate-buffered saline (pH 7.4), trypsinized using Ethylenediaminetetraacetic acid (EDTA), and counted in a hemocytometer using 0.4% trypan blue.
4.3. Phantom Construction
Tissue-mimicking phantom construction was previously described by our group [
13]. Briefly, gel phantoms were constructed using a combination of intralipid as the scattering agent, India ink as the absorber, and agar powder to make the substrate. The intralipid and India ink concentrations were chosen to produce phantoms with reduced scattering coefficient (µ
s’) of 7 cm
−1 and an absorption coefficient (µ
a) of 0.26 cm
−1 at 630 nm, and µ
s’ of 7.05 cm
−1 and µ
a of 0.24 cm
−1 at 532 nm [
13]. The phantoms were 3- or 5-mm thick.
4.4. In Vitro PDT
Cells were plated in wells at a seeding density of 2500 cells in 100 µL of media. After incubation for 24 h, the media was replaced with media containing TLD1433 to give final concentrations of 0.01 µM to 60 µM. Following 60 min incubation, the media (containing any TLD1433 not taken up by cells) was replaced with fresh media without TLD1433. Within 3-5 min, the TLD1433-treated cells were exposed to the therapeutic light. This procedure of TLD1433 media replacement follows previous methods described by Kaspler et al. [
9].
The cells were illuminated with either a 630-nm or 532-nm light. In one set of experiments, the light was delivered through a laser fiber with a micro-lens (front diffuser, FD1, Medlight SA, Ecublens, Switzerland). A 630-nm diode laser (ML-6500-630, Modulight Inc., Tampere, Finland) was used to illuminate the plate with an irradiance of 65 mW/cm2 and fluence of 230 J/cm2 with a spot size of 3-cm. A 2-W fiber coupled 532-nm diode laser (LSR532H-2W-FC, CivilLaser, Hangzhou, Zhejiang, China) was used to deliver 20 J/cm2 with an irradiance of 28 mW/cm2 with a spot size of 5-cm. After light treatment, the plates were returned to the incubator for 24 h.
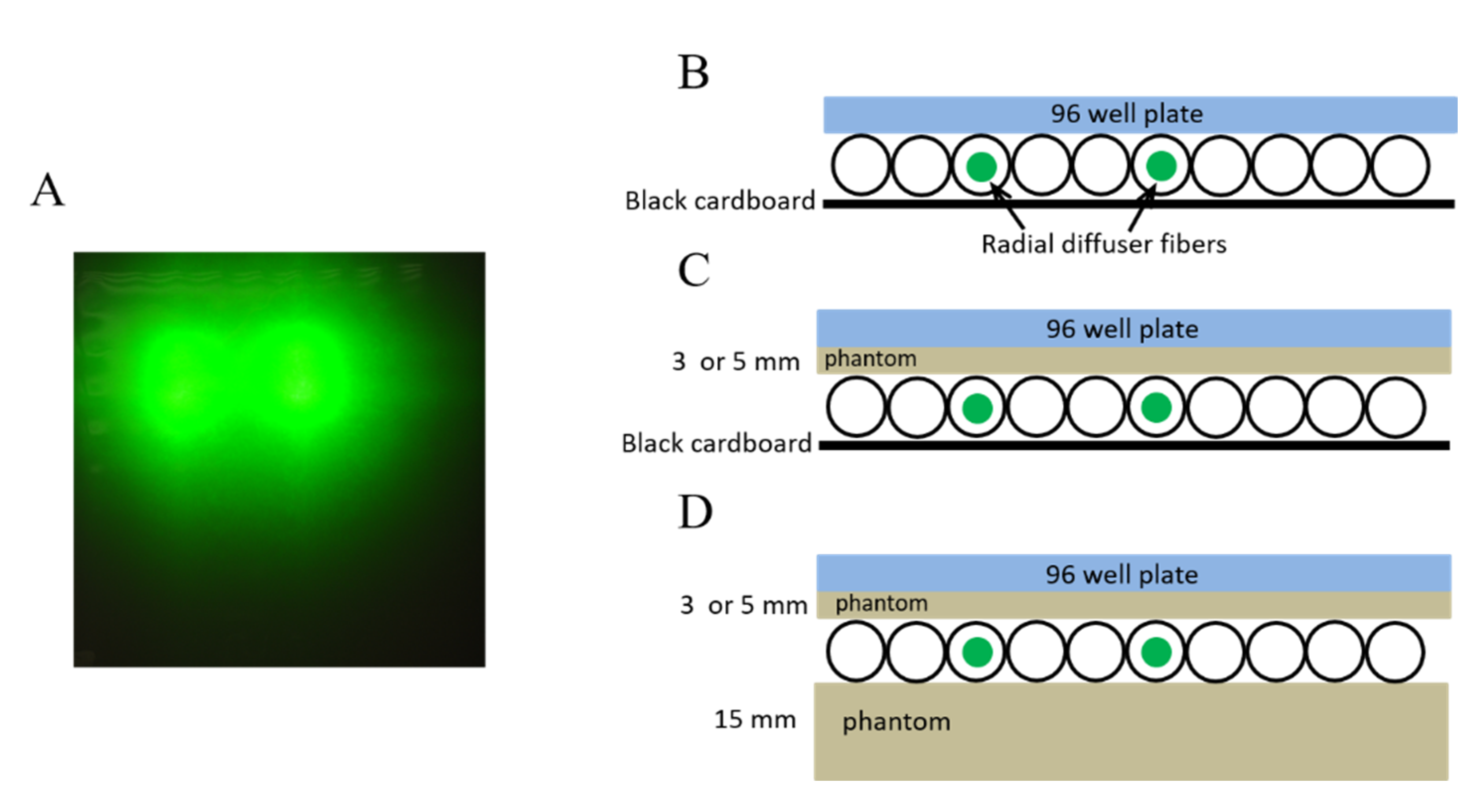
In another set of experiments, the cells were illuminated with the OSA, using two 2-cm radial diffusers (RD20, Medlight SA, Ecublens, Switzerland) that were placed 3 cm apart in parallel channels (
Figure 3A). Each fiber transmitted 532-nm light at 50 mW/cm for 278 s (a total 200 mW/cm for 55.6 J/cm). Plates were either placed in direct contact with the OSA or on top of a 3-mm to 5-mm phantom (
Figure 3B–D). This set up mimics the configuration where the OSA light is delivered in one direction towards the pleural in the chest cavity (
Figure 3B,C). In another configuration, the OSA was placed on a phantom that acted as a backscattering layer (
Figure 3D) that could be present due to surrounding tissue as we previously reported [
13]. All PDT-treated cells were evaluated alongside controls, including cells treated with light but not TLD1433 and cells treated with TLD1433 but not light. Controls for assessing cell growth in the absence of TLD1433 or light were also included. All experiments were conducted in triplicate.
After light treatment, the plates were returned to the incubator (the 37 °C, 5% CO2) for 24 h. Cell viability was then measured with a resazurin cell viability assay. Resazurin was incubated (37 °C, 5% CO2) with the cells for 4 h, then measured by quantifying fluorescence (excitation at 570 nm, emission at 585 nm) using a Varian, Cary Eclipse Fluorescence Spectrophotometer plate reader. The cell viability was normalized to growth control. Results of PDT response were plotted using Graphpad Prism, while viability maps were generated using SigmaPlot.
4.5. Treatment Planning
Light simulations were performed using an open source Monte Carlo (MC) software package (FullMonte,
https://gitlab.com/FullMonte) [
19]. A geometrical model was generated to mimic the in vitro experimental set ups shown in
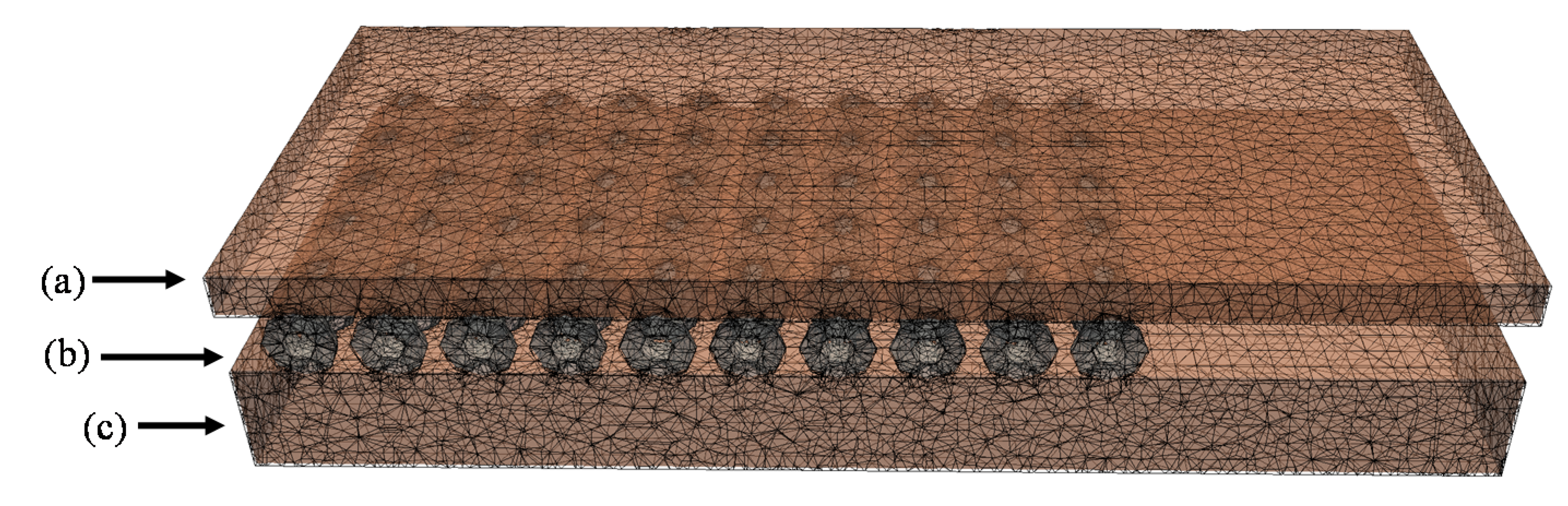
Figure 3. The model includes the OSA with the light sources and phantoms. The FullMonte’s Meshtool was used to generate the tetrahedral mesh (with up to 1.8 × 10
6 elements) shown in
Figure 5. For the Meshtool input parameters, the Cell Radius Edge Ratio, which defines the shape of the elements using the ratio of the circumradius of the tetrahedron and the shortest edge length, was kept at 2.0. In addition, the Smooth parameter, a unitless value used to smooth the surface of the mesh in Meshtool, was between 50–100, in order to keep the complex geometry of the beads in the OSA. Setting Smooth above 200 resulted in loss of beads in the OSA. The largest element size was 2.0 mm.
The light propagation was simulated with 10
7 photon packets emitted from two 2-cm line sources at 3 cm apart within the OSA. This configuration was identical to the set-up of the in vitro OSA light administration. Simulation results were output in photon packet weight. Irradiance calculated as the output* (Total input power (mW/cm2))/(number of simulated photon packets). Fluence was then acquired by multiplying the irradiance by the total time of light delivery. The 96-well plate was not included in the computation. It was assumed that the 96-well is placed at the surface of the OSA or at 3- or 5-mm of phantoms on top of the OSA surface (as in the configurations shown in
Figure 4B–D).
4.6. Patents
G.S. and D.B. are co-inventors of a patent application owned by Roswell Park for the OSA that was licensed to Lumeda Inc. S.A.M. is the inventor of two issued patents (9,345,769 and 9,676,806 B2) for TLD1433 that are licensed to Theralase Technologies, Inc.