Mantonico and Pecorello Grape Seed Extracts: Chemical Characterization and Evaluation of In Vitro Wound-Healing and Anti-Inflammatory Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction and Chemical Characterization of the Extracts
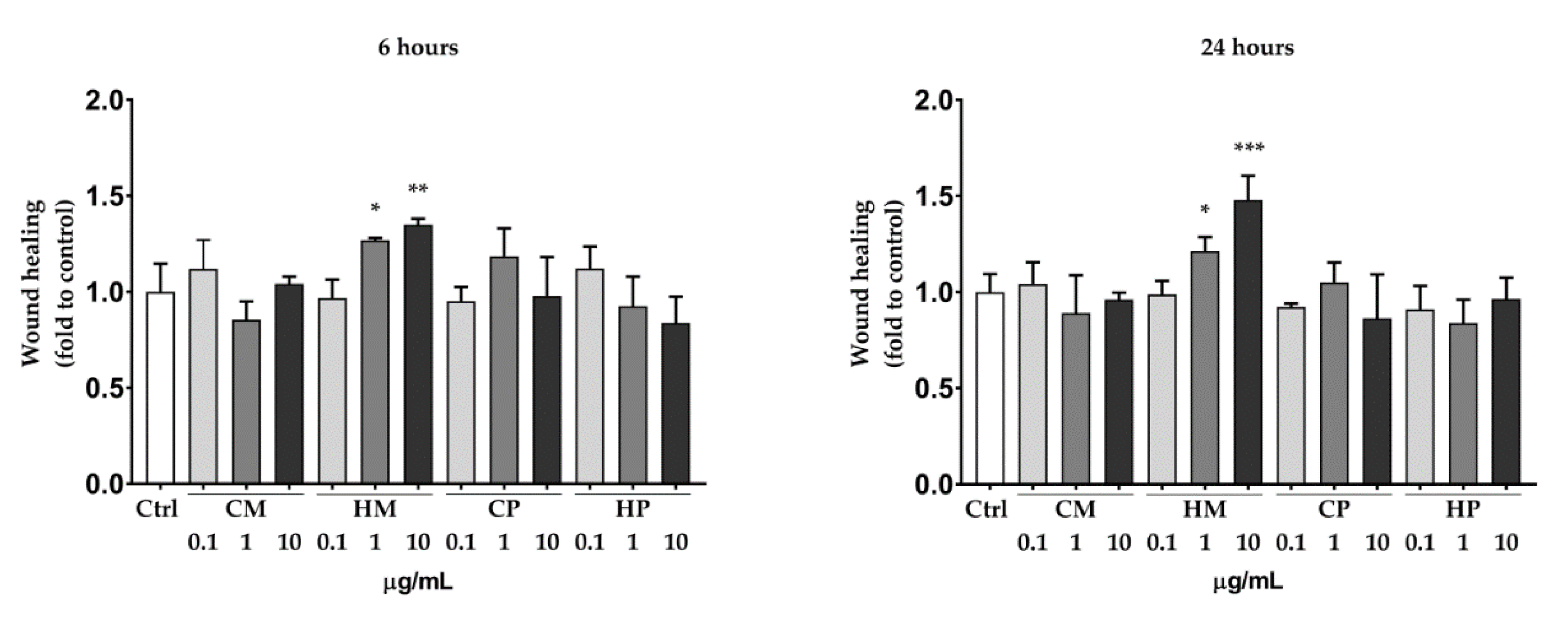
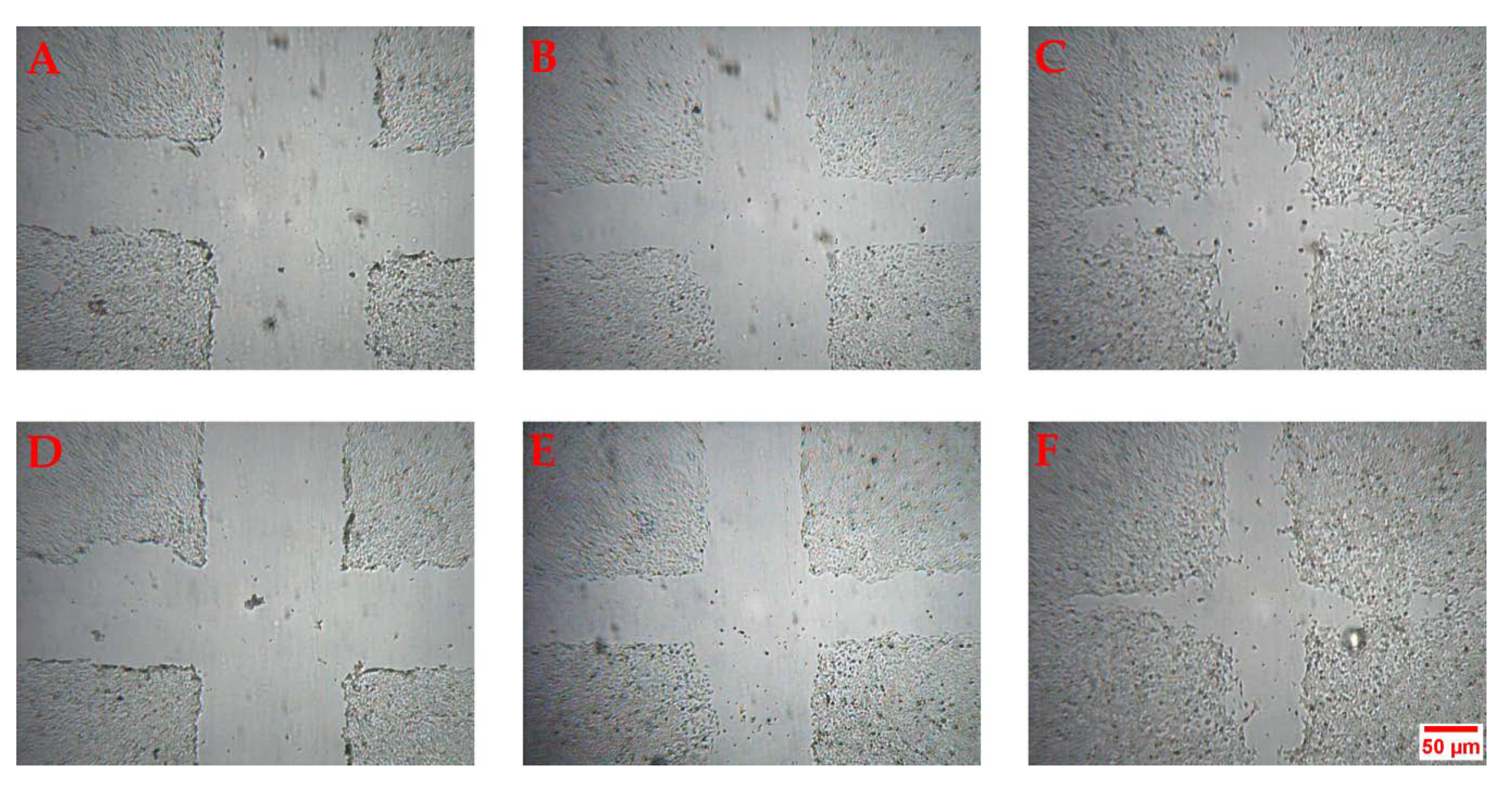
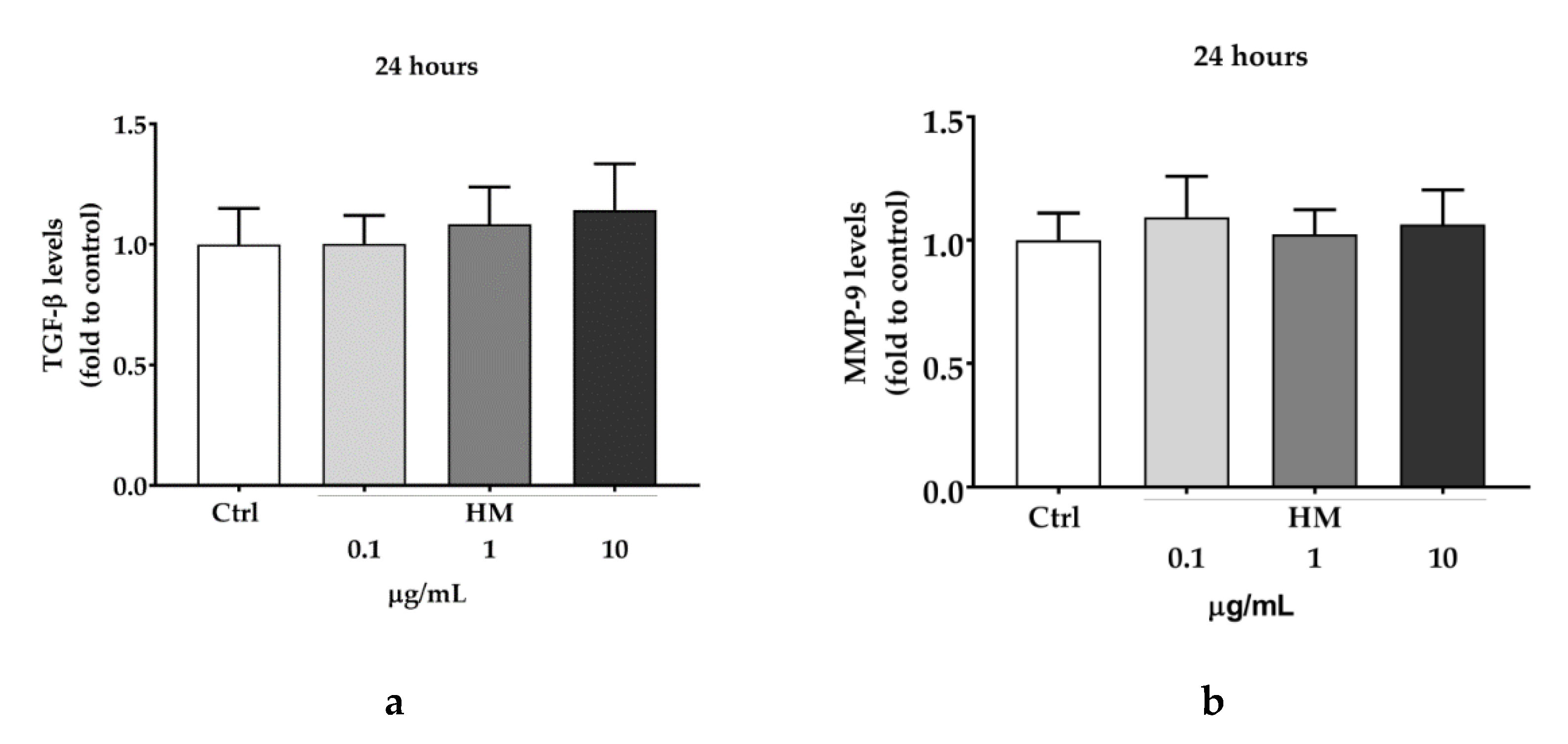
2.2. Scratch Wound Healing Assay in HaCaT Cell Line
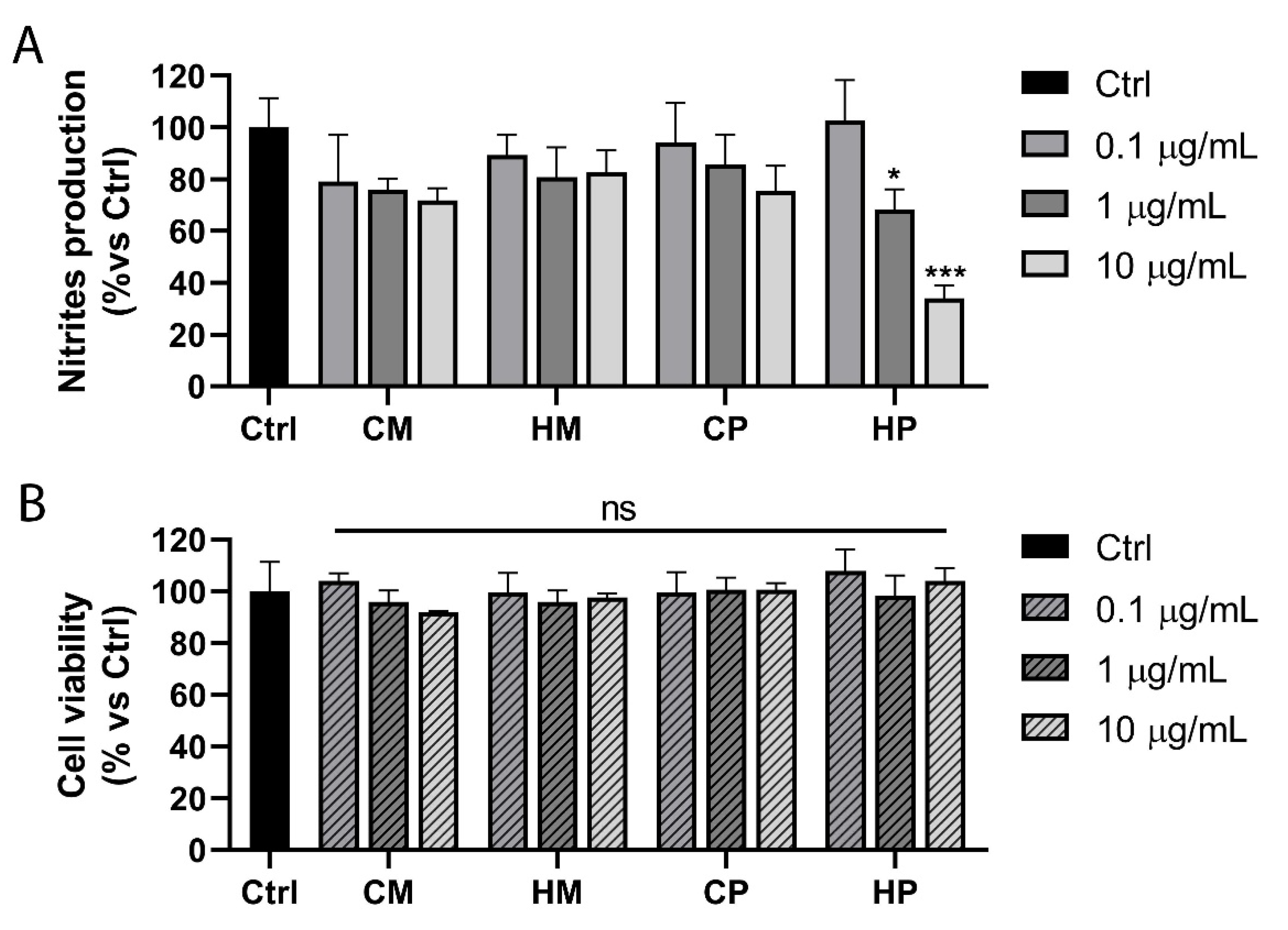
2.3. Inhibition of Nitric Oxide Production in Lipopolysaccharide (LPS)-Stimulated RAW 264.7 Macrophages
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Extraction Procedure
3.4. Gas-Chromatography/Mass Spectrometry (GC/MS) Analysis
3.5. Nuclear Magnetic Resonance (NMR) Analysis
3.6. Cell Cultures and Treatments
3.7. HaCaT Cell Viability Assay
3.8. HaCaT Scratch Wound Healing Assay
3.9. HaCaT Cell Proliferation Assay
3.10. TGF-β Production and MMP-9 Release
3.11. Inhibition of Nitric Oxide (NO) Production in Lipopolysaccharide (LPS)-Stimulated RAW 264.7 Cells
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Restuccia, D.; Giorgi, G.; Gianfranco Spizzirri, U.; Sciubba, F.; Capuani, G.; Rago, V.; Carullo, G.; Aiello, F. Autochthonous white grape pomaces as bioactive source for functional jams. Int. J. Food Sci. Technol. 2019, 54, 1313–1320. [Google Scholar] [CrossRef]
- Rubio, M.; Alvarez-Ortí, M.; Alvarruiz, A.; Fernández, E.; Pardo, J.E. Characterization of oil obtained from grape seeds collected during berry development. J. Agric. Food Chem. 2009, 57, 2812–2815. [Google Scholar] [CrossRef]
- Carullo, G.; Governa, P.; Spizzirri, U.G.; Biagi, M.; Sciubba, F.; Giorgi, G.; Loizzo, M.R.; Di Cocco, M.E.; Aiello, F.; Restuccia, D. Sangiovese cv pomace seeds extract-fortified kefir exerts anti-inflammatory activity in an in vitro model of intestinal epithelium using caco-2 cells. Antioxidants 2020, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, E.I.; Varelas, V.; Liouni, M.; Nerantzis, E.T. Grape seed oil: From a winery waste to a value added cosmetic product-a review. Edible Med. Non-Med. Plants 2012, 2, 867–878. [Google Scholar]
- Carullo, G.; Durante, M.; Sciubba, F.; Restuccia, D.; Spizzirri, U.G.; Ahmed, A.; Di Cocco, M.E.; Saponara, S.; Aiello, F.; Fusi, F. Vasoactivity of Mantonico and Pecorello grape pomaces on rat aorta rings: An insight into nutraceutical development. J. Funct. Foods 2019, 57, 328–334. [Google Scholar] [CrossRef]
- García-Lomillo, J.; González-SanJosé, M.L.; Del Pino-García, R.; Rivero-Pérez, M.D.; Muñiz-Rodríguez, P. Antioxidant and antimicrobial properties of wine byproducts and their potential uses in the food industry. J. Agric. Food Chem. 2014, 62, 12595–12602. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, A.; Xiao, W.; Zhang, P.; Han, X.; Zhou, T.; Chen, Y.; Jin, J.; Ma, X. Morin induces endothelium-dependent relaxation by activating TRPV4 channels in rat mesenteric arteries. Eur. J. Pharmacol. 2019, 859, 172561. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape seed oil compounds: Biological and chemical actions for health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Romani, A.; Campo, M.; Lombardi-Boccia, G.; Cecchini, F. Bio-based compounds from grape seeds: A biorefinery approach. Molecules 2018, 23, 1888. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Kiefer, J.; Santini, A.; Lombardi-Boccia, G.; Souto, E.B.; Romani, A.; Lampe, A.; Nicoli, S.F.; Gabrielli, P.; et al. Grape seeds: Chromatographic profile of fatty acids and phenolic compounds and qualitative analysis by FTIR-ATR spectroscopy. Foods 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Governa, P.; Carullo, G.; Biagi, M.; Rago, V.; Aiello, F. Evaluation of the in vitro wound-healing activity of calabrian honeys. Antioxidants 2019, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, A.A.; Aghel, N.; Rashidi, I.; Gholampur-Aghdami, A. Topical grape (Vitis vinifera) seed extract promotes repair of full thickness wound in rabbit. Int. Wound J. 2011, 8, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Venojarvi, M.; Roy, S.; Sharma, N.; Trikha, P.; Bagchi, D.; Bagchi, M.; Sen, C.K. Dermal wound healing properties of redox-active grape seed proanthocyanidins. Free Radic. Biol. Med. 2002, 33, 1089–1096. [Google Scholar] [CrossRef]
- Hemmati, A.A.; Foroozan, M.; Houshmand, G.; Moosavi, Z.B.; Bahadoram, M.; Maram, N.S. The topical effect of grape seed extract 2% cream on surgery wound healing. Glob. J. Health Sci. 2015, 7, 52–58. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, Q.; Chang, J.; Wu, C. Grape seed-inspired smart hydrogel scaffolds for melanoma therapy and wound healing. ACS Nano 2019, 13, 4302–4311. [Google Scholar] [CrossRef]
- Moalla Rekik, D.; Ben Khedir, S.; Ksouda Moalla, K.; Kammoun, N.G.; Rebai, T.; Sahnoun, Z. Evaluation of wound healing properties of grape seed, sesame, and fenugreek oils. Evid. Based. Complement Alternat. Med. 2016, 2016, 7965689. [Google Scholar] [CrossRef]
- McDaniel, J.C.; Belury, M.; Ahijevych, K.; Blakely, W. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008, 16, 337–345. [Google Scholar] [CrossRef]
- Silva, J.R.; Burger, B.; Kühl, C.M.C.; Candreva, T.; dos Anjos, M.B.P.; Rodrigues, H.G. Wound healing and omega-6 fatty acids: From inflammation to repair. Mediat. Inflamm. 2018, 2018, 2503950. [Google Scholar] [CrossRef]
- Pereira, L.M.; Hatanaka, E.; Martins, E.F.; Oliveira, F.; Liberti, E.A.; Farsky, S.H.; Curi, R.; Pithon-Curi, T.C. Effect of oleic and linoleic acids on the inflammatory phase of wound healing in rats. Cell Biochem. Funct. 2008, 26, 197–204. [Google Scholar] [CrossRef]
- Carullo, G.; Governa, P.; Leo, A.; Gallelli, L.; Citraro, R.; Cione, E.; Caroleo, M.C.; Biagi, M.; Aiello, F.; Manetti, F. Quercetin-3-Oleate contributes to skin wound healing targeting FFA1/GPR40. ChemistrySelect 2019, 4, 8429–8433. [Google Scholar] [CrossRef]
- Carullo, G.; Perri, M.; Manetti, F.; Aiello, F.; Caroleo, M.C.; Cione, E. Quercetin-3-oleoyl derivatives as new GPR40 agonists: Molecular docking studies and functional evaluation. Bioorganic Med. Chem. Lett. 2019, 29, 1761–1764. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Hao, H.; Fu, X.; Han, W. Insight into reepithelialization: How do mesenchymal stem cells perform? Stem Cells Int. 2016, 2016, 6120173. [Google Scholar] [CrossRef] [PubMed]
- Mazgaeen, L.; Gurung, P. Recent advances in lipopolysaccharide recognition systems. Int. J. Mol. Sci. 2020, 21, 379. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress--implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Kawata, A.; Murakami, Y.; Suzuki, S.; Fujisawa, S. Anti-inflammatory activity of β-carotene, lycopene and Tri-n-butylborane, a scavenger of reactive oxygen species. In Vivo 2018, 32, 255–264. [Google Scholar]
- Perri, F.; Frattaruolo, L.; Haworth, I.; Brindisi, M.; El-magboub, A.; Ferrario, A.; Gomer, C.; Aiello, F.; Adams, J.D. Naturally occurring sesquiterpene lactones and their semi-synthetic derivatives modulate PGE2 levels by decreasing COX2 activity and expression. Heliyon 2019, 5, e01366. [Google Scholar] [CrossRef]
- Borgonetti, V.; Governa, P.; Biagi, M.; Dalia, P.; Corsi, L. Rhodiola rosea L. modulates inflammatory processes in a CRH-activated BV2 cell model. Phytomedicine 2020, 68, 153143. [Google Scholar] [CrossRef]
- Chiocchio, I.; Poli, F.; Governa, P.; Biagi, M.; Lianza, M. Wound healing and in vitro antiradical activity of five Sedum species grown within two sites of community importance in Emilia-Romagna (Italy). Plant Biosyst.-An Int. J. Deal. All Asp. Plant Biol. 2019, 153, 610–615. [Google Scholar] [CrossRef]
- He, F. Bradford protein assay. Bio-protocol 2011, 1, e45. [Google Scholar] [CrossRef]
- Mazzotta, S.; Frattaruolo, L.; Brindisi, M.; Ulivieri, C.; Vanni, F.; Brizzi, A.; Carullo, G.; Cappello, A.R.; Aiello, F. 3-Amino-alkylated indoles: Unexplored green products acting as anti-inflammatory agents. Future Med. Chem. 2020, 12, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A.R. Antioxidant and anti-inflammatory activities of flavanones from Glycyrrhiza glabra L. (licorice) leaf phytocomplexes: Identification of licoflavanone as a modulator of NF-kB/MAPK pathway. Antioxidants 2019, 8, 186. [Google Scholar] [CrossRef] [PubMed]
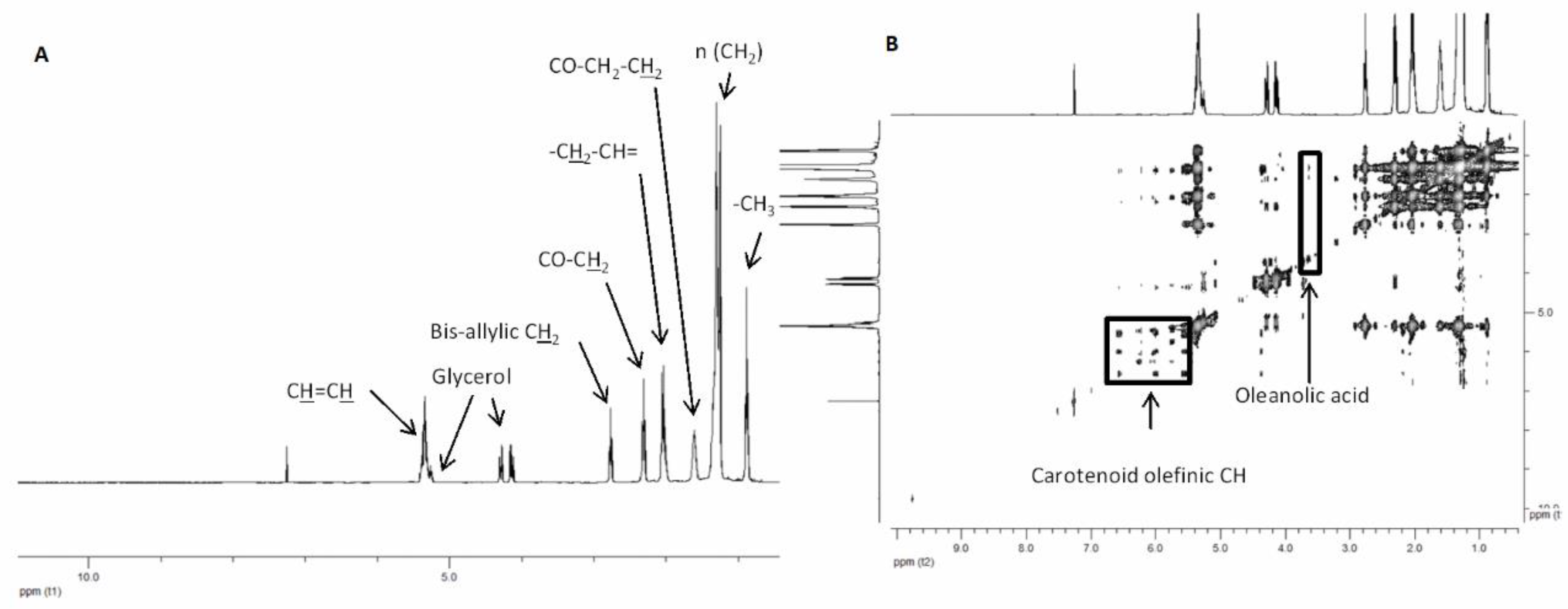

| Compounds 1 | Assignment 2 | Multiplicity 3 | δ H (ppm) | Amount (µmol/g) 4 | |||
|---|---|---|---|---|---|---|---|
| CM | CP | HM | HP | ||||
| Stearic acid | CH2-CO2- | t | 2.30 | 24.71 | 14.21 | 269.58 | 69.87 |
| Oleic acid | CH2-CH=CH | m | 2.03 | 33.93 | 18.47 | 375.04 | 97.44 |
| Linoleic acid | =CH-CH2-CH= | t | 2.76 | 92.71 | 64.04 | 1177.66 | 277.85 |
| Oleanoic acid | CH-3 | m | 3.60 | 3.24 | 1.79 | 17.79 | 4.21 |
| Glycerol | CH2 | dd | 3.65–3.55 | 48.57 | 31.31 | 587.87 | 142.58 |
| Carotenoids | CH-11,11′ | m | 6.68 | 0.77 | 0.54 | 2.69 | 7.19 |
| Total Phenols | Aromatic moieties | m | 6.8–7.0 | 3.11 | 1.46 | 3.02 | 0.00 |
| Aldehydes | CHO | brs | 9.76 | 0.35 | 0.09 | 2.90 | 0.87 |
| CM | CP | HM | HP | |
|---|---|---|---|---|
| Compounds | % of compound | |||
| 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | - | - | 2.19 | 0.53 |
| 2,4 Decadienal | 13.43 | - | 9.76 | 10.37 |
| 2,4-Decadienal, (E,E)- | 9.17 | 15.59 | - | 5.83 |
| 2-Decenal, (E)- | - | 5.01 | - | 1.48 |
| 2-Decenal, (Z)- | - | - | 4.02 | - |
| E-2-dodecenal | - | - | - | 0.93 |
| Eicosane | - | - | 5.57 | 0.48 |
| 2-Heptenal, (E)- | 12.45 | 12.59 | 12.45 | 4.53 |
| 13-Hexacosyne | - | - | 2.41 | - |
| Hexadecanoic acid | 5.10 | 5.58 | 3.96 | 8.03 |
| Hexadecanoic acid, ethyl ester | - | - | - | 0.89 |
| Hexane, 1,1-diethoxy- | - | 1.58 | 5.27 | 1.18 |
| Linoleic acid, butyl ester | 2.91 | - | - | - |
| Linoleic acid, ethyl ester | - | 9.87 | - | - |
| 9,12-Octadecadienoic acid, ethyl ester | - | - | - | 12.30 |
| 9,12-Octadecadienoic acid (Z,Z)- | 42.50 | 31.19 | 6.59 | 49.60 |
| Octadecane | - | - | 2.53 | - |
| Octadecanoic acid | 11.22 | - | 7.58 | - |
| 9-Octadecenoic acid (Z)- | - | - | 14.09 | - |
| 1-Octanamine, N-methyl-N-octyl- | - | - | 3.31 | - |
| 1-Octanamine, N,N-dioctyl- | - | - | 2.13 | - |
| 2 Octenal | - | 1.58 | - | - |
| 2-Octenal, (E)- | - | - | - | 0.59 |
| 1-Octen, 3-ol | - | 2.09 | - | - |
| Phthalic acid, isobutyl nonyl ester | - | 0.51 | - | - |
| Tetradecanoic acid, ethyl ester | - | 0.98 | - | - |
| Treatment | 6 h | 24 h |
|---|---|---|
| Ctrl | 19.39 ± 3.18 | 36.64 ± 3.86 |
| TGF-β a | 17.06 ± 1.72 | 43.87 ± 2.58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carullo, G.; Sciubba, F.; Governa, P.; Mazzotta, S.; Frattaruolo, L.; Grillo, G.; Cappello, A.R.; Cravotto, G.; Di Cocco, M.E.; Aiello, F. Mantonico and Pecorello Grape Seed Extracts: Chemical Characterization and Evaluation of In Vitro Wound-Healing and Anti-Inflammatory Activities. Pharmaceuticals 2020, 13, 97. https://doi.org/10.3390/ph13050097
Carullo G, Sciubba F, Governa P, Mazzotta S, Frattaruolo L, Grillo G, Cappello AR, Cravotto G, Di Cocco ME, Aiello F. Mantonico and Pecorello Grape Seed Extracts: Chemical Characterization and Evaluation of In Vitro Wound-Healing and Anti-Inflammatory Activities. Pharmaceuticals. 2020; 13(5):97. https://doi.org/10.3390/ph13050097
Chicago/Turabian StyleCarullo, Gabriele, Fabio Sciubba, Paolo Governa, Sarah Mazzotta, Luca Frattaruolo, Giorgio Grillo, Anna Rita Cappello, Giancarlo Cravotto, Maria Enrica Di Cocco, and Francesca Aiello. 2020. "Mantonico and Pecorello Grape Seed Extracts: Chemical Characterization and Evaluation of In Vitro Wound-Healing and Anti-Inflammatory Activities" Pharmaceuticals 13, no. 5: 97. https://doi.org/10.3390/ph13050097
APA StyleCarullo, G., Sciubba, F., Governa, P., Mazzotta, S., Frattaruolo, L., Grillo, G., Cappello, A. R., Cravotto, G., Di Cocco, M. E., & Aiello, F. (2020). Mantonico and Pecorello Grape Seed Extracts: Chemical Characterization and Evaluation of In Vitro Wound-Healing and Anti-Inflammatory Activities. Pharmaceuticals, 13(5), 97. https://doi.org/10.3390/ph13050097












