Electroanalysis Applied to Compatibility and Stability Assays of Drugs: Carvedilol Study Case
Abstract
1. Introduction
2. Results
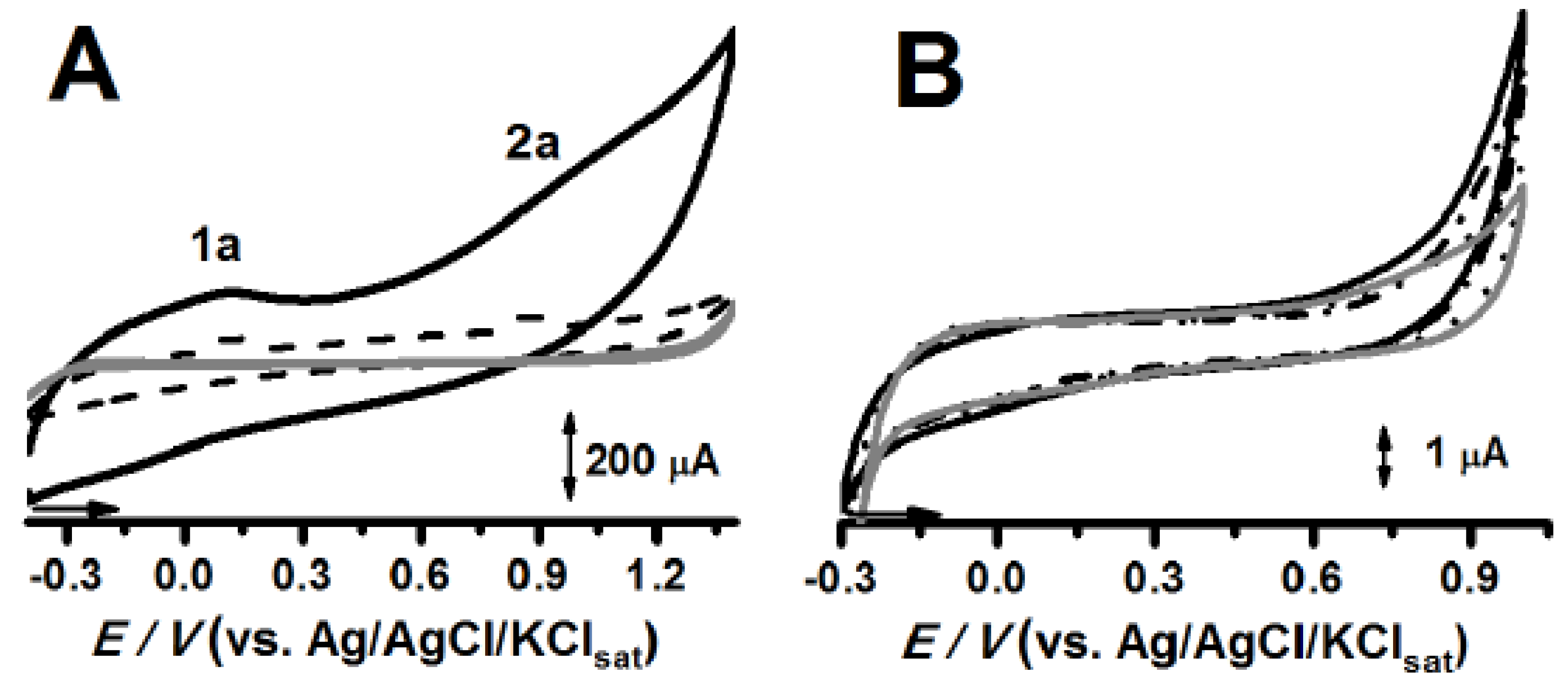
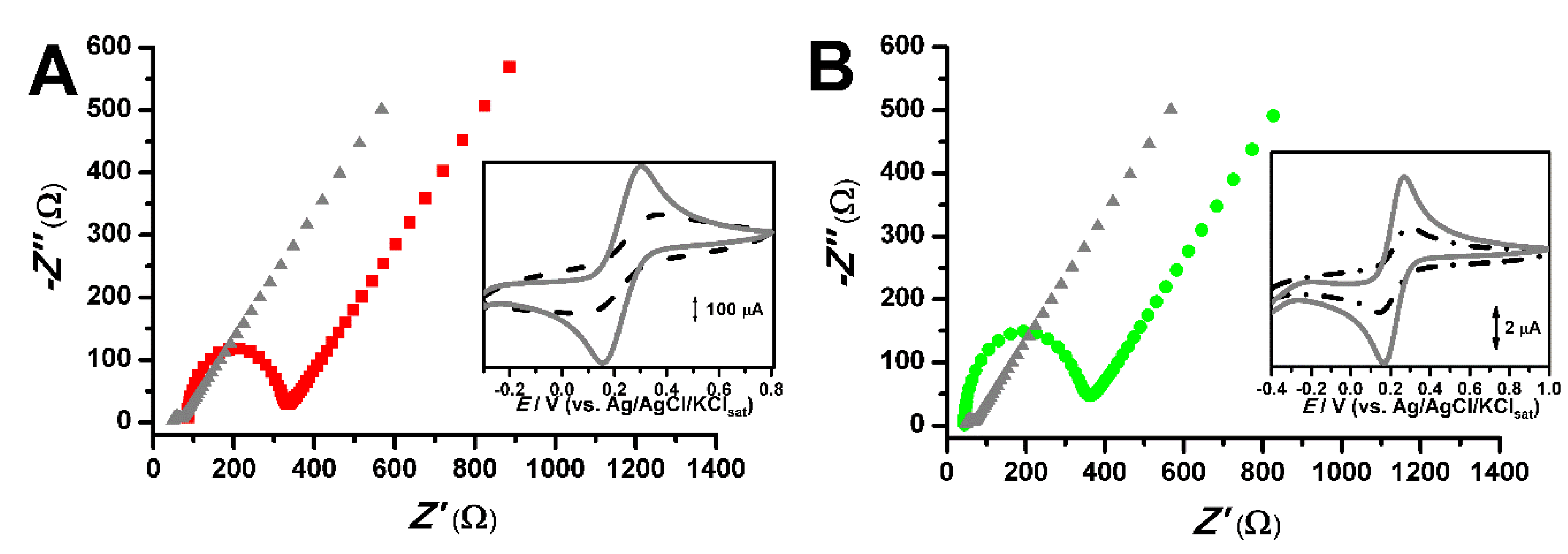
2.1. Electrochemical Characterization of Carbon-Paste Electrodes (CPEs)
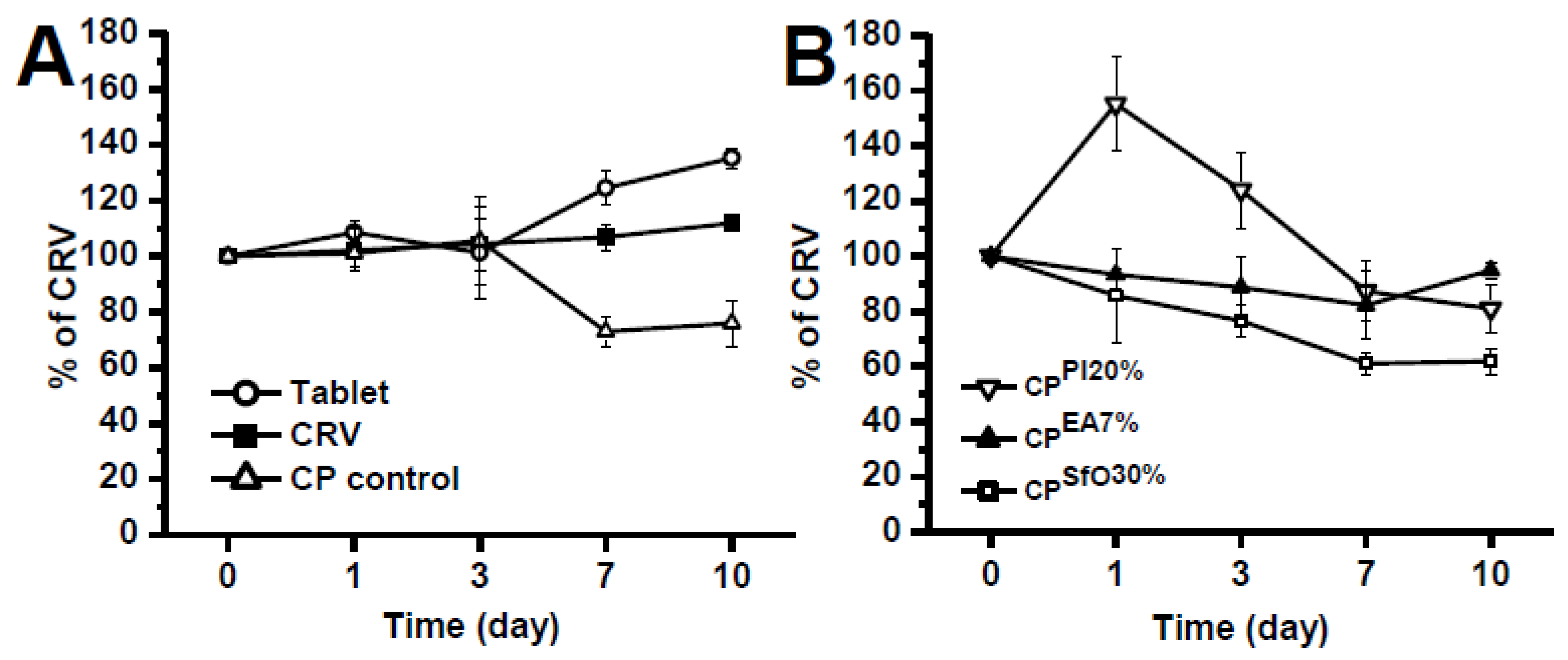
2.2. Electrochemical Evaluation of CRV and Binary Systems at Solid State
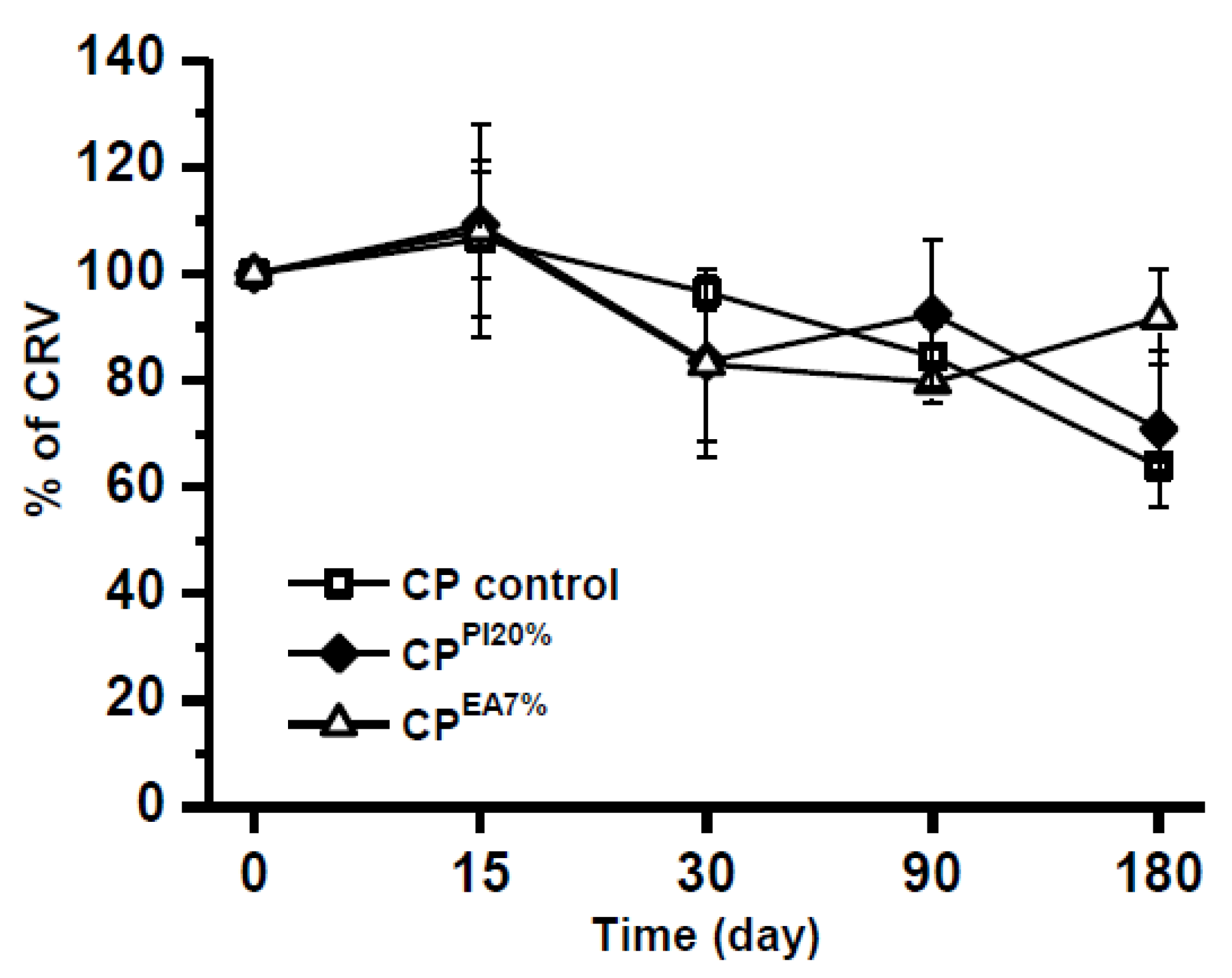
2.3. Effect of Time and Temperature
3. Discussion
4. Materials and Methods
4.1. Reagents and Solutions
4.2. Electrochemical Assays of Stability and Compatibility
4.2.1. Voltammetric Assays
4.2.2. EIS Characterization
4.3. Electrochemical Evaluation of Oxidative Stability of Lipophilic Compounds
4.3.1. Electrochemical Evaluation of Redox Compatibility
Effect of Time and Temperature
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Helal, M.G.; Said, E. Carvedilol attenuates experimentally induced silicosis in rats via modulation of P-AKT/mTOR/TGFβ1 signaling. Int. Immunopharmacol. 2019, 70, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Abuosa, A.M.; Elshiekh, A.H.; Qureshi, K.; Abrar, M.B.; Kholeif, M.A.; Kinsara, A.J.; Andejani, A.; Ahmed, A.H.; Cleland, J.G.F. Prophylactic use of carvedilol to prevent ventricular dysfunction in patients with cancer treated with doxorubicin. Indian Heart J. 2018, 70, S96–S100. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.F.D.; Da Silva, A.T.M.; Borges, K.B.; Nascimento, C.S. Carvedilol-imprinted polymer: Rational design and selectivity studies. J. Mol. Struct. 2019, 1177, 101–106. [Google Scholar] [CrossRef]
- Singh, B.; Singh, R.; Bandyopadhyay, S.; Kapil, R.; Garg, B. Optimized nanoemulsifying systems with enhanced bioavailability of carvedilol. Colloids Surf. B Biointerfaces 2013, 101, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.C.P.; Riccomini, K.; Silva, L.A.D.; Galter, D.; Lima, E.M.; Durig, T.; Taveira, S.F.; Martins, F.T.; Cunha-Filho, M.S.S.; Marreto, R.N. Development of carvedilol-cyclodextrin inclusion complexes using fluid-bed granulation: A novel solid-state complexation alternative with technological advantages. J. Pharm. Pharmacol. 2016, 68, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jain, C.P.; Tanwar, Y.S. Preparation and characterization of solid dispersions of carvedilol with poloxamer 188. J. Chil. Chem. Soc. 2013, 58, 1553–1557. [Google Scholar] [CrossRef]
- Gannu, R.; Vishnu, Y.V.; Kishan, V.; Rao, Y.M. In vitro permeation of carvedilol through porcine skin: Effect of vehicles and penetration enhancers. PDA J. Pharm. Sci. Technol. 2008, 62, 256–263. [Google Scholar]
- Taveira, S.F.; Varela-Garcia, A.; Dos Santos Souza, B.; Marreto, R.N.; Martin-Pastor, M.; Concheiro, A.; Alvarez-Lorenzo, C. Cyclodextrin-based poly(pseudo)rotaxanes for transdermal delivery of carvedilol. Carbohydr. Polym. 2018, 200, 278–288. [Google Scholar] [CrossRef]
- El-Say, K.M.; Hosny, K.M. Optimization of carvedilol solid lipid nanoparticles: An approach to control the release and enhance the oral bioavailability on rabbits. PLoS ONE 2018, 13, e0203405. [Google Scholar] [CrossRef]
- Arregui, J.R.; Kovvasu, S.P.; Kunamaneni, P.; Betageri, G. V Carvedilol solid dispersion for enhanced oral bioavailability using rat model. J. Appl. Pharm. Sci. 2019, 9, 42–50. [Google Scholar]
- Zaid Alkilani, A.; Hamed, R.; Al-Marabeh, S.; Kamal, A.; Abu-Huwaij, R.; Hamad, I. Nanoemulsion-based film formulation for transdermal delivery of carvedilol. J. Drug Deliv. Sci. Technol. 2018, 46, 122–128. [Google Scholar] [CrossRef]
- Meng, D.; Li, Z.; Wang, G.; Ling, L.; Wu, Y.; Zhang, C. Carvedilol attenuates liver fibrosis by suppressing autophagy and promoting apoptosis in hepatic stellate cells. Biomed. Pharmacother. 2018, 108, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Kajdič, S.; Vrečer, F.; Kocbek, P. Preparation of poloxamer-based nanofibers for enhanced dissolution of carvedilol. Eur. J. Pharm. Sci. 2018, 117, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.D.; Teixeira, F.V.; Serpa, R.C.; Esteves, N.L.; Dos Santos, R.R.; Lima, E.M.; Da Cunha-Filho, M.S.S.; De Souza Araújo, A.A.; Taveira, S.F.; Marreto, R.N. Evaluation of carvedilol compatibility with lipid excipients for the development of lipid-based drug delivery systems. J. Therm. Anal. Calorim. 2016, 123, 2337–2344. [Google Scholar] [CrossRef]
- Chadha, R.; Bhandari, S. Drug-excipient compatibility screening-role of thermoanalytical and spectroscopic techniques. J. Pharm. Biomed. Anal. 2014, 87, 82–97. [Google Scholar] [CrossRef]
- Jamrógiewicz, M.; Pieńkowska, K. Recent breakthroughs in the stability testing of pharmaceutical compounds. TrAC Trends Anal. Chem. 2019, 111, 118–127. [Google Scholar] [CrossRef]
- Zilker, M.; Sörgel, F.; Holzgrabe, U. A systematic review of the stability of finished pharmaceutical products and drug substances beyond their labeled expiry dates. J. Pharm. Biomed. Anal. 2019, 166, 222–235. [Google Scholar] [CrossRef]
- Sengupta, P.; Chatterjee, B.; Tekade, R.K. Current regulatory requirements and practical approaches for stability analysis of pharmaceutical products: A comprehensive review. Int. J. Pharm. 2018, 543, 328–344. [Google Scholar] [CrossRef]
- Costentin, C.; Savéant, J.M. Theoretical and mechanistic aspects of proton-coupled electron transfer in electrochemistry. Curr. Opin. Electrochem. 2017, 1, 104–109. [Google Scholar] [CrossRef]
- Torres, S.; Brown, R.; Szucs, R.; Hawkins, J.M.; Zelesky, T.; Scrivens, G.; Pettman, A.; Taylor, M.R. The application of electrochemistry to pharmaceutical stability testing—Comparison with in silico prediction and chemical forced degradation approaches. J. Pharm. Biomed. Anal. 2015, 115, 487–501. [Google Scholar] [CrossRef]
- Jaikaew, W.; Ruff, A.; Khunkaewla, P.; Erichsen, T.; Schuhmann, W.; Schulte, A. Robotic microplate voltammetry for real-time hydrogel drug release testing. Anal. Chim. Acta 2018, 1041, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Djekic, L.; Primorac, M. The influence of cosurfactants and oils on the formation of pharmaceutical microemulsions based on PEG-8 caprylic/capric glycerides. Int. J. Pharm. 2008, 352, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.; Couto, R.O.; Conceição, E.C.; Reis, N.S.; Gil, E.S. Solid state differential pulse voltammetry (DPV) from spots of thin layer chromatography (TLC): A new method for analysis of antioxidant phytoactives. Quimica Nova 2011, 34, 330–334. [Google Scholar]
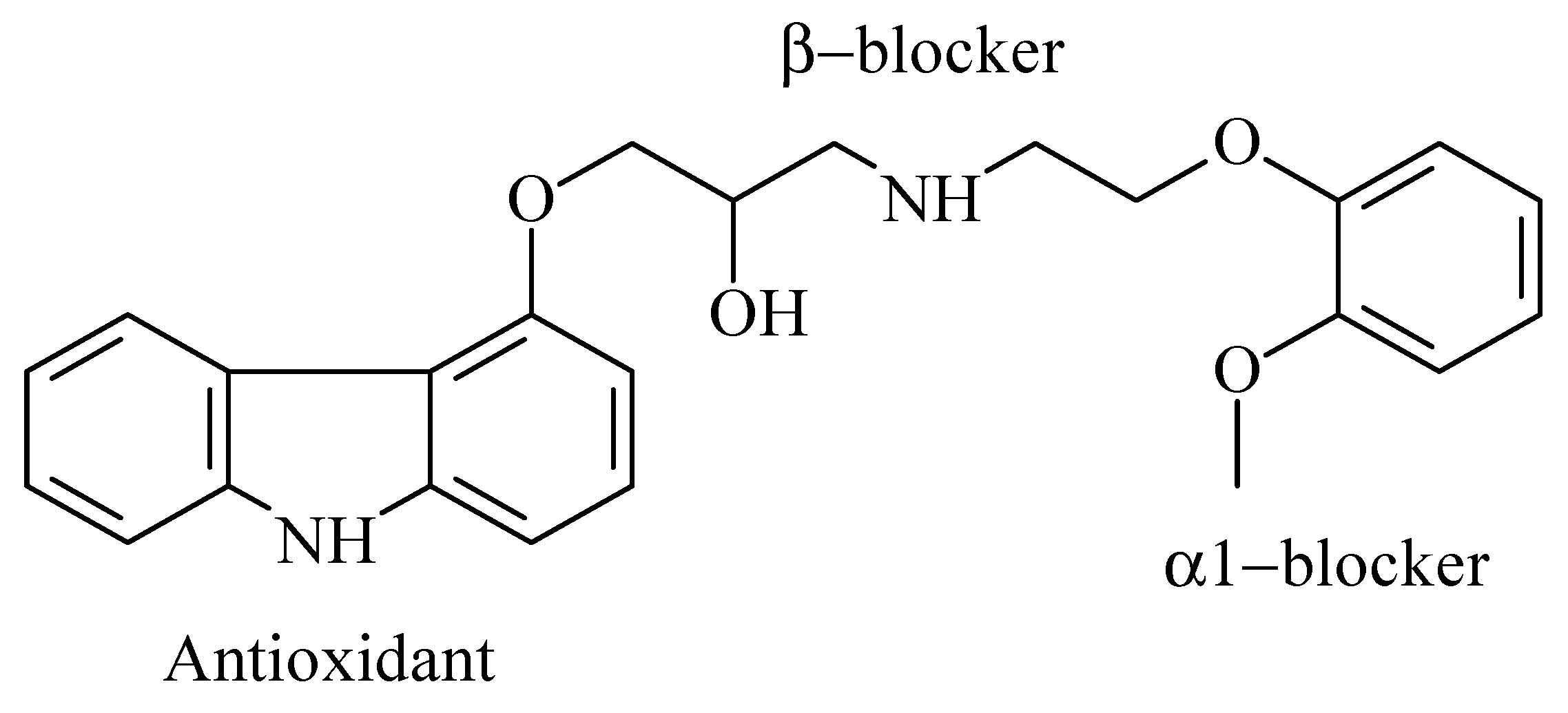

| Lipophilic Modifiers | (mg) | Mineral Oil (mg) | Graphite Powder (mg) |
|---|---|---|---|
| Liquid excipients 1 | 10 | 20 | 70 |
| 15 | 15 | 70 | |
| 20 | 10 | 70 | |
| 30 | - | 70 | |
| Solid excipients 2 | 1.5 | 30 | 68.5 |
| 3 | 30 | 67 | |
| 7 | 30 | 63 | |
| Carvedilol (CRV) control | 1 | 30 | 70 |
| Carbon Paste (CP) control | - | 30 | 70 |
| Excipients | Ep1a (V) | Ip1a (µA) | ΔEp1a (Ep1a–Cp Control Ep1a) |
|---|---|---|---|
| CP control | 0.625 ± 0.025 | 1.881 ± 0.285 | --- |
| Liquid Excipients | |||
| CPOA | 0.670 ± 0.004 | 5.679 ± 0.283 | 0.045 |
| CPSeO | 0.689 ± 0.062 | 1.937 ± 0.236 | 0.064 |
| CPCO | 0.706 ± 0.048 | 2.013 ± 0.490 | 0.081 |
| ѣ CPSfO | 0.727 ± 0.006 | 4.089 ± 0.179 | 0.102 |
| ѣ CPPI | 0.919 ± 0.001 | 3.105 ± 0.523 | 0.294 |
| Solid Excipients | |||
| CPEmul | 0.660 ± 0.001 | 3.410 ± 2.405 | 0.035 |
| ѣ CPCpt | 0.930 ± 0.010 | 0.205 ± 0.056 | 0.305 |
| ѣ CPEA | 1.043 ± 0.015 | 4.850 ± 1.816 | 0.418 |
| Circuit Elements | Nujol® | CPE Pl20% | CPE EA7% |
|---|---|---|---|
| Rs | 48.78 Ω | 85.25 Ω | 44.79 Ω |
| Rct | 17.31 Ω | 232.75 Ω | 291.5 Ω |
| C | 5.95 µF | 1.89 µF | 1.04 µF |
| Y | 5.63 mMho.s1/2 | 3.94 mMho.s1/2 | 1.82 mMho.s1/2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho, M.F.; Garcia, L.F.; de Macedo, I.Y.L.; Marreto, R.N.; de Oliveira, M.T.; do Couto, R.O.; da Cunha, C.E.P.; de Siqueira Leite, K.C.; Rezende, K.R.; Machado, F.B.; et al. Electroanalysis Applied to Compatibility and Stability Assays of Drugs: Carvedilol Study Case. Pharmaceuticals 2020, 13, 70. https://doi.org/10.3390/ph13040070
de Carvalho MF, Garcia LF, de Macedo IYL, Marreto RN, de Oliveira MT, do Couto RO, da Cunha CEP, de Siqueira Leite KC, Rezende KR, Machado FB, et al. Electroanalysis Applied to Compatibility and Stability Assays of Drugs: Carvedilol Study Case. Pharmaceuticals. 2020; 13(4):70. https://doi.org/10.3390/ph13040070
Chicago/Turabian Stylede Carvalho, Murilo Ferreira, Luane Ferreira Garcia, Isaac Yves Lopes de Macedo, Ricardo Neves Marreto, Mayk Teles de Oliveira, Renê Oliveira do Couto, Carlos Eduardo Peixoto da Cunha, Karla Carneiro de Siqueira Leite, Kênnia Rocha Rezende, Fabio Bahls Machado, and et al. 2020. "Electroanalysis Applied to Compatibility and Stability Assays of Drugs: Carvedilol Study Case" Pharmaceuticals 13, no. 4: 70. https://doi.org/10.3390/ph13040070
APA Stylede Carvalho, M. F., Garcia, L. F., de Macedo, I. Y. L., Marreto, R. N., de Oliveira, M. T., do Couto, R. O., da Cunha, C. E. P., de Siqueira Leite, K. C., Rezende, K. R., Machado, F. B., Somerset, V., & Gil, E. d. S. (2020). Electroanalysis Applied to Compatibility and Stability Assays of Drugs: Carvedilol Study Case. Pharmaceuticals, 13(4), 70. https://doi.org/10.3390/ph13040070





