Abstract
On 11 March 2020, the coronavirus disease (COVID-19) was defined by the World Health Organization as a pandemic. Severe acute respiratory syndrome-2 (SARS-CoV-2) is the newly evolving human coronavirus infection that causes COVID-19, and it first appeared in Wuhan, China in December 2019 and spread rapidly all over the world. COVID-19 is being increasingly investigated through virology, epidemiology, and clinical management strategies. There is currently no established consensus on the standard of care in the pharmacological treatment of COVID-19 patients. However, certain medications suggested for other diseases have been shown to be potentially effective for treating this infection, though there has yet to be clear evidence. Therapies include new agents that are currently tested in several clinical trials, in addition to other medications that have been repurposed as antiviral and immune-modulating therapies. Previous high-morbidity human coronavirus epidemics such as the 2003 SARS-CoV and the 2012 Middle East respiratory syndrome coronavirus (MERS-CoV) prompted the identification of compounds that could theoretically be active against the emerging coronavirus SARS-CoV-2. Moreover, advances in molecular biology techniques and computational analysis have allowed for the better recognition of the virus structure and the quicker screening of chemical libraries to suggest potential therapies. This review aims to summarize rationalized pharmacotherapy considerations in COVID-19 patients in order to serve as a tool for health care professionals at the forefront of clinical care during this pandemic. All the reviewed therapies require either additional drug development or randomized large-scale clinical trials to be justified for clinical use.
Keywords:
COVID-19; SARS-CoV-2; remdesivir; chloroquine; lopinavir; favipiravir; baricitinib; interferons; ACE2; TMPRSS2 1. Introduction
The pandemic of the coronavirus disease (COVID-19) has quickly spread to affect almost all countries and territories around the world. The agent causing the infection was rapidly detected to be a beta coronavirus, first named the novel coronavirus (2019-nCoV). The International Committee of Taxonomy of Viruses changed the name to severe acute respiratory syndrome-2 (SARS-CoV-2), denoting the coronavirus as causing severe acute respiratory syndrome-2 [1].
The reported COVID-19 case fatality ranges from 1% to 7%. According to reports of the World Health Organization (WHO), these values must be interpreted with caution. In countries that have implemented massive screening for the whole population, like South Korea, the overall case fatality may be less than 1%, as a large number of mild or asymptomatic cases have been counted in the denominator of their equation. Due to the huge, unprecedented scale of the pandemic, the actual numbers of deaths are considerable [2].
Respiratory failure was obviously a major cause of death in previous viral pandemics, including Spanish flu in 1918 and Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012, and this is also the case in the new COVID-19 [3]. Besides conventional treatment, antiviral therapy (both classic and novel repurposed medications) may shorten the course of the disease and improve its outcome. However, there is currently no supporting evidence for these therapies apart from small studies or case series. Severe cases of COVID-19 may need assisted ventilation through various modalities [4]. A high number of severe cases (especially elderly) may progress to acute severe lung injury and/or multiple organ failure due to a deranged immune response or a “cytokine storm,” the treatment of which may involve immunomodulators. This review covers the key medications suggested as potential agents under investigation in registered clinical trials for the treatment of COVID-19. Broadly, they are either repurposed antiviral, other anti-infective, or immunomodulatory agents, in addition to drugs that act on host cell receptors. The ventilatory and hemodynamic support of critically-ill COVID-19 patients has been reviewed elsewhere [5,6].
The significant advances and continuous progress of computational analysis have greatly served the study of epidemiological aspects COVID-19 through data collection, compilation, and dissemination. In parallel, advances in molecular technology and the availability of the virus genome database have allowed for the identification of the virus and its mutations. An important approach to drug discovery is the screening of chemical libraries, including a large number of available molecules or databases of transcriptional signatures in various cell lines. Ongoing studies are conducted to validate such molecules as potential therapies [7].
On the other hand, several products have appeared in the market with strong claims of effectiveness against SARS-CoV-2 infection. Some constitute a risk on patients’ health, e.g., chlorine dioxide products, known as a “Miracle Mineral Solution” that was warned against by the FDA (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-warns-seller-marketing-dangerous-chlorine-dioxide-products-claim).
The broad strategies for COVID-19 treatment include the targeting of key enzymes of SARS-CoV-2, thus interfering with the viral cycle inside the host cell by using repurposed antiviral drugs previously tested for other coronaviruses like SARS-CoV and MERS-CoV, as well as other RNA virus infections, e.g., HIV and HCV. Other anti-infective agents have also been proposed. In addition, targets on the host cell surface may represent potential COVID-19 therapy-inhibiting virus entries. The second strategy is immunomodulation by using non-specific immunoglobulins and interferons or specific agents, e.g., tyrosine kinase inhibitors and monoclonal antibodies. Immunomodulation is particularly needed early in the disease to boost antiviral immunity, as well as in severe cases where an uncontrolled immune response may lead to acute lung injury and other organ damage.
This review covers key medications proposed for COVID-19 that have been registered in various clinical trials (https://clinicaltrials.gov/), including the Chinese clinical trial database (http://www.chictr.org.cn/index.aspx).
2. The SARS-CoV-2 Infection
2.1. The Structure of the Virus
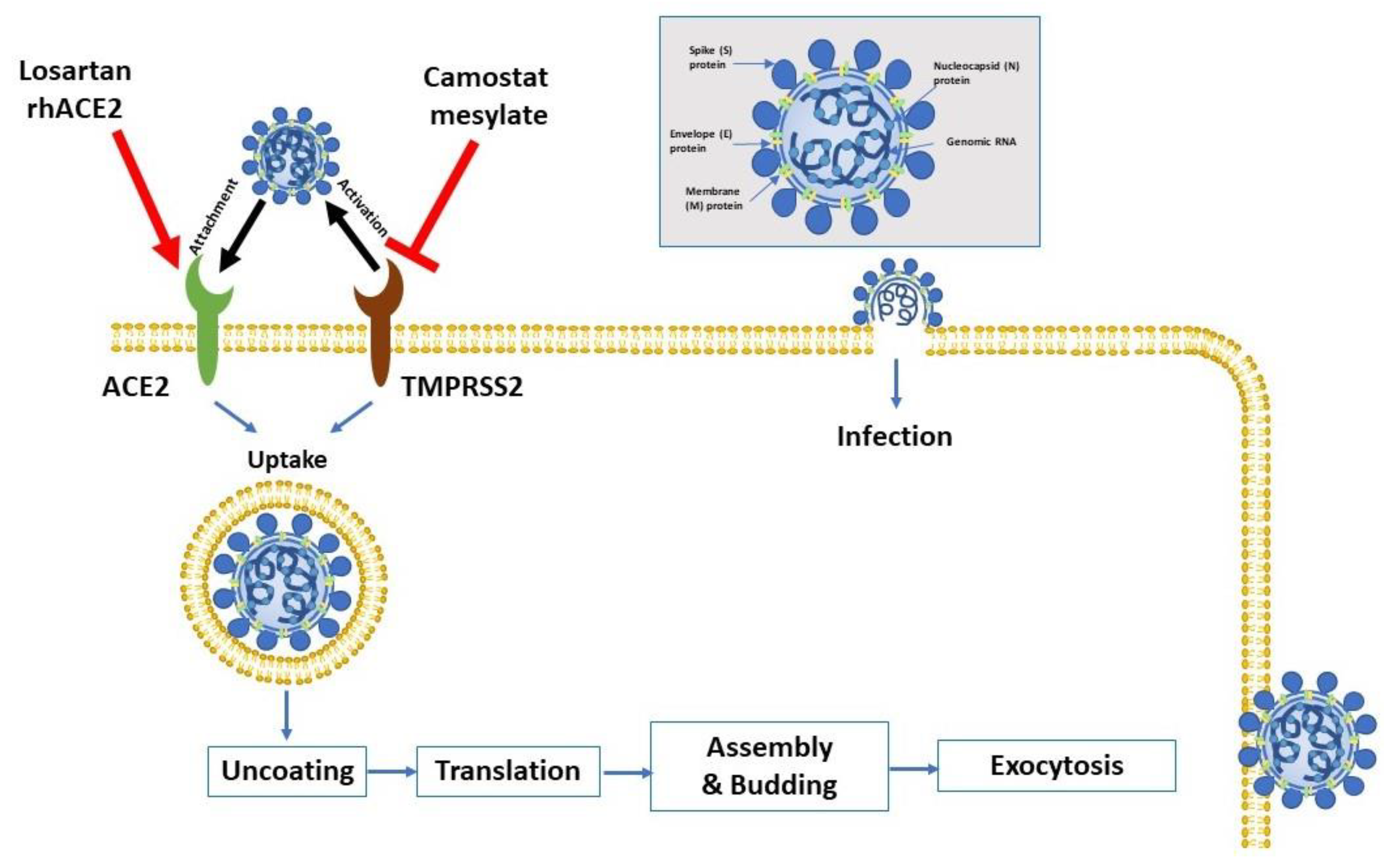
Coronaviruses belong to Nidovirales order; viruses depending on a nested group of mRNAs for their replication (“nido-” = “nest”). They contain the biggest known viral RNA genomes (27–32 kb in length). These viruses are enveloped positive-sense single-stranded RNA viruses. Figure 1 shows the key virus proteins. Under electron microscopy, the virus has a characteristic crown-like shape due to the presence of the spike (S) protein [8,9].
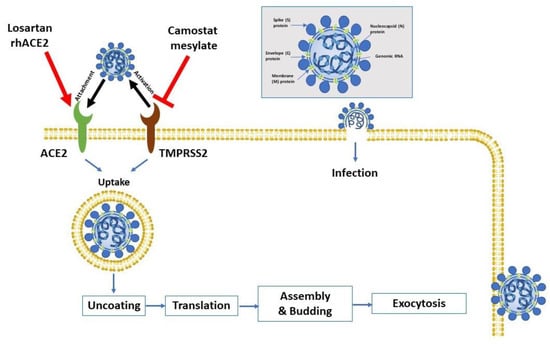
Figure 1.
Virus entry into the host cell. The attachment protein “-spike glycoprotein” of the severe acute respiratory syndrome-2 (SARS-CoV-2) uses a cellular attachment factor (angiotensin-converting enzyme 2 (ACE2)) and uses the cellular protease TMPRSS2 (transmembrane protease serine 2) for its activation. ACE2 can be activated via either losartan or recombinant human ACE 2 (rhACE2). Potential pharmacotherapeutic approaches include the use of camostat mesylate (which is a TMPRSS2 inhibitor) to block the priming of the spike protein, increasing the number of ACE2 receptors via losartan, and the use of soluble recombinant human ACE2 (which should slow viral entry into cells via competitive binding with SARS-CoV-2). The structure of SARS-CoV-2 is shown in the upper right.
The S protein is extensively glycosylated, forming a homotrimer. It mediates virus entry through binding to specific receptors (e.g., angiotensin-converting enzyme 2 (ACE2)) and fusion with the cell membrane of the host (Figure 1). The S protein harbors the major antigen that stimulates the formation of neutralizing antibodies, in addition to targets of cytotoxic lymphocytes. The membrane (M) protein has a major role in viral assembly [10]. The nucleocapsid (N) protein is responsible for the regulation of viral RNA synthesis—it interacts with M protein during the budding of the virus. It forms part of the nucleocapsid (in association with the RNA). SARS-CoV-2 has sequence homology with the influenza virus through hemagglutinin-esterase glycoprotein (HE). The HE binds to neuraminic acid on the host cell surface, leading to the adsorption of the virus on the cell surface. Such homology may denote an early recombination between the two viruses. Finally, the function of the envelope (E)protein is not fully elucidated. Proteins M, N, and E are essential for the virus’ assembly and release [11].
2.2. Clinical Picture
The incubation period is thought to be 14 days after exposure [12]. In more than 80% of patients, the symptoms are mild. However, in 14% of cases, especially in the elderly, the symptoms of dyspnea and hypoxia may develop (with more than 50% lung involvement on computed tomography (CT) scan). In around 5% of cases, in a late phase of the disease, respiratory failure (due to acute lung injury), shock, and/or multiple organ dysfunction (due to the cytokine storm) may develop [13]. Severe cases of COVID-19 that require intensive care unit (ICU) admission have been shown to have increased plasma levels of key inflammatory cytokines including interleukin-2 (IL-2) and tumor necrosis factor-alpha (TNF-α), among many others, thus indicating a cytokine storm that correlates with disease severity [13]. Uncontrolled pulmonary inflammation is a major cause of death in SARS-CoV-2 infection. More than 80% of patients with COVID-19 have lymphopenia [13], suggesting the possible pulmonary recruitment of lymphocytes. Another explanation of lymphopenia is immune-related cell apoptosis or pyroptosis.
4. Immuno-Modulatory Agents
4.1. Baricitinib
Baricitinib is a small, orally active molecule that inhibits Janus kinase 1 and2. The Janus kinase family, including JAK1, JAK2, JAK3, and tyrosine kinase 2 (Tyk2), has important roles in some signaling pathways through its triggering of the cytokine-induced phosphorylation of STAT that is later transported to the nucleus to regulate gene transcription [64,65].
Baricitinib is reported to be effective in the treatment of rheumatoid arthritis in several clinical trials [65], with excellent results in terms of clinical response and a good safety profile [66]. The literature has reported that the drug blocks the phosphorylation of STATs induced by different cytokines in the blood, and it causes a transient alteration in neutrophil and lymphocyte counts [66,67,68,69].
Receptor-mediated endocytosis is the most common pathway for virus entry to the cells. ACE2, a cell surface protein expressed by cells in the kidney, blood vessels, heart, and, most importantly, lung AT2 alveolar epithelial cells, is the receptor used by SARS-CoV-2 to infect lung cells [70]. AP2-associated protein kinase 1 (AAK1) is a pivotal regulator of clathrin-mediated endocytosis; thus, the disruption of AAK1 would interfere with the virus passage into the cells and further prevent the intracellular assembly of virus particles [71].
A benevolent Artificial intelligence (AI)s knowledge graph and customizations bespoke to SARS-CoV-2 were used together to identify drugs that may halt the viral infection process. Baricitinib was expected to have a high binding affinity to AAK1 and cyclin G-associated kinase, another regulator of endocytosis [72]. Accordingly, baricitinib is suggested to be trialed on patients with SARS-CoV-2 acute respiratory disease in order to reduce the viral entry and the associated inflammation (NCT04321993).
A great chance for potential combination therapy with baracitinib is present due to its favorable pharmacokinetic properties such as low plasma protein binding affinity, minimal interaction with CYP enzymes, and drug transporters [73]. Currently, in the COVID-19 outbreak, baricitinib is being combined with different antivirals, such as lopinavir, ritonavir, and remdesivir, since this combination would decrease viral infectivity, viral replication, and the exaggerated host inflammatory response.
On the other hand, using baricitinib is associated with an increased risk of developing severe infections due to opportunistic pathogens, especially if it is taken concomitantly with immunosuppressants such as corticosteroids [74]. Tuberculosis was identified in patients receiving baricitinib, and patients should be tested prior to and during therapy for latent tuberculosis infection. In addition, thrombosis, including deep venous thrombosis and pulmonary embolism, has been documented more than placebo in patients treated with baricitinib [74].
4.2. Ruxolitinib
Ruxolitinib is an orally available and potent JAK1 and JAK2 inhibitor. It was the first drug for myelofibrosis to be approved by the FDA in November 2011 [75]. The pharmacokinetic analysis of ruxolitinib predicted that the unbound plasma concentration required to inhibit clathrin-mediated endocytosis greatly exceeds the currently tolerated therapeutic dose [73]. Therefore, viral infectivity at therapeutic doses is unlikely to decrease, but the drug may reduce the inflammatory response of the host by inhibiting JAK. Ruxolitinib is currently being studied to combine it with COVID-19 mesenchymal stem cell infusion (NCT04252118).
4.3. Camrelizumab
Camrelizumab is a humanized monoclonal antibody against programmed cell death 1 (PD-1) receptors. PD-1 receptors are expressed on the surface of T cells and act as negative regulators of T cell function. Camrelizumab has been reported to block PD-1′s binding to its ligand, PD-L1, thus inhibiting the immune escape of tumor cells [76]. Recently, in China, the drug has received conditional approval for the treatment of relapsed or refractory Hodgkin’s lymphoma. Camrelizumab is also being investigated as a possible therapy for other malignancies [76].
After antigen exposure, the expression of PD-1 on T-cells is induced rapidly, following the engagement between the T-cell receptor (TCR) and the loaded major histocompatibility complex (MHC) molecule in the draining lymph nodes [77]. The presence of various pro-inflammatory mediators such as IL-2, IL-6, IL-7, and IL-15 in the affected tissue contribute further to PD-1′s up-regulation of T-cells [78]. The interaction between PD-1 and PD-1L provides signals that tolerize T-cells to their antigenic targets, neutralizing their effector functions [78]. Furthermore, in sepsis patients, PD-1 and PD-L1 are known to be the primary mediators in T cell depletion [79]. Animal models have shown that blocking PD-1 or PD-L1 prevents T cell death, regulates cytokine production, and reduces both organ dysfunction and death in sepsis [80]. Researchers are therefore attempting to perform clinical trials to determine the effectiveness of PD-1 inhibitors in 2019 novel coronavirus infection in patients with serious lymphocytopenia-associated pneumonia.
On the other hand, the use of camrelizumab is associated with a good safety profile; the high incidence of all adverse drug reactions is related to reactive capillary hemangiomas, which are generally unthreatening and self-limited [81]. Currently, camrelizumab is being investigated for its effectiveness in COVID-19 (NCT04268537).
4.4. Eculizumab
Eculizumab is a humanized monoclonal IgG antibody that binds to the C5 complement protein, preventing cleavage into C5a and C5b. Blocking the formation of C5b prevents terminal complex C5b-9 or membrane attack complex (MAC) from subsequent formation. In the United States, eculizumab is approved for treating paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome [82,83].
The literature has identified the complement system as an essential host mediator for both SARS-CoV- and MERS-CoV-induced disease and has suggested that complement activation controls the systemic pro-inflammatory response against the viral infection [84,85]. Accordingly, eculizumab is expected to stop immune-mediated death in patients infected with SARS-CoV-2. Currently, the drug is under clinical trials for its effectiveness in patients with confirmed COVID-19 infection who are in ICU due to ARDS (NCT04288713).
4.5. Meplazumab
Meplazumab is a humanized anti-CD147 antibody. A recent study showed that SARS-CoV-2 can invade human host cells via a novel route of the CD147-spike protein (SP), [86]. The SP was found to bind to CD147, a receptor on the host cells, thus mediating the invasion of the virus. The study also showed that meplazumab could competitively inhibit the binding of SP and CD147, and it could prevent viruses from invading host cells; this has provided a potential target for developing antiviral cures or protocols.
Recently, the drug was evaluated for its effectiveness and safety as an add-on treatment in patients with COVID-19 pneumonia. Meplazumab was found to speed up the recovery of patients with pneumonia associated with COVID-19 infection with a favorable safety profile [87]. The findings of this study support the carrying out of a large-scale investigation of meplazumab as a possible treatment for COVID-19 pneumonia.
4.6. Tocilizumab
Tocilizumab is a humanized monoclonal antibody against the interleukin-6 receptor (IL-6R). IL-6 is a cytokine that plays a major role in immune response and is associated with the pathogenesis of many autoimmune diseases [88]. IL-6 is produced by different cells, including T-cells and B-cells, lymphocytes, monocytes, and fibroblasts. IL-6 is involved in various physiological processes such as T-cell activation, the induction of immunoglobulin secretion, the initiation of hepatic acute-phase protein synthesis, and the stimulation of hematopoietic cells precursor proliferation and differentiation. The drug is already approved for the treatment of serious or life-threatening cytokine release syndrome (CRS). The FDA has approved a randomized, double-blind, placebo-controlled phase III clinical trial to assess the safety and efficacy of combining tocilizumab to the standard of care in hospitalized patients with severe COVID-19 pneumonia [89].
The seventh edition of the Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment published by China National Health Commission on 4 March 2020 included tocilizumab as an option for patients with severe COVID-19, extensive lung lesions, and elevated IL-6 levels [90]. This followed reports of positive outcomes from the use of tocilizumab to control dangerous lung inflammation in 21 patients with severe COVID-19 in China [91].
However, Health Canada notified healthcare providers that severe cases of drug-induced liver injuries had been reported in patients treated with tocilizumab, including cases of acute liver failure that required a transplant [92]. Patients should be closely monitored for the emergence of signs and symptoms of infection during and after treatment with tocilizumab, including the potential development of tuberculosis in patients who have tested negative for latent tuberculosis infection before starting therapy. Many patients who acquired these infections were taking concomitant immunosuppressants, such as methotrexate or corticosteroids, and, as such, the drug should be only used according to the specialist instructions [93].
4.7. Sarilumab
Sarilumab is another human monoclonal antibody against the interleukin-6 receptor. It binds to soluble and membrane-bound IL-6 receptors and inhibits IL-6-mediated signaling via these receptors. There is a growing body of evidence confirming the benefit of targeting the IL-6 pathway in patients with COVID-19, most notably in a recent single-arm study in China with tocilizumab in critically ill COVID-19 patients [94].
Currently, the clinical efficacy of sarilumab against SARS-CoV-2 is being evaluated in various clinical trials (Table 1).
Similar to tocilizumab, sarilumab-treated patients are at an elevated risk of developing severe opportunistic infections that may result in hospitalization or death [95].
4.8. Bevacizumab
Bevacizumab is a recombinant, humanized monoclonal antibody that has been used in anti-tumor therapy for 16 years. It binds to and neutralizes vascular endothelial growth factor (VEGF), preventing its interaction with endothelial receptors, Flt-1 and KDR. The suppression of vascular permeability in patients with severe/critical COVID-19 is expected to reduce pulmonary edema. The autopsies of patients who died from COVID-19 have shown the significant exudation of the pulmonary mucus, more evident than infection with SARS [96]. Pulmonary CT scanning and pathological observation have recognized inflammatory exudation as a distinctive characteristic of COVID-19 [97]. Evidence has suggested that bevacizumab is a promising treatment for critically ill COVID-19 patients. Currently, the drug is being evaluated for its efficacy and safety in severe or critical patients with COVID-19 in a multicenter, randomized, controlled clinical trial [98].
Bevacizumab has been reported by the American Heart Association to be an agent that may either cause reversible direct myocardial toxicity or exacerbate underlying myocardial dysfunction Moreover, bevacizumab has been reported to cause and/or worsen hypertension [98]. Thus, blood pressure monitoring during bevacizumab treatment and regularly after discontinuation is recommended.
4.9. Fingolimod
Fingolimod is a sphingosine analog that modulates the sphingosine-1-phosphate receptor and thus alters the migration of lymphocytes, resulting in lymph node sequestration [99]. As an immunomodulatory agent, it may confine the over-exuberant inflammatory response and slow down the progress of lung injury.
Fingolimod is mainly contra-indicated in patients with myocardial infarction (within six months), unstable angina, stroke, heart failure, and heart block. Prolonged QT interval above 500 m/s is another important contra-indication, given the high incidence of myocarditis as a complication of severe COVID-19. Patients with diabetes may get macular edema upon fingolimod treatment [100]. A clinical trial has been registered to use fingolimod at a dose of 0.5 mg daily for three consecutive days in the treatment of severe COVID-19 infection (NCT04280588).
5. Other Anti-Infective Agents Repurposed to Treat COVID-19
5.1. Chloroquine and Hydroxychloroquine
Both chloroquine (CQ) and hydroxychloroquine (HCQ) are antimalarial drugs that are also prescribed in treating autoimmune diseases [101]. According to the literature, both chloroquine and hydroxychloroquine possess antiviral activity against several viruses. CQ, through the inhibition of specific viral replication steps, restricts HIV. [102], influenza virus [103], dengue virus (DENV), [104], West Nile virus (WNV) infection, Japanese encephalitis virus (JEV), and Zika virus infection [105]. Those specific viral replication steps depend on the pH.
The investigation of the anti-SARS-CoV-2 activity of CQ and HCQ is also encouraged by several factors. First, both CQ and HCQ have been used for the treatment and prophylaxis of malaria and rheumatic diseases for more than 50 years. Second, both drugs are very cheap, e.g., when compared with immunotherapy. Finally, CQ inhibits the synthesis of a myriad of pro-inflammatory cytokines such as TNF-α, IL-1, and IL-6 [106], which may have deleterious effects on COVID-19 prognosis.
CQ and HCQ, as weak bases, exert their antiviral mechanism by affecting the acid vesicles, which leads to the disruption of enzymes required for the post-translational modifications of proteins. Both drugs concentrate in the acidic cell organelles, thus inhibiting virus replication by preventing endosome/lysosome trafficking. Another potential mechanism is the intervention with the viral protein maturation during virion maturation [107]. CQ has been shown to alkalinize the endosomal pH, thus preventing fusion between the virus and the cell and interfering with the glycosylation of the ACE2 receptor and its binding to the SARS-CoV-2 spike protein [108].
To date, clinical studies investigating hydroxychloroquine or chloroquine have been limited, and their role in COVID-19 treatment is still to be elucidated. Recent reports have found that CQ can also inhibit the growth of SARS-CoV-2 [20,108]. Chinese experts recommend that all cases of COVID-19 who have no contraindications to CQ should receive the drug in a dose of 500 milligrams twice daily for ten days [109].
In the United States, the FDA endorsed an emergency use authorization to permit CQ and HCQ treatment for hospitalized patients for COVID-19 [109]. There is a risk of potential toxicity with those medications, including QTc prolongation, cardiomyopathy, and retinal toxicity. Given that, and because of the previously described cases of myocarditis in COVID-19, CQ and HCQ should be cautiously prescribed under close clinical supervision by specialists. Patients with myocarditis are expected to be more susceptible to cardiac adverse effects. In addition, drug–drug interactions should be considered before use, and the patients should be monitored closely for adverse effects during use. The American College of Cardiology has recommended QTc monitoring parameters in that setting [110].
In a study on 36 patients with COVID-19, patients on HCQ (200 mg three times per day for 10 days) showed a higher rate of undetectable SARS-CoV-2 RNA on nasopharyngeal specimens at day six compared to patients who received no specific treatment (70% versus 12.5%, respectively) [111]. In this study, the use of azithromycin in combination with HCQ was apparently associated with a more rapid decline in viral RNA; however, the control groups for the study were a concern, and the biologic basis for using azithromycin in this setting is unclear. Another small observational study in patients with more severe illness did not suggest rapid viral RNA clearance with the combination [112].
5.2. Ivermectin
Ivermectin is derived from avermectins produced by the microorganism Streptomyces avermitilis, which was discovered by Ōmura [113]. Ivermectin, which is a highly safe compound, was later marketed to cover agricultural and aquaculture as well as the veterinary areas in 1981. Ivermectin, in the oral tablet dosage form, is used to treat infections of parasites, including the intestinal tract, skin, and eyes parasitic infections.
Ivermectin has numerous medical uses in which antiviral activity is considered one of the experimental targets. Ivermectin in sub-nanomolar range EC50 inhibits the replication of the yellow fever virus [113]. Ivermectin is also able to inhibit other flavivirus replication, including: dengue, tick-borne encephalitis, and Japanese encephalitis viruses. One of the mechanisms involved in this inhibition is the targeting of the activity of the non-structural enzyme 3 helicase [113]. Moreover, ivermectin inhibits dengue viruses and interrupts virus replication [114]. Ivermectin has also been demonstrated to be a potent inhibitor of importin α/β, which mediates the nuclear transport of several RNA viruses. Thus, ivermectin blocks the nuclear trafficking of RNA viral proteins. Ivermectin has been shown to have potent antiviral activity against HIV-1 and dengue viruses. Both types of viruses depend on the importin protein system. Ivermectin may also disrupt the HIV-1 integrase in HIV-1 in addition to the NS-5 (non-structural protein 5) polymerase in dengue viruses [115,116].
To investigate the effect of ivermectin on SARS-CoV-2, Vero/hSLAM cells with SARS-CoV-2 that were isolated Australia/VIC01/2020 were treated with ivermectin [117]. Ivermectin administration resulted in a successful viral loss by 48 h with no further reduction in viral RNA at 72 h. The authors hypothesized that ivermectin exerts its antiviral activity by inhibiting the nuclear import of viral proteins that is mediated by importinα/β1—the same mechanism discussed before for other RNA viruses.
5.3. Azithromycin
Macrolides are a unique class of antibiotics that include erythromycin, clarithromycin, and azithromycin. They have not only antimicrobial activity but also immunomodulatory reactions, including anti-inflammatory effects. Lately, the anti-viral effects of macrolides have been investigated.
Erythromycin is the first macrolide proved to have efficacy in the treatment of rhinovirus and influenza virus [118]. On the other hand, clarithromycin and azithromycin have shown higher effectivity towards rhinovirus, respiratory syncytial virus, and influenza virus [118,119]. In addition, Zika and Ebola viruses have been shown to be inhibited by azithromycin [120,121]. During the early phase of influenza virus infection, azithromycin has been found to block influenza virus endocytosis into host cells [119].
Regarding macrolide treatment for COVID-19, Gautret et al. hypothesized that hydroxychloroquine, in addition to azithromycin, might be an effective treatment for COVID-19. The mechanism of azithromycin against SARS-CoV-2 is unclear; however, it may be similar to the mechanistic pathways of macrolides against the influenza virus [111].
Several pathways for the suspected antiviral effects found with azithromycin have been suggested. Firstly, as mentioned regarding CQ and HCQ, azithromycin is a weak base that could inhibit endocytosis, thus limiting viral entry and replication [122]. Secondly, azithromycin directly affects the activity of bronchial epithelial cells and decreases mucus production, thus improving lung function [123]. Thirdly, there is evidence suggesting that azithromycin increases the production of IFN via the stimulation of IFN genes, leading to a reduction of viral replication [124]. Lastly and recently, azithromycin was shown to directly intervene with the SARS-CoV-2 host cell entry by inhibiting the interaction between SARS-CoV-2 spike protein and the host cell receptor, ACE2 [125].
6. Drugs Acting on Host Cell Receptors
6.1. Angiotensin-Converting Enzyme Inhibitors (ACEis) and Angiotensin II Receptor Blockers (ARBs)
Similar to SARS-CoV, SARS-CoV-2 binds first with its S glycoprotein to angiotensin-converting enzyme 2 (ACE-2), a homologs enzyme to ACE expresses on the membrane of lung epithelial cells, which is followed by virus-cell fusion and viral entry; see Figure 1, [125,126,127,128]. ACE-2 is a zinc carboxypeptidase whose catalytic active site is exposed at the extracellular surface [126,129]. Angiotensin II (ANGII) is generated from angiotensin I (ANGI) after being cleaved by ACE. ANGII binds to angiotensin type 1 receptor (AT1R) and functionally results in blood pressure elevation. ACE2 terminates the ANGII effect by degrading it into angiotensin-(1–7), which binds to Mas receptors on blood vessels, thus resulting in a vasodilator effect [12]. Furthermore, ACE2 degrades ANGI into angiotensin-(1–9), which is later converted into angiotensin-(1–7) via ACE [130]. Notably, ACE2 acts as a negative regulator in the renin–aldosterone–angiotensin system and opposes the deleterious cardiovascular effects induced by that system [126,129]. ANGII plays an important role in regulating ACE2. In the absence of abnormally elevated levels of Ang II, complex formation between angiotensin receptor (AT1R) and ACE2 on the cell membrane is predominant [129]. When ANGII levels increase, the AT1R/ACE2 complex is disrupted and triggers ACE2 cellular internalization, with subsequent lysosomal degradation via an AT1R-dependent pathway [129,131].
Angiotensin-converting enzyme inhibitor (ACEi) and angiotensin II receptor blocker (ARB) are two related categories of antihypertensive treatment that are extensively used to treat hypertension and other cardiovascular diseases. ACEi and ARB increase the expression of ACE2 on the lung epithelial cell surface [126,129]. Therefore, it has been suggested that treatment with ACEi or ARB could have a higher expression of cell membrane-bound ACE2, exposing essential binding sites to SARS-CoV-2 spike glycoprotein. However, the reduction of ANGII synthesis by ACEi or the inhibition of ANGII’s physiological effect via ARB is thought to leave AT1R in interaction with ACE2 [129]. This interaction could block the affinity of SARS-CoV-2 spike glycoprotein to ACE2 and hence reduce SARS-CoV-2 viral entry [129]. Furthermore, Dijkman et al. suggested that viral replication directly diminishes cellular ACE2 levels [130]. This may explain the lung protection role for ACEi and ARB via increasing the ACE2 levels and opposing the SARS-CoV-2 infection consequences.
Losartan is an AT1R blocker that is widely applied in the management of hypertension and to slow down the nephropathy progression in patients with diabetes mellitus. It is a safe drug with infrequent adverse drug events [132]. Interestingly, around 50% of hospitalized SARS-CoV patients have developed hypotension during their hospital stay [133]. No published clinical data are yet available on hypotension rates among hospitalized (NCT04312009) or non-hospitalized (NCT04311177). SARS-CoV-2 patients; it is thus too early to estimate how safe AT1R blockers in targeting COVID-19 without developing exacerbated hypotension.
Another approach for treatment of COVID-19 is to use a soluble form of ACE2. A recombinant human ACE2 (rhACE2; APN01, and GSK2586881) is safe in patients with acute respiratory distress syndrome [134,135]. Treatment with rhACE2 rapidly abolishes the elevated levels angiotensin-II, with a trend to decrease the plasma concentration of IL-6. A small clinical trial of rhACE2 in patients with severe SARS-CoV-2 infection was recently initiated (NCT04287686).
Logically, low cellular membrane ACE2 expression may reduce the chance of cells to be entered by SARS-CoV-2, but at the same time, it leads to the activation of AT1R with subsequent lung tissue injury. However, the higher the expression of ACE2 on the lung cell surface, the higher the chance of viral entry and the lower chance lung injury, due to minimal AT1R activation. This is the main reason why ACEi/ARB or rhACE2 may be useful in COVD-19 treatment. These opposing effects of ACE2 and, hence, ACEi/ARB or rhACE2, treatment in SARS-CoV-2 infection need more investigation.
6.2. Camostat
In-vitro studies have shown that endosomal cysteine proteases, namely cathepsin B (CTSB) and L (CTSL), can activate the glycoproteins of filoviruses, SARS-CoV, other coronaviruses, Nipah virus (NiV), and Hendra virus (HeV) through a mechanism that facilitates virus entry into certain cell lines [136]. In addition, other molecules that can lead to the activation of coronaviruses include TMPRSS2, and other serine proteases present extracellularly or present at the cell surface [137]. TMPRSS2 activity is crucial for viral spread and pathogenesis in an infected host [16,138], and camostat mesylate is a serine protease inhibitor that inhibits SARS-CoV activation by TMPRSS [139]. Camostat mesylate also inhibits the replication of both influenza and parainfluenza viruses. In experimentally infected mice, camostat was also found to prevent the development of pneumonia and viral myocarditis [140]. Moreover, camostat mesylate has been found to help to slow down or inhibit chronic pancreatitis in animal models and has been used for the treatment of chronic pancreatitis patients in Japan [141]. Therefore, camostat mesylate could also be of benefit in patients infected with SARS-CoV-2 (Table 1).
7. Conclusions
The drugs presented in this review are possible pharmacological modalities to COVID 19, but most of them are proposed based on their possible pharmacological mechanism to target virus entry, replication, and associated complications. The management of COVID 19 is rapidly evolving, as all available therapies are currently being investigated in clinical trials, and information will continue to emerge regarding pharmacological therapy for SARS-CoV-2. While awaiting the further development of clinical guidelines, the rational use of therapies should be ensured by local protocols based on the clinical expertise.
Author Contributions
M.S.-A. suggested the idea and wrote the “antiviral” section, designed the graphics and compiled the manuscript, E.A.-G. wrote the “immunomodulators” section, M.A.S. wrote “other anti-infective medications” section. All authors participate in manuscript preparation and revision. All authors have read and agreed to the published version of the manuscript
Funding
M.S.-A. is funded by the University of Sharjah targeted grant (1801090141--P) and the MBRU-Al-Mahmeed Research Award 2019.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Managing Epidemics: Key Facts about Major Deadly Diseases; World Health Organization: Geneva, Switzerland, 2018; 257p. [Google Scholar]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.-M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Intensive care management of coronavirus disease. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef]
- Poston, J.T.; Patel, B.K.; Davis, A.M. Management of critically ill adults with COVID-19. JAMA 2020. [Google Scholar] [CrossRef] [PubMed]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving sepsis campaign: Guidelines on the management of critically ill adults with coronavirus disease. Intensive Care Med. 2020. [Google Scholar] [CrossRef]
- Contini, A. Virtual screening of an FDA approved drugs database on two COVID-19 coronavirus proteins. ChemRxiv 2020. pre-print. [Google Scholar]
- Condit, R.C. Principles of virology. In Fields Virology, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Baez-Santos, Y.M.; Mielech, A.M.; Deng, X.; Baker, S.; Mesecar, A.D. Catalytic function and substrate specificity of the papain-like protease domain of nsp3 from the Middle East respiratory syndrome coronavirus. J. Virol. 2014, 88, 12511–12527. [Google Scholar] [CrossRef]
- Tseng, Y.-T.; Wang, S.-M.; Huang, K.-J.; Lee, A.I.-R.; Chiang, C.-C.; Wang, C.-T. Self-assembly of severe acute respiratory syndrome coronavirus membrane protein. J. Biol. Chem. 2010, 285, 12862–12872. [Google Scholar] [CrossRef]
- Siu, Y.L.; Teoh, K.T.; Lo, J.; Chan, C.M.; Kien, F.; Escriou, N.; Tsao, S.W.; Nicholls, J.M.; Altmeyer, R.; Peiris, J.S.M.; et al. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J. Virol. 2008, 82, 11318–11330. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Klann, K.; Koch, B.; Krause, D. SARS-CoV-2 infected host cell proteomics reveal potential therapy targets. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Lo, M.K.; Feldmann, F.; Gary, J.M.; Jordan, R.; Bannister, R.; Cronin, J.; Patel, N.R.; Klena, J.D.; Nichol, S.T.; Cihlar, T.; et al. Remdesivir (GS-5734) protects African green monkeys from Nipah virus challenge. Sci. Transl. Med. 2019, 11, eaau9242. [Google Scholar] [CrossRef] [PubMed]
- Madelain, V.; Baize, S.; Jacquot, F.; Reynard, S.; Fizet, A.; Barron, S.; Solas, C.; Lacarelle, B.; Carbonnelle, C.; Mentré, F.; et al. Ebola viral dynamics in nonhuman primates provides insights into virus immuno-pathogenesis and antiviral strategies. Nat. Commun. 2018, 9, 4013. [Google Scholar] [CrossRef]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016, 531, 381–385. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Feng, J.Y.; Porter, D.P.; Gotte, M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020, 295, 4772–4779. [Google Scholar] [CrossRef]
- De Wit, E.; Feldmann, F.; Cronin, J.; Jordan, R.; Okumura, A.; Thomas, T.; Scott, D.; Cihlar, T.; Feldmann, H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. USA 2020, 117, 6771–6776. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Lescure, F.-X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.-H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Le Hingrat, Q.; et al. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Holshue, M.L.; DeBolt, C.; Lindquist, S.; Lofy, K.H.; Wiesman, J.; Bruce, H.; Spitters, C.; Ericson, K.; Wilkerson, S.; Tural, A.; et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020, 382, 929–936. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Yao, Y.; Yeung, M.-L.; Deng, W.; Bao, L.; Jia, L.; Li, F.; Xiao, C.; Gao, H.; Yu, P.; et al. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015, 212, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, V.; Plosker, G.L. Lopinavir/ritonavir. Drugs 2006, 66, 1275–1299. [Google Scholar] [CrossRef] [PubMed]
- Chandwani, A.; Shuter, J. Lopinavir/ritonavir in the treatment of HIV-1 infection: A review. Ther. Clin. Risk Manag. 2008, 4, 1023–1033. [Google Scholar] [PubMed]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Muralidharan, N.; Sakthivel, R.; Velmurugan, D.; Gromiha, M.M. Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 Protease against COVID-19. J. Biomol. Struct. Dyn. 2020, 1–7. [Google Scholar] [CrossRef]
- Kakuda, T.N.; Van De Casteele, T.; Petrovic, R.; Neujens, M.; Salih, H.; Opsomer, M.; Hoetelmans, R. Bioequivalence of a darunavir/cobicistat fixed-dose combination tablet versus single agents and food effect in healthy volunteers. Antivir. Ther. 2014, 19, 597–606. [Google Scholar] [CrossRef]
- Mathias, A.A.; German, P.; Murray, B.P.; Wei, L.; Jain, A.; West, S.; Warren, D.; Hui, J.; Kearney, B.P. Pharmacokinetics and pharmacodynamics of GS-9350: A novel pharmacokinetic enhancer without anti-HIV activity. Clin. Pharmacol. Ther. 2010, 87, 322–329. [Google Scholar] [CrossRef]
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B 2017, 93, 449–463. [Google Scholar] [CrossRef]
- Booth, C.M.; Matukas, L.M.; Tomlinson, G.A.; Rachlis, A.R.; Rose, D.B.; Dwosh, H.A.; Walmsley, S.L.; Mazzulli, T.; Avendano, M.; Derkach, P.; et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 2003, 289, 2801–2809. [Google Scholar] [CrossRef]
- Lee, N.; Hui, D.; Wu, A.; Chan, P.; Cameron, P.; Joynt, G.M.; Ahuja, A.; Yung, M.Y.; Leung, C.B.; To, K.F.; et al. A Major Outbreak of Severe Acute Respiratory Syndrome in Hong Kong. N. Engl. J. Med. 2003, 348, 1986–1994. [Google Scholar] [CrossRef]
- Chu, C.M.; Cheng, V.C.C.; Hung, I.F.N.; Wong, M.M.L.; Chan, K.H.; Chan, K.S.; Kao, R.Y.T.; Poon, L.L.M.; Wong, C.L.P.; Guan, Y.; et al. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax 2004, 59, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawfiq, J.A.; Momattin, H.; Dib, J.; Memish, Z.A. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: An observational study. Int. J. Infect. Dis. 2014, 20, 42–46. [Google Scholar] [CrossRef]
- Omrani, A.S.; Saad, M.M.; Baig, K.; Bahloul, A.; Abdul-Matin, M.; Alaidaroos, A.Y.; Almakhlafi, G.A.; Albarrak, M.M.; Memish, Z.A.; Albarrak, A.M. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: A retrospective cohort study. Lancet Infect. Dis. 2014, 14, 1090–1095. [Google Scholar] [CrossRef]
- Spanakis, N.; Tsiodras, S.; Haagmans, B.L.; Raj, V.S.; Pontikis, K.; Koutsoukou, A.; Koulouris, N.G.; Osterhaus, A.D.M.E.; Koopmans, M.P.G.; Tsakris, A. Virological and serological analysis of a recent Middle East respiratory syndrome coronavirus infection case on a triple combination antiviral regimen. Int. J. Antimicrob. Agents 2014, 44, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Boriskin, Y.; Leneva, I.; Pecheur, E.-I.; Polyak, S. Arbidol: A broad-spectrum antiviral compound that blocks viral fusion. Curr. Med. Chem. 2008, 15, 997–1005. [Google Scholar] [CrossRef]
- Haviernik, J.; Štefánik, M.; Fojtíková, M.; Kali, S.; Tordo, N.; Rudolf, I.; Hubálek, Z.; Eyer, L.; Ruzek, D. Arbidol (Umifenovir): A broad-spectrum antiviral drug that inhibits medically important arthropod-borne flaviviruses. Viruses 2018, 10, 184. [Google Scholar] [CrossRef]
- Moscona, A. Neuraminidase inhibitors for influenza. N. Engl. J. Med. 2005, 353, 1363–1373. [Google Scholar] [CrossRef]
- Leneva, I.A.; Roberts, N.; Govorkova, E.A.; Goloubeva, O.G.; Webster, R.G. The neuraminidase inhibitor GS4104 (oseltamivir phosphate) is efficacious against A/Hong Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza viruses. Antiviral Res. 2000, 48, 101–115. [Google Scholar] [CrossRef]
- Tumpey, T.M.; García-Sastre, A.; Mikulasova, A.; Taubenberger, J.K.; Swayne, D.E.; Palese, P.; Basler, C.F. Existing antivirals are effective against influenza viruses with genes from the 1918 pandemic virus. Proc. Natl. Acad. Sci. USA 2002, 99, 13849–13854. [Google Scholar] [CrossRef]
- Davies, B.E. Pharmacokinetics of oseltamivir: An oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J. Antimicrob. Chemother. 2010, 65, ii5–ii10. [Google Scholar] [CrossRef]
- Hoffman, K.B.; Demakas, A.; Erdman, C.B.; Dimbil, M.; Doraiswamy, P.M. Neuropsychiatric adverse effects of oseltamivir in the FDA adverse event reporting system, 1999–2012. BMJ 2013, 347, f4656. [Google Scholar] [CrossRef] [PubMed]
- Toovey, S.; Prinssen, E.P.; Rayner, C.R.; Thakrar, B.T.; Dutkowski, R.; Koerner, A.; Chu, T.; Sirzen-Zelenskaya, A.; Britschgi, M.; Bansod, S.; et al. Post-marketing assessment of neuropsychiatric adverse events in influenza patients treated with oseltamivir: An updated review. Adv. Ther. 2012, 29, 826–848. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cheng, Y.; Wu, Y. Understanding SARS-CoV-2-mediated inflammatory responses: From mechanisms to potential therapeutic tools. Virol. Sin. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-Y.; Moriyama, M.; Chang, M.-F.; Ichinohe, T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Sheridan, C. Fast, portable tests come online to curb coronavirus pandemic. Nat. Biotechnol. 2020. [Google Scholar] [CrossRef]
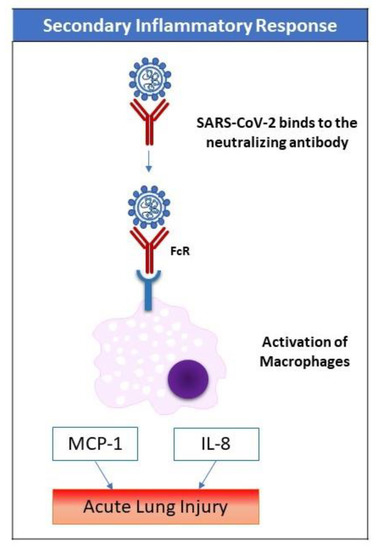
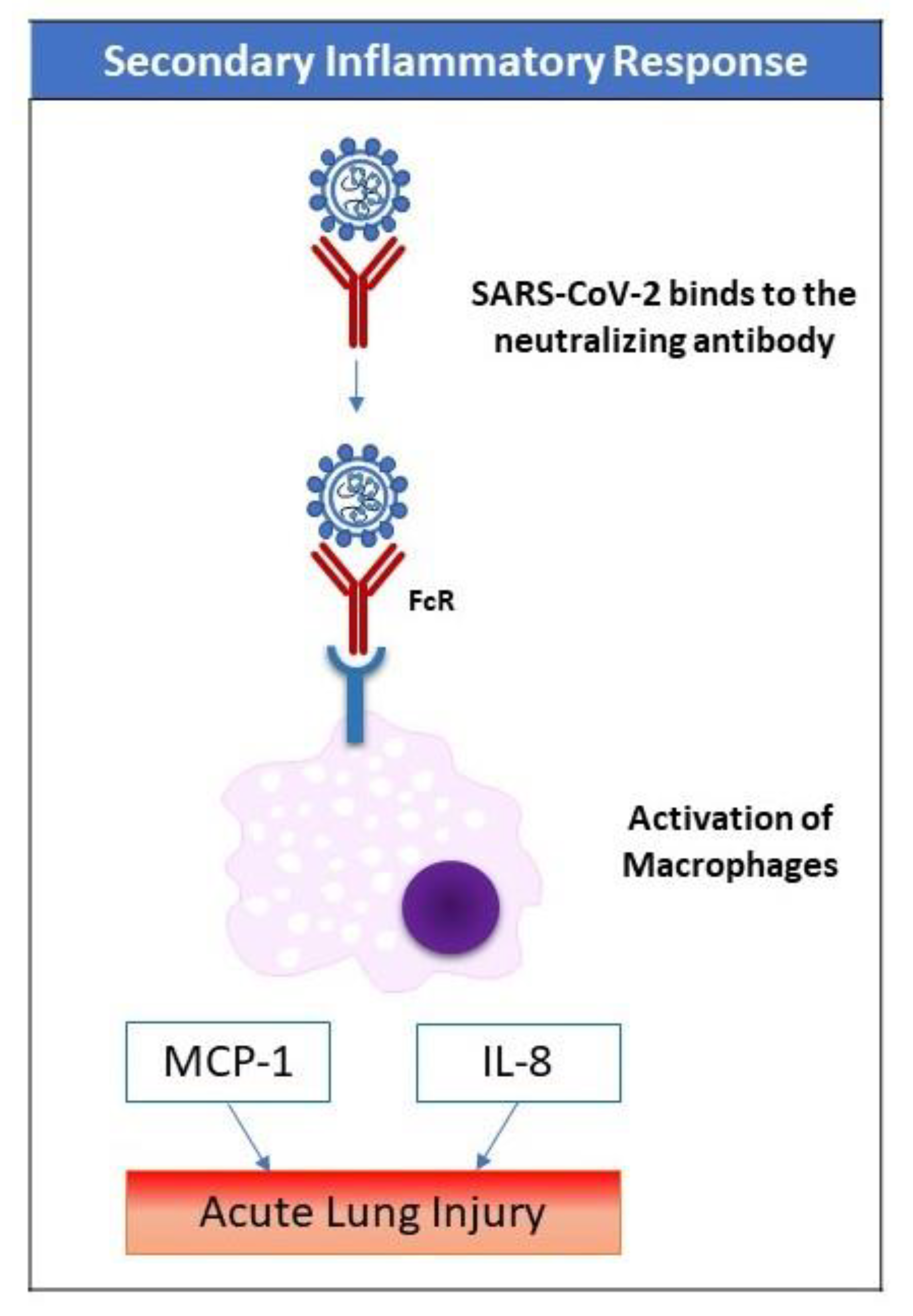
- Liu, L.; Wei, Q.; Lin, Q.; Fang, J.; Wang, H.; Kwok, H.; Tang, H.; Nishiura, K.; Peng, J.; Tan, Z.; et al. Anti–spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Tseng, C.-T.; Sbrana, E.; Iwata-Yoshikawa, N.; Newman, P.C.; Garron, T.; Atmar, R.L.; Peters, C.J.; Couch, R.B. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS ONE 2012, 7, e35421. [Google Scholar] [CrossRef]
- Bolles, M.; Deming, D.; Long, K.; Agnihothram, S.; Whitmore, A.; Ferris, M.; Funkhouser, W.; Gralinski, L.; Totura, A.; Heise, M.; et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J. Virol. 2011, 85, 12201–12215. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Yu, W.; He, T.; Yu, J.; Yi, C.E.; Ba, L.; Li, W.; Farzan, M.; Chen, Z.; et al. Antibody responses against SARS coronavirus are correlated with disease outcome of infected individuals. J. Med. Virol. 2006, 78, 1–8. [Google Scholar] [CrossRef]
- Haslwanter, D.; Blaas, D.; Heinz, F.X.; Stiasny, K. A novel mechanism of antibody-mediated enhancement of flavivirus infection. PLoS Pathog. 2017, 13, e1006643. [Google Scholar] [CrossRef]
- Werdan, K.; Pilz, G.; Bujdoso, O.; Fraunberger, P.; Neeser, G.; Schmieder, R.E.; Viell, B.; Marget, W.; Seewald, M.; Walger, P.; et al. Score-based immunoglobulin G therapy of patients with sepsis: The SBITS study. Crit. Care Med. 2007, 35, 2693–2701. [Google Scholar] [PubMed]
- Hamano, N.; Nishi, K.; Onose, A.; Okamoto, A.; Umegaki, T.; Yamazaki, E.; Hirota, K.; Ookura, H.; Takahashi, H.; Shingu, K. Efficacy of single-dose intravenous immunoglobulin administration for severe sepsis and septic shock. J. Intensive Care 2013, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.B.; Kirkpatrick, A.W.; Delaney, A. Polyclonal intravenous immunoglobulin for the treatment of severe sepsis and septic shock in critically ill adults: A systematic review and meta-analysis. Crit. Care Med. 2007, 35, 2686–2692. [Google Scholar] [PubMed]
- Kreymann, K.G.; De Heer, G.; Nierhaus, A.; Kluge, S. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit. Care Med. 2007, 35, 2677–2685. [Google Scholar]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef]
- Bellingan, G.; Maksimow, M.; Howell, D.C.; Stotz, M.; Beale, R.; Beatty, M.; Walsh, T.; Binning, A.; Davidson, A.; Kuper, M.; et al. The effect of intravenous interferon-beta-1a (FP-1201) on lung CD73 expression and on acute respiratory distress syndrome mortality: An open-label study. Lancet Respir. Med. 2014, 2, 98–107. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Pettilä, V.; Karvonen, M.K.; Jalkanen, J.; Nightingale, P.; Brealey, D.; Mancebo, J.; Ferrer, R.; Mercat, A.; Patroniti, N.; et al. Effect of intravenous interferon β-1a on death and days free from mechanical ventilation among patients with moderate to severe acute respiratory distress syndrome. JAMA 2020, 323, 725. [Google Scholar] [CrossRef]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J. Hear. Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Zhang, F.; Wang, Q.; Li, T.; Liu, Z.; Wang, J.; Qin, Y.; Zhang, X.; Yan, X.; et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The perspectives of clinical immunologists from China. Clin. Immunol. 2020, 214, 108393. [Google Scholar] [CrossRef]
- Dong, L.; Hu, S.; Gao, J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov. Ther. 2020, 14, 58–60. [Google Scholar] [CrossRef]
- Costantini, C.; Bellet, M.M.; Pariano, M.; Renga, G.; Stincardini, C.; Goldstein, A.L.; Garaci, E.; Romani, L. A reappraisal of thymosin Alpha1 in cancer therapy. Front. Oncol. 2019, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- Iwata, S.; Tanaka, Y. Progress in understanding the safety and efficacy of Janus kinase inhibitors for treatment of rheumatoid arthritis. Expert Rev. Clin. Immunol. 2016, 12, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Koumaki, D.; Koumaki, V.; Lagoudaki, E.; Bertsias, G. Palmoplantar pustulosis-like eruption induced by baricitinib for treatment of rheumatoid arthritis. Eur. J. Case Rep. Intern. Med. 2019, 7, 001383. [Google Scholar] [PubMed]
- Kunwar, S.; Collins, C.E.; Constantinescu, F. Baricitinib, a Janus kinase inhibitor, in the treatment of rheumatoid arthritis: A systematic literature review and meta-analysis of randomized controlled trials. Clin. Rheumatol. 2018, 37, 2611–2620. [Google Scholar] [CrossRef]
- Emery, P.; McInnes, I.; Genovese, M.C.; Smolen, J.S.; Kremer, J.; Dougados, M.; Schlichting, D.E.; Rooney, T.; Issa, M.; De Bono, S.; et al. A7.16 Characterisation of changes in lymphocyte subsets in baricitinib-treated patients with rheumatoid arthritis in two phase 3 studies. Ann. Rheum. Dis. 2016, 75, A62. [Google Scholar] [CrossRef]
- Kubo, S.; Nakayamada, S.; Nakano, K.; Tanaka, Y. THU0203 baricitinib targets the type I IFN/STAT-medicated activities of human T cells and dendritic cells. Ann. Rheum. Dis. 2016, 75, 260. [Google Scholar] [CrossRef]
- Shi, J.G.; Chen, X.; Lee, F.; Emm, T.; Scherle, P.A.; Lo, Y.; Punwani, N.; Williams, W.V.; Yeleswaram, S. The pharmacokinetics, pharmacodynamics, and safety of baricitinib, an oral JAK 1/2 inhibitor, in healthy volunteers. J. Clin. Pharmacol. 2014, 54, 1354–1361. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Z.; Wang, Y.; Zhou, Y.; Ma, Y.; Zuo, W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Sorrell, F.J.; Szklarz, M.; Azeez, K.R.A.; Elkins, J.M.; Knapp, S. Family-wide structural analysis of human numb-associated protein kinases. Structure 2016, 24, 401–411. [Google Scholar] [CrossRef]
- Stebbing, J.; Phelan, A.; Griffin, I.; Tucker, C.; Oechsle, O.; Smith, D.; Richardson, P. COVID-19: Combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Yoo, D.H.; Lee, C.K.; Li, K.-J.; Won, J.-E.; Wu, W.-S.; Zhong, J.; Nicolay, C.; Walls, C.D.; Tanaka, Y. Safety of baricitinib in East Asian patients with moderate-to-severe active rheumatoid arthritis: An integrated analysis from clinical trials. Int. J. Rheum. Dis. 2020, 23, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Verstovsek, S.; Kantarjian, H.; Mesa, R.A.; Pardanani, A.D.; Cortes-Franco, J.; Thomas, D.A.; Estrov, Z.; Fridman, J.S.; Bradley, E.C.; Erickson-Viitanen, S.; et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N. Engl. J. Med. 2010, 363, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Markham, A.; Keam, S.J. Camrelizumab: First global approval. Drugs 2019, 79, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Patil, N.K.; Guo, Y.; Luan, L.; Sherwood, E.R. Targeting immune cell checkpoints during sepsis. Int. J. Mol. Sci. 2017, 18, 2413. [Google Scholar] [CrossRef]
- Patera, A.C.; Drewry, A.M.; Chang, K.; Beiter, E.R.; Osborne, D.; Hotchkiss, R.S. Frontline Science: Defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. J. Leukoc. Biol. 2016, 100, 1239–1254. [Google Scholar] [CrossRef]
- Fang, W.; Yang, Y.; Ma, Y.; Hong, S.; Lin, L.; He, X.; Xiong, J.; Li, P.; Zhao, H.; Huang, Y.; et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: Results from two single-arm, phase 1 trials. Lancet Oncol. 2018, 19, 1338–1350. [Google Scholar] [CrossRef]
- Food and Drug Administration. Soliris Product Insert; US Department of Health and Human Services, Food and Drug Administration: Silver Spring, MD, USA, 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125166s417lbl.pdf (accessed on 20 April 2020).
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, G.; Song, N.; Li, P.; Chen, Y.; Guo, Y.; Sun, S.; Zhou, Y. Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg. Microbes Infect. 2018, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Gralinski, L.E.; Sheahan, T.P.; Morrison, T.E.; Menachery, V.D.; Jensen, K.; Leist, S.R.; Whitmore, A.; Heise, M.T.; Baric, R.S. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio 2018, 9, e01753-18. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, W.; Zhou, Y.-S.; Lian, J.-Q.; Zhang, Z.; Du, P.; Gong, L.; Zhang, Y.; Cui, H.-Y.; Geng, J.-J.; et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv 2020. [Google Scholar] [CrossRef]
- Bian, H.; Zheng, Z.-H.; Wei, D.; Zhang, Z.; Kang, W.-Z.; Hao, C.-Q.; Dong, K.; Kang, W.; Xia, J.-L.; Miao, J.-L.; et al. Meplazumab treats COVID-19 pneumonia: An open-labelled, concurrent controlled add-on clinical trial. medRxiv 2020. [Google Scholar] [CrossRef]
- Venkiteshwaran, A. Tocilizumab. MAbs 2009, 1, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Genentech. Genentech Announces FDA Approval of Clinical Trial for Actemra to Treat Hospitalized Patients with Severe COVID-19 Pneumonia. Genentech. 23 March 2020. Available online: https://www.gene.com/media/press-releases/14843/2020-03-23/genentech-announces-fda-approval-of-clin (accessed on 20 April 2020).
- Commission TCNH. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment 2020. Available online: https://www.acc.org/latest-in-cardiology/articles/2020/03/17/11/22/chinese-clinical-guidance-for-covid-19-pneumonia-diagnosis-and-treatment (accessed on 20 April 2020).
- Liu, A. Roche Launches Clinical Trial of COVID-19 Pneumonia Hopeful Actemra after Backing from China. FiercePharma. 19 March 2020. Available online: https://www.fiercepharma.com/pharma/roche-launches-clinical-trial-covid-19-pneumonia-hopeful-actemra-after-backing-from-china (accessed on 20 April 2020).
- Health Canada. Important Safety Information on ACTEMRA® (tocilizumab)—Risk of Hepatotoxicity. 2019. Available online: https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2019/69991a-eng.php (accessed on 20 April 2020).
- Cantini, F.; Nannini, C.; Niccoli, L.; Petrone, L.; Ippolito, G.; Goletti, D. Risk of tuberculosis reactivation in patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis receiving non-anti-TNF-targeted biologics. Mediators Inflamm. 2017, 2017, 8909834. [Google Scholar] [CrossRef]
- McCarty, D.; Robinson, A. Efficacy and safety of sarilumab in patients with active rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2018, 10, 61–67. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Cuomo, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation and Treatment Coronavirus (COVID-19); StatPearls: Treasure Island, FL, USA, 2020. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32150360 (accessed on 20 April 2020).
- Cascella, M.; Rajnik, M.; Cuomo, A.; Dulebohn, S.C.; Di Napoli, R.; Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020, 395, 565–574. [Google Scholar]
- Totzeck, M.; Mincu, R.I.; Rassaf, T. Cardiovascular adverse events in patients with cancer treated with bevacizumab: A meta-analysis of more than 20,000 patients. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Cohen, J.A.; Chun, J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann. Neurol. 2011, 69, 759–777. [Google Scholar] [CrossRef] [PubMed]
- Coppes, O.J.M.; Gutierrez, I.; Reder, A.T.; Ksiazek, S.; Bernard, J. Severe early bilateral macular edema following fingolimod therapy. Mult. Scler. Relat. Disord. 2013, 2, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Silverman, E.; Bargman, J.M. The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat. Rev. Nephrol. 2011, 7, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.P.; Nara, P.L.; Kung, H.F.; Oroszlan, S. Inhibition of human immunodeficiency virus infectivity by chloroquine. AIDS Res. Hum. Retroviruses 1990, 6, 481–489. [Google Scholar] [CrossRef]
- Ooi, E.E.; Chew, J.S.; Loh, J.P.; Chua, R.C. In vitro inhibition of human influenza a virus replication by chloroquine. Virol. J. 2006, 3, 39. [Google Scholar] [CrossRef]
- Farias, K.J.; Machado, P.R.; Da Fonseca, B.A. Chloroquine inhibits dengue virus type 2 replication in Vero cells but not in C6/36 cells. ScientificWorldJournal 2013, 2013, 282734. [Google Scholar] [CrossRef]
- Delvecchio, R.; Higa, L.M.; Pezzuto, P.; Valadao, A.L.; Garcez, P.P.; Monteiro, F.L.; Loiola, E.C.; Dias, A.A.; Silva, F.J.; Aliota, M.T.; et al. Chloroquine, an endocytosis blocking agent, inhibits zika virus infection in different cell models. Viruses 2016, 8, 322. [Google Scholar] [CrossRef]
- Jang, C.H.; Choi, J.H.; Byun, M.S.; Jue, D.M. Chloroquine inhibits production of TNF-alpha, IL-1beta and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes. Rheumatology 2006, 45, 703–710. [Google Scholar] [CrossRef]
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An old drug against today’s diseases? Lancet Infect. Dis 2003, 3, 722–727. [Google Scholar] [CrossRef]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020. [Google Scholar] [CrossRef]
- Jie, Z.; He, H.; Xi, H.; Zhi, Z. Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Chin. J. Tuberc. Respir. Dis. 2020, 43, 185–188. [Google Scholar]
- American College of Cardiology. Ventricular Arrhythmia Risk Due to Hydroxychloroquine-Azithromycin Treatment for COVID-19. 2020. Available online: https://www.acc.org/latest-in-cardiology/articles/2020/03/27/14/00/ventricular-arrhythmia-risk-due-to-hydroxychloroquine-azithromycin-treatment-for-covid-19 (accessed on 20 April 2020).
- Gautret, P.; Lagier, J.-C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 105949. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.M.; Delaugerre, C.; Le Goff, J.; Mela-Lima, B.; Ponscarme, D.; Goldwirt, L.; De Castro, N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Mal. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Burg, R.W.; Miller, B.M.; Baker, E.E.; Birnbaum, J.; Currie, S.A.; Hartman, R.; Kong, Y.L.; Monaghan, R.L.; Olson, G.; Putter, I.; et al. Avermectins, new family of potent anthelmintic agents: Producing organism and fermentation. Antimicrob. Agents Chemother. 1979, 15, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Y.; Fraser, J.E.; Chan, W.K.; Moreland, N.J.; Rathore, A.P.; Wang, C.; Vasudevan, S.G.; Jans, D.A. Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antivir. Res. 2013, 99, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Kosyna, F.K.; Nagel, M.; Kluxen, L.; Kraushaar, K.; Depping, R. The importin alpha/beta-specific inhibitor Ivermectin affects HIF-dependent hypoxia response pathways. Biol. Chem. 2015, 396, 1357–1367. [Google Scholar] [CrossRef]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef]
- Min, J.Y.; Jang, Y.J. Macrolide therapy in respiratory viral infections. Mediat. Inflamm. 2012, 2012, 649570. [Google Scholar] [CrossRef]
- Tran, D.H.; Sugamata, R.; Hirose, T.; Suzuki, S.; Noguchi, Y.; Sugawara, A.; Ito, F.; Yamamoto, T.; Kawachi, S.; Akagawa, K.S.; et al. Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A(H1N1) pdm09 virus infection by interfering with virus internalization process. J. Antibiot. 2019, 72, 759–768. [Google Scholar] [CrossRef]
- Madrid, P.B.; Panchal, R.G.; Warren, T.K.; Shurtleff, A.C.; Endsley, A.N.; Green, C.E.; Kolokoltsov, A.; Davey, R.; Manger, I.D.; Gilfillan, L.; et al. Evaluation of Ebola virus inhibitors for drug repurposing. ACS Infect. Dis. 2015, 1, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Iannetta, M.; Ippolito, G.; Nicastri, E. Azithromycin shows anti-zika virus activity in human glial cells. Antimicrob. Agents Chemother. 2017, 61, e01152-17. [Google Scholar] [CrossRef] [PubMed]
- Tyteca, D. Azithromycin, a lysosomotropic antibiotic, has distinct effects on fluid-phase and receptor-mediated endocytosis, but does not impair phagocytosis in J774 macrophages. Exp. Cell Res. 2002, 281, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Cramer, C.L.; Patterson, A.; Alchakaki, A.; Soubani, A.O. Immunomodulatory indications of azithromycin in respiratory disease: A concise review for the clinician. Postgrad. Med. 2017, 129, 493–499. [Google Scholar] [CrossRef]
- Li, C.; Zu, S.; Deng, Y.-Q.; Li, D.; Parvatiyar, K.; Quanquin, N.; Shang, J.; Sun, N.; Su, J.; Liu, Z.; et al. Azithromycin protects against zika virus infection by upregulating virus-induced type I and III interferon responses. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Sandeep, S.; McGregor, K. Energetics based modeling of hydroxychloroquine and azithromycin binding to the SARS-CoV-2 spike (S)protein—ACE2 Complex. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Ohto-Nakanishi, T.; Penninger, J.M. Trilogy of ACE2: A peptidase in the renin–angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010, 128, 119–128. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Kassiri, Z.; Jiang, C.; Liu, P.P.; Poutanen, S.M.; Penninger, J.M.; Butany, J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Investig. 2009, 39, 618–625. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Chen, M.; Feng, Y.; Xiong, C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020, 116, 1097–1100. [Google Scholar] [CrossRef]
- Deshotels, M.R.; Xia, H.; Sriramula, S.; Lazartigues, E.; Filipeanu, C.M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor–dependent mechanism. Hypertension 2014, 64, 1368–1375. [Google Scholar] [CrossRef]
- Hamming, I.; Cooper, M.; Haagmans, B.; Hooper, N.; Korstanje, R.; Osterhaus, A.; Timens, W.; Turner, A.; Navis, G.; Van Goor, H. The emerging role of ACE2 in physiology and disease. J. Pathol. 2007, 212, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dijkman, R.; Jebbink, M.F.; Deijs, M.; Milewska, A.; Pyrc, K.; Buelow, E.; Van der Bijl, A.; Van der Hoek, L. Replication-dependent downregulation of cellular angiotensin-converting enzyme 2 protein expression by human coronavirus NL63. J. Gen. Virol. 2012, 93, 1924–1929. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, M.; Caffe, S.E.; Michalak, R.A.; Reid, J.L. Losartan, an orally active angiotensin (AT1) receptor antagonist: A review of its efficacy and safety in essential hypertension. Pharmacol. Ther. 1997, 74, 181–194. [Google Scholar] [CrossRef]
- Yu, C.M.; Wong, R.S.; Wu, E.B.; Kong, S.L.; Wong, J.; Yip, G.W.; Soo, Y.O.; Chiu, M.L.; Chan, Y.S.; Hui, D.; et al. Cardiovascular complications of severe acute respiratory syndrome. Postgr. Med. J. 2006, 82, 140–144. [Google Scholar] [CrossRef]
- Haschke, M.; Schuster, M.; Poglitsch, M.; Loibner, H.; Salzberg, M.; Bruggisser, M.; Penninger, J.; Krahenbuhl, S. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin. Pharmacokinet. 2013, 52, 783–792. [Google Scholar] [CrossRef]
- Khan, A.; Benthin, C.; Zeno, B.; Albertson, T.E.; Boyd, J.; Christie, J.D.; Hall, R.; Poirier, G.; Ronco, J.J.; Tidswell, M.; et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care 2017, 21, 234. [Google Scholar] [CrossRef]
- Zhou, Y.; Vedantham, P.; Lu, K.; Agudelo, J.; Carrion, R., Jr.; Nunneley, J.W.; Barnard, D.; Pohlmann, S.; McKerrow, J.H.; Renslo, A.R.; et al. Protease inhibitors targeting coronavirus and filovirus entry. Antivir. Res. 2015, 116, 76–84. [Google Scholar] [CrossRef]
- Simmons, G.; Zmora, P.; Gierer, S.; Heurich, A.; Pohlmann, S. Proteolytic activation of the SARS-coronavirus spike protein: Cutting enzymes at the cutting edge of antiviral research. Antivir. Res. 2013, 100, 605–614. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Kawase, M.; Shirato, K.; Van der Hoek, L.; Taguchi, F.; Matsuyama, S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J. Virol. 2012, 86, 6537–6545. [Google Scholar] [CrossRef]
- Zhirnov, O.P.; Klenk, H.D.; Wright, P.F. Aprotinin and similar protease inhibitors as drugs against influenza. Antivir. Res. 2011, 92, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Taguchi, M.; Otsuki, M. Preventive and therapeutic effects of the protease inhibitor camostat on pancreatic fibrosis and atrophy in CCK-1 receptor-deficient rats. Pancreas 2005, 30, 54–61. [Google Scholar] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).