The Emerging Therapeutic Landscape of ALK Inhibitors in Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. ALK Inhibitors
2.1. Crizotinib
2.2. Ceritinib
2.3. Alectinib
2.4. Brigatinib
2.5. Lorlatinib
2.6. Ensartinib
2.7. Entrectinib
3. Intracranial Efficacy
4. Safety
5. Mechanisms of Resistance
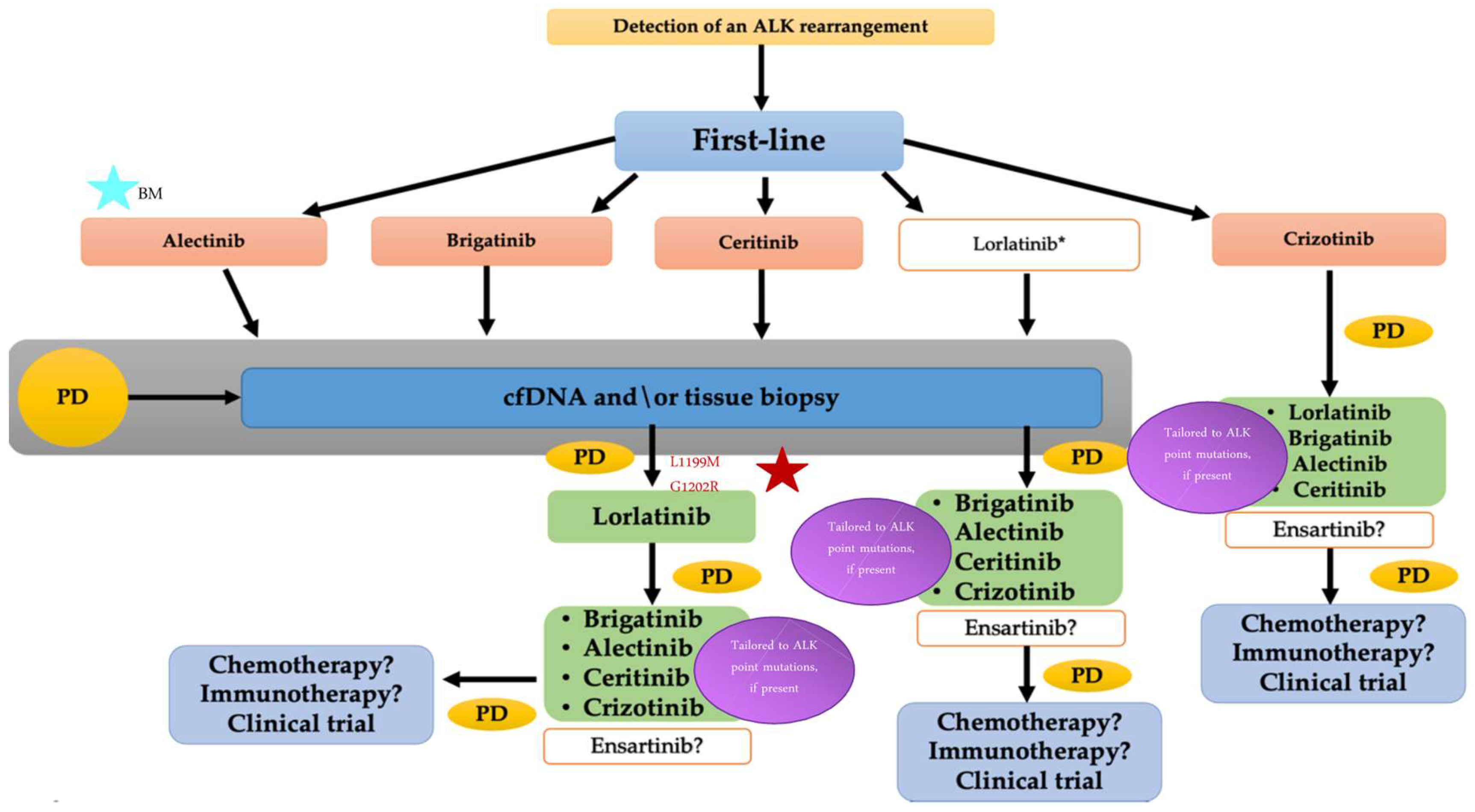
6. Sequence of Therapy
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Franchina, T.; Ricciardi, G.R.; Ferraro, G.; Scimone, A.; Bronte, G.; Russo, A.; Rolfo, C.; Adamo, V. Central nervous system involvement in ALK-rearranged NSCLC: Promising strategies to overcome crizotinib resistance. Expert Rev. Anticancer Ther. 2016, 16, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.M.; Shaw, A.T. ALK inhibitors in non-small cell lung cancer: Crizotinib and beyond. Clin. Adv. Hematol. Oncol. 2014, 12, 429–439. [Google Scholar] [PubMed]
- Chia, P.L.; Mitchell, P.; Dobrovic, A.; John, T. Prevalence and natural history ofALK positive non-small-cell lung cancer and the clinical impact oftargeted therapy with ALK inhibitors. Clin. Epidemiol. 2014, 6, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, B.; Palmer, R.H. The role of the ALK receptor in cancer biology. Ann. Oncol. 2016, 27, iii4–iii15. [Google Scholar] [CrossRef]
- Khan, M.; Lin, J.; Liao, G.; Tian, Y.; Liang, Y.; Li, R.; Liu, M.; Yuan, Y. ALK Inhibitors in the treatment of ALK positive NSCLC. Front. Oncol. 2019, 9, 557. [Google Scholar] [CrossRef]
- McCusker, M.G.; Russo, A.; Scilla, K.A.; Mehra, R.; Rolfo, C. How I treat ALK-positive non-small cell lung cancer. ESMO Open 2019, 4, e000524. [Google Scholar] [CrossRef]
- Wynes, M.W.; Sholl, L.M.; Dietel, M.; Schuuring, E.; Tsao, M.S.; Yatabe, Y.; Tubbs, R.R.; Hirsch, F.R. An international interpretation study using the ALK IHC antibody D5F3 and a sensitive detection kit demonstrates high concordance between ALK IHC and ALK FISH and between evaluators. J. Thorac. Oncol. 2014, 9, 631–638. [Google Scholar] [CrossRef]
- Katayama, R.; Lovly, C.M.; Shaw, A.T. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: A paradigm for precision cancer medicine. Clin. Cancer Res. 2015, 21, 2227–2235. [Google Scholar] [CrossRef]
- Xalkori® (Crizotinib) [Prescribing Information]. Available online: http://labeling.pfizer.com/showlabeling.aspx?id=676 (accessed on 9 December 2020).
- Camidge, D.R.; Bang, Y.J.; Kwak, E.L.; Iafrate, A.J.; Varella-Garcia, M.; Fox, S.B.; Riely, G.J.; Solomon, B.; Ou, S.H.; Kim, D.W.; et al. Activity and safety of crizotinibin patients with ALK-positive non-small-cell lung cancer:updated results from a phase 1 study. Lancet Oncol. 2012, 13, 1011–1019. [Google Scholar] [CrossRef]
- Blackhall, F.; Ross Camidge, D.; Shaw, A.T.; Soria, J.C.; Solomon, B.J.; Mok, T.; Hirsh, V.; Jänne, P.A.; Shi, Y.; Yang, P.C.; et al. Final results of thelarge-scale multinational trial PROFILE 1005: Efficacy and safety ofcrizotinib in previously treated patients with advanced/metastaticALK-positive non-small cell lung cancer. ESMO Open 2017, 2, e000219. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Kim, D.W.; Wu, Y.L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; Tang, Y.; et al. Final overall survival analysisfrom a study comparing first-line crizotinib versus chemotherapy inALK-mutation-positive non–small-cell lung cancer. J. Clin. Oncol. 2018, 36, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Mok, T.; Kim, D.W.; Wu, Y.L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Massarelli, E.; Papadimitrakopoulou, V. Ceritinib for the treatment of late-stage (metastatic) non-small cell lung cancer. Clin. Cancer. Res. 2015, 21, 670–674. [Google Scholar] [CrossRef]
- Friboulet, L.; Li, N.; Katayama, R.; Lee, C.C.; Gainor, J.F.; Crystal, A.S.; Michellys, P.Y.; Awad, M.M.; Yanagitani, N.; Kim, S.; et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014, 4, 662–673. [Google Scholar] [CrossRef]
- Zykadia® (Ceritinib) [Prescribing Information]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205755s009lbl.pdf (accessed on 9 December 2020).
- Kim, D.W.; Mehra, R.; Tan, D.S.W.; Felip, E.; Chow, L.Q.M.; Camidge, D.R.; Vansteenkiste, J.; Sharma, S.; De Pas, T.; Riely, G.J.; et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): Updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016, 17, 452–463. [Google Scholar] [CrossRef]
- Crinò, L.; Ahn, M.J.; De Marinis, F.; Groen, H.J.; Wakelee, H.; Hida, T.; Mok, T.; Spigel, D.; Felip, E.; Nishio, M.; et al. ASCEND-2: A single-arm, openlabel, multicenter phase ii study of ceritinib in adult patients (pts)with ALK-rearranged (ALK+) non-small cell lung cancer(NSCLC) previously treated with chemotherapy and crizotinib(crz). J. Clin. Oncol. 2015, 34, 2866–2873. [Google Scholar] [CrossRef]
- Soria, J.C.; Tan, D.S.W.; Chiari, R.; Wu, Y.L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.J.; et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, T.M.; Crinò, L.; Gridelli, C.; Kiura, K.; Liu, G.; Novello, S.; Bearz, A.; Gautschi, O.; Mok, T.; et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 874–886. [Google Scholar] [CrossRef]
- Highlights of Prescribing Information: Alecensa® (Alectinib) Capsules. Available online: https://www.gene.com/download/pdf/alecensa_prescribing.pdf (accessed on 9 December 2020).
- Seto, T.; Kiura, K.; Nishio, M.; Nakagawa, K.; Maemondo, M.; Inoue, A.; Hida, T.; Yamamoto, N.; Yoshioka, H.; Harada, M.; et al. CH5424802 (RO5424802) forpatients with ALK-rearranged advanced non-small-cell lung cancer(AF-001JP study): A single-arm, open-label, phase 1–2 study. Lancet Oncol. 2013, 14, 590–598. [Google Scholar] [CrossRef]
- Shaw, A.T.; Gandhi, L.; Gadgeel, S.; Riely, G.J.; Cetnar, J.; West, H.; Camidge, D.R.; Socinski, M.A.; Chiappori, A.; Mekhail, T.; et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: A single-group, multicentre, Phase 2 trial. Lancet Oncol. 2016, 17, 234–242. [Google Scholar] [CrossRef]
- Seto, T.; Nishio, M.; Hida, T.; Nokihara, H.; Morise, M.; Kim, Y.W.; Azuma, K.; Takiguchi, Y.; Yoshioka, H.; Kumagai, T. Final PFS analysis and safety data from thephase III J-ALEX study of alectinib (ALC) vs. crizotinib (CRZ) in ALKinhibitornaïve ALK-positive non-small cell lung cancer (ALK+NSCLC). J. Clin. Oncol. 2019, 37, 9092. [Google Scholar] [CrossRef]
- Camidge, D.R.; Dziadziuszko, R.; Peters, S.; Mok, T.; Noe, J.; Nowicka, M.; Gadgeel, S.M.; Cheema, P.; Pavlakis, N.; De Marinis, F.; et al. Updated efficacy andsafety data and impact of the EML4-ALK fusion variant on theefficacy of alectinib in untreated ALK-positive advanced non-smallcell lung cancer in the global phase III ALEX study. J. Thorac. Oncol. 2019, 14, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in untreated ALK-positive non-small-cell lung. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Kim, S.W.; Reungwetwattana, T.; Zhou, J.; Zhang, Y.; He, J.; Yang, J.J.; Cheng, Y.; Lee, S.H.; Bu, L.; et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): A randomised phase 3 study. Lancet Respir. Med. 2019, 7, 437–446. [Google Scholar] [CrossRef]
- Passiglia, F.; Pilotto, S.; Facchinetti, F.; Bertolaccini, L.; Del Re, M.; Ferrara, R.; Franchina, T.; Malapelle, U.; Menis, J.; Passaro, A.; et al. Treatment of advanced non-small-cell lung cancer: The 2019 AIOM (Italian Association of Medical Oncology) clinical practice guidelines. Crit. Rev. Oncol. Hematol. 2020, 146, 102858. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aggarwal, C.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J. Natl. Compr. Canc. Netw. 2019, 17, 1464–1472. [Google Scholar] [CrossRef]
- Alunbrig® (Brigatinib) Tablets. [Prescribing Information]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208772s008lbl.pdf (accessed on 9 December 2020).
- Kim, D.W.; Tiseo, M.; Ahn, M.J.; Reckamp, K.L.; Hansen, K.H.; Kim, S.W.; Huber, R.M.; West, H.L.; Groen, H.J.M.; Hochmair, M.J.; et al. Brigatinib in patients withcrizotinib-refractory anaplastic lymphoma kinase-positivenon-small-cell lung cancer: A randomized, multicenter phase II trial. J. Clin Oncol. 2017, 35, 2490–2498. [Google Scholar] [CrossRef]
- Huber, R.M.; Hansen, K.H.; Paz-Ares Rodríguez, L.; West, H.L.; Reckamp, K.L.; Leighl, N.B.; Tiseo, M.; Smit, E.F.; Kim, D.W.; Gettinger, S.N.; et al. Brigatinib incrizotinib-refractory ALK+ non-small cell lung cancer: 2-year follow-up on systemic and intracranial outcomes in the phase 2 ALTA trial. J. Thorac. Oncol. 2020, 15, 404–415. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, H.R.; Ahn, M.J.; Yang, J.C.H.; Han, J.Y.; Hochmair, M.J.; Lee, K.H.; Delmonte, A.; García Campelo, M.R.; Kim, D.-W.; et al. Brigatinib versus Crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: Second interim analysis of the phase III ALTA-1L trial. J. Clin. Oncol. 2020, 38, 3592–3603. [Google Scholar] [CrossRef] [PubMed]
- An Efficacy Study Comparing Brigatinib Versus Alectinib in Advanced Anaplastic Lymphoma Kinase-Positive (ALK+) Non-Small-Cell Lung Cancer (NSCLC) Participants Who Have Progressed on Crizotinib (ALTA-3). Available online: https://clinicaltrials.gov/ct2/show/NCT03596866 (accessed on 9 December 2020).
- Friedlaender, A.; Banna, G.; Patel, S.; Addeo, A. Diagnosis and treatment of ALK aberrations in metastatic NSCLC. Curr. Treat. Options Oncol. 2019, 20, 79. [Google Scholar] [CrossRef] [PubMed]
- Lorbrena® (Lorlatinib) Tablets. [Prescribing Information]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210868s000lbl.pdf (accessed on 9 December 2020).
- El Darsa, H.; Abdel-Rahman, O.; Sangha, R. Pharmacological and clinical properties of lorlatinib in the treatment of ALK-rearranged advanced non-small cell lung cancer. Expert Opin. Pharmacother. 2020, 21, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Besse, B.; Bauer, T.M.; Felip, E.; Soo, R.A.; Camidge, D.R.; Chiari, R.; Bearz, A.; Lin, C.C.; Gadgeel, S.M.; et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: Results from a global phase 2 study. Lancet Oncol. 2018, 19, 1654–1667. [Google Scholar] [CrossRef]
- Shaw, A.T.; Solomon, B.J.; Besse, B.; Bauer, T.M.; Lin, C.C.; Soo, R.A.; Riely, G.J.; Ou, S.I.; Clancy, J.S.; Li, S.; et al. ALK resistance mutations andefficacy of lorlatinib in advanced anaplastic lymphomakinase-positive non-small-cell lung cancer. J. Clin. Oncol. 2019, 37, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.; Bauer, T.M.; De Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, J.; Mok, T.; Polli, A.; et al. Lorlatinib vs crizotinib in the first-line treatment of patients (pts) with advanced ALK-positive non-small cell lung cancer (NSCLC): Results of the phase III CROWN study; Presented at ESMO virtual congress 2020, 19 September 2020. Ann. Oncol. 2020, 31, S1142–S1215. [Google Scholar] [CrossRef]
- Akamine, T.; Toyokawa, G.; Tagawa, T.; Seto, T. Spotlight on lorlatinib and its potential in the treatment of NSCLC: The evidence to date. Oncol. Targets Ther. 2018, 11, 5093–5101. [Google Scholar] [CrossRef]
- Horn, L.; Infante, J.R.; Reckamp, K.L.; Blumenschein, G.R.; Leal, T.A.; Waqar, S.N.; Gitlitz, B.J.; Sanborn, R.E.; Whisenant, J.G.; Du, L.; et al. Ensartinib (X-396) inALK-positive non-small cell lung cancer: Results from a first-inhumanPhaseI/II, multicenter study. Clin. Cancer Res. 2018, 24, 2771–2779. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, J.; Zhou, J.; Feng, J.; Zhuang, W.; Chen, J.; Zhao, J.; Zhong, W.; Zhao, Y.; Zhang, Y.; et al. Efficacy, safety, and biomarkeranalysis of ensartinib in crizotinib-resistant, ALK-positive non-small-cell-lung cancer: A multicentre, phase 2 trial. Lancet Respir. Med. 2020, 8, 45–53. [Google Scholar] [CrossRef]
- Selvaggi, G.; Wakelee, H.A.; Mok, T.; Wu, Y.L.; Reck, M.; Chiappori, A.; Cicin, I.; Lee, D.H.; Breder, V.; Fan, Y. ID:1882 phase III randomized study of ensartinib vs crizotinib in anaplastic lymphoma kinase (ALK) positive NSCLC patients: eXalt3. J. Thor. Oncol. 2020, 15, E41–E42. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Ou, S.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: Combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Bauer, T.; Liu, S.V.; Drilon, A.E.; Wheler, J.J.; Shaw, A.T.; Farago, A.F.; Ou, S.H.I.; Luo, D.; Yeh, L.; et al. STARTRK-1: Phase 1/2a study of entrectinib, an oral Pan-Trk, ROS1, and ALK inhibitor, in patients with advanced solid tumors with relevant molecular alterations. J. Clin. Oncol. 2015, 33, 2596. [Google Scholar] [CrossRef]
- Rangachari, D.; Yamaguchi, N.; VanderLaan, P.A.; Folch, E.; Mahadevan, A.; Floyd, S.R.; Uhlmann, E.J.; Wong, E.T.; Dahlberg, S.E.; Huberman, H.; et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015, 88, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, X.; Hui, Z. Treatment optimization for brain metastasis from anaplastic lymphoma kinase rearrangement non-small-cell lung cancer. Oncol. Res. Treat. 2019, 42, 599–606. [Google Scholar] [CrossRef]
- Alexander, M.; Lin, E.; Cheng, H. Leptomeningeal metastases in non-small cell lung cancer: Optimal systemic management in NSCLC with and without driver mutations. Curr. Treat. Options Oncol. 2020, 21, 72. [Google Scholar] [CrossRef]
- Costa, D.B.; Shaw, A.T.; Ou, S.H.; Solomon, B.J.; Riely, G.J.; Ahn, M.J.; Zhou, C.; Shreeve, S.M.; Selaru, P.; Polli, A.; et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J. Clin. Oncol. 2015, 33, 1881–1888. [Google Scholar] [CrossRef]
- Solomon, B.J.; Cappuzzo, F.; Felip, E.; Blackhall, F.H.; Costa, D.B.; Kim, D.W.; Nakagawa, K.; Wu, Y.L.; Mekhail, T.; Paolini, J.; et al. Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced alk-positive non-small-cell lung cancer: Results from PROFILE 1014. J. Clin. Oncol. 2016, 34, 2858–2865. [Google Scholar] [CrossRef]
- Kort, A.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Brain accumulation of the EML4-ALK inhibitor ceritinib is restricted by P-glycoprotein (P-GP/ABCB1) and breast cancer resistance protein (BCRP/ABCG2). Pharmacol. Res. 2015, 102, 200–207. [Google Scholar] [CrossRef]
- Crinò, L.; Ahn, M.J.; De Marinis, F.; Groen, H.J.; Wakelee, H.; Hida, T.; Mok, T.; Spigel, D.; Felip, E.; Nishio, M.; et al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: Results from ASCEND-2. J. Clin. Oncol. 2016, 34, 2866–2873. [Google Scholar] [CrossRef]
- Nishio, M.; Felip, E.; Orlov, S.; Park, K.; Yu, C.J.; Tsai, C.M.; Cobo, M.; McKeage, M.; Su, W.C.; Mok, T.; et al. Final overall survival and other efficacy and safety results from ASCEND-3: Phase II study of ceritinib in ALKi-naive patients with ALK-rearranged NSCLC. J. Thorac. Oncol. 2020, 15, 609–617. [Google Scholar] [CrossRef]
- Cho, B.C.; Obermannova, R.; Bearz, A.; McKeage, M.; Kim, D.W.; Batra, U.; Borra, G.; Orlov, S.; Kim, S.W.; Geater, S.L.; et al. Efficacy and safety of ceritinib (450 mg/d or 600 mg/d) with food versus 750-mg/d fasted in patients with ALK receptor tyrosine kinase (ALK)-positive NSCLC: Primary efficacy results from the ASCEND-8 study. J. Thorac. Oncol. 2019, 14, 1255–1265. [Google Scholar] [CrossRef]
- Hirota, T.; Muraki, S.; Ieiri, I. Clinical pharmacokinetics of anaplastic lymphoma kinase inhibitors in non-small-cell lung cancer. Clin. Pharmacokinet 2019, 58, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Kodama, T.; Hasegawa, M.; Takanashi, K.; Sakurai, Y.; Kondoh, O.; Sakamoto, H. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother. Pharmacol. 2014, 74, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Kodama, T.; Tsukaguchi, T.; Satoh, Y.; Yoshida, M.; Watanabe, Y.; Kondoh, O.; Sakamoto, H. Alectinib shows potent antitumor activity against RET-rearranged non-small cell lung cancer. Mol. Cancer Ther. 2014, 13, 2910–2918. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Besse, B. Brain metastases in oncogene-addicted non-small cell lung cancer patients: Incidence and treatment. Front. Oncol. 2018, 8, 88. [Google Scholar] [CrossRef]
- Gadgeel, S.M.; Shaw, A.T.; Govindan, R.; Gandhi, L.; Socinski, M.A.; Camidge, D.R.; De Petris, L.; Kim, D.W.; Chiappori, A.; Moro-Sibilot, D.L.; et al. Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non-small-cell lung cancer. J. Clin. Oncol. 2016, 34, 4079–4085. [Google Scholar] [CrossRef]
- Yang, J.C.; Ou, S.I.; De Petris, L.; Gadgeel, S.; Gandhi, L.; Kim, D.W.; Barlesi, F.; Govindan, R.; Dingemans, A.C.; Crino, L.; et al. Pooled systemic efficacy and safety data from the pivotal phase II studies (NP28673 and NP28761) of alectinib in ALK-positive non-small cell lung cancer. J. Thorac. Oncol. 2017, 12, 1552–1560. [Google Scholar] [CrossRef]
- Novello, S.; Mazières, J.; Oh, I.J.; de Castro, J.; Migliorino, M.R.; Helland, Å.; Dziadziuszko, R.; Griesinger, F.; Kotb, A.; Zeaiter, A.; et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: Results from the phase III ALUR study. Ann. Oncol. 2018, 29, 1409–1416. [Google Scholar] [CrossRef]
- Hida, T.; Nokihara, H.; Kondo, M.; Kim, Y.H.; Azuma, K.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; Imamura, F.; et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J.-ALEX): An open-label, randomised phase 3 trial. Lancet 2017, 390, 29–39. [Google Scholar] [CrossRef]
- Nishio, M.; Nakagawa, K.; Mitsudomi, T.; Yamamoto, N.; Tanaka, T.; Kuriki, H.; Zeaiter, A.; Tamura, T. Analysis of central nervous system efficacy in the J.-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer 2018, 121, 37–40. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, H.R.; Ahn, M.J.; Yang, J.C.H.; Han, J.Y.; Hochmair, M.J.; Lee, K.H.; Delmonte, A.; García Campelo, M.R.; Kim, D.W.; et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Felip, E.; Bauer, T.M.; Besse, B.; Navarro, A.; Postel-Vinay, S.; Gainor, J.F.; Johnson, M.; Dietrich, J.; James, L.P.; et al. Lorltinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: An international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017, 18, 1590–1599. [Google Scholar] [CrossRef]
- Kassem, L.; Shohdy, K.S.; Lasheen, S.; Abdel-Rahman, O.; Ali, A.; Abdel-Malek, R.R. Safety issues with the ALK inhibitors in the treatment of NSCLC: A systematic review. Crit. Rev. Oncol. Hematol. 2019, 134, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Rothenstein, J.M.; Letarte, N. Managing treatment-related adverse events associated with ALK inhibitors. Curr. Oncol. 2014, 21, 19–26. [Google Scholar] [CrossRef]
- Ou, S.H.; Ahn, J.S.; De Petris, L.; Govindan, R.; Yang, J.C.; Hughes, B.; Lena, H.; Moro-Sibilot, D.; Bearz, A.; Ramirez, S.V.; et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: A phase II global study. J. Clin. Oncol. 2016, 34, 661–668. [Google Scholar] [CrossRef]
- Indini, A.; Rijavec, E.; Ghidini, M.; Bareggi, C.; Gambini, D.; Galassi, B.; Antonelli, P.; Bettio, G.; Di Nubila, C.; Grossi, F. Pharmacotherapeutic advances with anaplastic lymphoma kinase inhibitors for the treatment of non-small cell lung cancer. Expert Opin. Pharmacother. 2020, 21, 931–940. [Google Scholar] [CrossRef]
- Ravaud, A. Treatment-associated adverse event management in the advanced renal cell carcinoma patient treated with targeted therapies. Oncologist 2011, 16, 32–44. [Google Scholar] [CrossRef]
- Gettinger, S.N.; Bazhenova, L.A.; Langer, C.J.; Salgia, R.; Gold, K.A.; Rosell, R.; Shaw, A.T.; Weiss, G.J.; Tugnait, M.; Narasimhan, N.I.; et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: A single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016, 17, 1683–1696. [Google Scholar] [CrossRef]
- Ferrara, M.G.; Di Noia, V.; D’Argento, E.; Vita, E.; Damiano, P.; Cannella, A.; Ribelli, M.; Pilotto, S.; Milella, M.; Tortora, G.; et al. Oncogene-addicted non-small-cell lung cancer: Treatment opportunities and future perspectives. Cancers 2020, 12, 1196. [Google Scholar] [CrossRef]
- Bauer, T.M.; Felip, E.; Solomon, B.J.; Thurm, H.; Peltz, G.; Chioda, M.D.; Shaw, A.T. Clinical management of adverse events associated with lorlatinib. Oncologist 2019, 24, 1103–1110. [Google Scholar] [CrossRef]
- Clinical and Research Information on Drug-Induced Liver Injury: Lorlatinib [Internet]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548564/ (accessed on 9 December 2020).
- Gainor, J.F.; Dardaei, L.; Yoda, S.; Friboulet, L.; Leshchiner, I.; Katayama, R.; Dagogo-Jack, I.; Gadgeel, S.; Schultz, K.; Singh, M.; et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK rearranged lung cancer. Cancer Discov. 2016, 6, 1118–1133. [Google Scholar] [CrossRef]
- Recondo, G.; Mezquita, L.; Facchinetti, F.; Planchard, D.; Gazzah, A.; Bigot, L.; Rizvi, A.Z.; Frias, R.L.; Thiery, J.P.; Scoazec, Y.J.; et al. Diverse resistancemechanisms to the third-generation ALK inhibitor lorlatinib inALK-rearranged lung cancer. Clin. Cancer Res. 2020, 26, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the college of american pathologists, the international association for the study of lung cancer, and the association for molecular pathology. J. Thor. Oncol. 2018, 13, 323–358. [Google Scholar] [CrossRef]
- Leighl, N.B.; Page, R.D.; Raymond, V.M.; Daniel, D.B.; Divers, S.G.; Reckamp, K.L.; Villalona-Calero, M.A.; Dix, D.; Odegaard, J.I.; Lanman, R.B.; et al. Clinical utility ofcomprehensive cell-free DNA analysis to identify genomic biomarkersin patients with newly diagnosed metastatic non-smallcell lung cancer. Clin. Cancer Res. 2019, 25, 4691–4700. [Google Scholar] [CrossRef]
- Russo, A.; De Miguel Perez, D.; Gunasekaran, M.; Scilla, K.; Lapidus, R.; Cooper, B.; Mehra, R.; Adamo, V.; Malapelle, U.; Rolfo, C. Liquid biopsy tracking of lung tumor evolutions over time. Expert Rev. Mol. Diagn. 2019, 19, 1099–1108. [Google Scholar] [CrossRef]
- Schwartzberg, L.S.; Horinouchi, H.; Chan, D.; Chernilo, S.; Tsai, M.L.; Isla, D.; Escriu, C.; Bennett, J.P.; Clark-Langone, K.; Svedman, C.; et al. Liquid biopsy mutation panel for non-small cell lung cancer: Analytical validation and clinical concordance. NPJ Precis. Oncol. 2020, 24, 15. [Google Scholar] [CrossRef] [PubMed]
- Bronte, G.; Passiglia, F.; Galvano, A.; Barraco, N.; Listì, A.; Castiglia, M.; Rizzo, S.; Fiorentino, E.; Bazan, V.; Russo, A. Nintedanib in NSCLC: Evidence to date and place in therapy. Ther. Adv. Med. Oncol. 2016, 8, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Passiglia, F.; Rizzo, S.; Rolfo, C.; Galvano, A.; Bronte, E.; Incorvaia, L.; Listi, A.; Barraco, N.; Castiglia, M.; Calo, V.; et al. Metastatic site location influences the diagnostic accuracy of ctDNA EGFR-mutation testing in NSCLC patients: A pooled analysis. Curr. Cancer. Drug Targets. 2018, 18, 697–705. [Google Scholar] [CrossRef] [PubMed]
- von Baumgarten, L.; Kumbrink, J.; Jung, A.; Reischer, A.; Flach, M.; Liebmann, S.; Metzeler, K.H.; Holch, J.W.; Niyazi, M.; Thon, N.; et al. Therapeutic management of neuro-oncologic patients—Potential relevance of CSF liquid biopsy. Theranostics 2020, 10, 856–866. [Google Scholar] [CrossRef]
- Mattox, A.K.; Yan, H.; Bettegowda, C. The potential of cerebrospinal fluid–based liquid biopsy approaches in CNS tumors. Neuro-Oncology 2019, 21, 1509–1518. [Google Scholar] [CrossRef]
- Boire, A.; Brandsma, D.; Brastianos, P.K.; Le Rhun, E.; Ahluwalia, M.; Junck, L.; Glantz, M.; Groves, M.D.; Lee, E.Q.; Lin, N.; et al. Liquid biopsy in central nervous system metastases: A RANO review and proposals for clinical applications. Neuro-Oncology 2019, 21, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.-M.; Li, Y.-S.; Jiang, B.J.; Tu, H.T.; Tang, W.F.; Yang, J.L.; Zhang, X.C.; Ye, Y.W.; Yan, H.H.; Su, J.; et al. Clinical utility of cerebrospinal fluid cell-free DNA as liquid biopsy for leptomeningeal metastases in ALK-rearranged NSCLC. J. Thorac. Oncol. 2019, 14, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Listì, A.; Barraco, N.; Bono, M.; Insalaco, I.; Castellana, L.; Cutaia, S.; Ricciardi, M.R.; Gristina, V.; Bronte, E.; Pantuso, G.; et al. Immuno-targeted combinations in oncogene-addicted non-small cell lung cancer. Transl. Cancer Res. 2019, 8, S55–S63. [Google Scholar] [CrossRef]
- Gristina, V.; Malapelle, U.; Galvano, A.; Pisapia, P.; Pepe, F.; Rolfo, C.; Tortorici, S.; Bazan, V.; Troncone, G.; Russo, A. The significance of epidermal growth factor receptor uncommon mutations in non-small cell lung cancer: A systematic review and critical appraisal. Cancer Treat. Rev. 2020, 85, 101994. [Google Scholar] [CrossRef]
- Nacchio, M.; Sgariglia, R.; Gristina, V.; Pisapia, P.; Pepe, F.; De Luca, C.; Migliatico, I.; Clery, E.; Greco, L.; Vigliar, E.; et al. KRAS mutations testing in non-small cell lung cancer: The role of Liquid biopsy in the basal setting. J. Thorac. Dis. 2020, 12, 3836–3843. [Google Scholar] [CrossRef]
- Incorvaia, L.; Fanale, D.; Badalamenti, G.; Barraco, N.; Bono, M.; Corsini, L.R.; Galvano, A.; Gristina, V.; Listì, A.; Vieni, S.; et al. Programmed death ligand 1 (PD-L1) as a predictive biomarker for pembrolizumab therapy in patients with advanced non-small-cell lung cancer (NSCLC). Adv. Ther. 2019, 36, 2600–2617. [Google Scholar] [CrossRef]
- D’Incecco, A.; Andreozzi, M.; Ludovini, V.; Rossi, E.; Capodanno, A.; Landi, L.; Tibaldi, C.; Minuti, G.; Salvini, J.; Coppi, E.; et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br. J. Cancer. 2015, 112, 95–102. [Google Scholar] [CrossRef]
- Ota, K.; Azuma, K.; Kawahara, A.; Hattori, S.; Iwama, E.; Tanizaki, J.; Harada, T.; Matsumoto, K.; Takayama, K.; Takamori, S.; et al. Induction of PD-L1 expression by the EML4-ALK oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin. Cancer Res. 2015, 21, 4014–4021. [Google Scholar] [CrossRef]
- Hong, S.; Chen, N.; Fang, W.; Zhan, J.; Liu, Q.; Kang, S.; He, X.; Liu, L.; Zhou, T.; Huang, J.; et al. Upregulation of PD-L1 by EML4-ALK fusion protein mediates the immune escape in ALK positive NSCLC: Implication for optional anti-PD-1/PD-L1 immune therapy for ALK-TKIs sensitive and resistant NSCLC patients. Oncoimmunology 2015, 5, e1094598. [Google Scholar] [CrossRef]
- Gainor, J.F.; Shaw, A.T.; Sequist, L.V.; Fu, X.; Azzoli, C.G.; Piotrowska, Z.; Huynh, T.G.; Zhao, L.; Fulton, L.; Schultz, K.R.; et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: A retrospective analysis. Clin. Cancer Res. 2016, 22, 4585–4593. [Google Scholar] [CrossRef]
- Spigel, D.R.; Reynolds, C.; Waterhouse, D.; Garon, E.B.; Chandler, J.; Babu, S.; Thurmes, P.; Spira, A.; Jotte, R.; Zhu, J.; et al. Phase 1/2 study of the safety and tolerability of nivolumab plus crizotinib for the first-line treatment of anaplastic lymphoma kinase translocation—Positive advanced non-small cell lung cancer (CheckMate 370). J. Thorac. Oncol. 2018, 13, 682–688. [Google Scholar] [CrossRef]
- Felip, E.; de Braud, F.G.; Maur, M.; Loong, H.H.; Shaw, A.T.; Vansteenkiste, J.F.; John, T.; Liu, G.; Lolkema, M.P.; Selvaggi, G.; et al. Ceritinib plus nivolumab in patients with advanced ALK-rearranged non-small cell lung cancer: Results of an open-label, multicenter, phase 1B study. J. Thorac. Oncol. 2020, 15, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Lee, S.H.; Ramalingham, S.S.; Bauer, T.M.; Boyer, M.J.; Costa, E.C.; Felip, E.; Han, Y.J.; Hida, T.; Hughes, B.G.M. Avelumab (anti–PD-L1) in combination with crizotinib or lorlatinib in patients with previously treated advanced NSCLC: Phase 1b results from JAVELIN Lung 101. J. Clin. Oncol. 2018, 36, 9008. [Google Scholar] [CrossRef]
- Gu, L.; Khadaroo, P.A.; Su, H.; Kong, L.; Chen, L.; Wang, X.; Li, X.; Zhu, H.; Zhong, X.; Pan, J.; et al. The safety and tolerability of combined immune checkpoint inhibitors (anti-PD-1/PD-L1 plus anti-CTLA-4): A systematic review and meta-analysis. BMC Cancer 2019, 19, 559. [Google Scholar] [CrossRef]
- Lin, J.J.; Schoenfeld, A.J.; Zhu, V.W.; Yeap, B.Y.; Chin, E.; Rooney, M.; Plodkowski, A.J.; Digumarthy, S.R.; Dagogo-Jack, I.; Gainor, J.F.; et al. Efficacy of platinum/pemetrexed combination chemotherapy in ALK-positive NSCLC refractory to second-generation ALK inhibitors. J. Thorac. Oncol. 2020, 15, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Eng. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
| Clinical Trial | Drugs | PFS | Primary Endpoint |
|---|---|---|---|
| PROFILE 1014 | Crizotinib vs. platinum-base CT | 10.9 vs. 7 mo. (HR 0.45; 95% CI 0.35–0.60) | PFS |
| ASCEND-4 | Ceritinib vs. platinum-based CT | 16.6 vs. 8.1 mo. (HR 0.55; 95% CI 0.42–0.73) | PFS |
| J-ALEX | Alectinib vs. crizotinib * | 34.1 vs. 10.2 mo. (HR 0.37; 95% CI 0.26–0.52) | PFS |
| ALEX | Alectinib vs. crizotinib ** | 34.8 vs. 10.9 mo. (HR 0.43; 95% CI 0.32–0.58) | PFS |
| ALESIA | Alectinib vs. crizotinib *** | NE vs. 11.1 mo. (HR 0.22; 95%CI 0.13–0.38) | PFS |
| ALTA 1L | Brigatinib vs. crizotinib | 24 vs. 11 mo. (HR 0.49; 95% CI 0.33−0.74) | PFS |
| eXalt3 | Ensartinib vs. crizotinib | 25.8 vs. 12.7 mo. (HR 0.52; 95% CI 0.36–0.75) | PFS |
| CROWN | Lorlatinib vs. crizotinib | NE vs. 9.3 mo. (HR 0.21; 95% CI 0.14–0.30) | PFS |
| Clinical Trial | Drugs/Phase | No. of pts w/BM | PFS | OS | IRR | IORR | IDCR | IDOR |
|---|---|---|---|---|---|---|---|---|
| PROFILE 1005 | Crizotinib, 2 | 166 | 8.4 mo. | 21.8 mo. | NA | 33% | 62% | NR |
| PROFILE 1007 | Crizotinib, 3 | 109 | 7.7 mo. | 12.2 mo. | NA | 18% | 56% | NR |
| PROFILE 1014 | Crizotinib, 3 | 39 | 9.0 mo. | 17.4 mo | 77% | 15% | NA | NR |
| ASCEND-1 | Ceritinib, 1 | 94 | 18.4 mo. | NR | NA | 72% | 79% | NA |
| ASCEND-2 | Ceritinib, 2 | 100 | 5.7 mo. | NR | NA | 45% | 80% | NA |
| ASCEND-3 | Ceritinib, 2 | 50 | 10.8 mo. | 36.2 mo. | NA | 20% | 80% | 9.1 mo. |
| ASCEND-4 | Ceritinib, 3 | 54 | 16.6 mo. | NR | NA | 73% | NA | 16.6 mo. |
| ASCEND-5 | Ceritinib, 3 | 66 | 4.4 mo. | NA | NA | 35% | NA | 6.9 mo. |
| ASCEND-6 | Ceritinib,1/2 | 103 | 5.7 mo. | NA | NA | 39.1% | 82.6% | NA |
| ASCEND-7 | Ceritinib, 2 | 138 | 5.4 mo. | NA | NA | 51.5% | 75.8% | 7.5 mo. |
| ALUR | Alectinib, 3 | 72 | 7.1 mo. | NA | NA | 54.2% | NA | NR |
| ALEX | Alectinib, 3 | 64 | 34.8 mo. | 48.2 mo | 59% | 81% | NA | 17.3 mo. |
| ALTA | Brigatinib, 3 | 41 | 29.4 mo. | NA | NA | 78% | NR | NA |
| CROWN | Lorlatinib, 3 | 30 | 18.3 mo. | NR | NA | 76% | NA | NE |
| NCT01625234 | Ensartinib, 1/2 | 35 | 9.2 mo. | NA | 69% | 64.3% | NA | 5.8 mo. |
| PROFILE 1014 | ASCEND4 | ALEX | ALTA-1L | CROWN | eXalt3 | |
|---|---|---|---|---|---|---|
| Grade 3/4 (%) | ||||||
| Any AEs | - | 78 | 41 | 61 | - | 23 |
| Diarrhea | 1 | 5 | 0 | 1 | - | 0 |
| Nausea | 1 | 3 | 1 | 1 | 0 | 1 |
| Anorexia | 30 | 29 | - | - | - | 1 |
| Weight reduction | - | 4 | - | 1 | - | - |
| Vomiting | 2 | 5 | 5 | 0 | - | 1 |
| Constipation | 2 | 0 | 0 | 0 | 0 | - |
| Blood creatinine increased | - | 2 | 2 | 0 | - | - |
| Peripheral edema | 1 | - | - | 1 | 0 | - |
| Rash | - | - | - | 0 | - | 12 |
| Dizziness | 0 | - | 0 | - | - | - |
| Visual impairment | 1 | - | 0 | - | 0 | - |
| Asthenia | 0 | 3 | - | - | - | - |
| AST and/or ALT increased | 14 | 31 | 5 | 1 | 2 | - |
| Bilirubin increased | - | - | 2 | - | - | - |
| Γ-glutamyltransferase increased | - | 29 | 1 | - | - | - |
| Myalgia | - | - | 0 | - | - | - |
| Headache | 1 | 0 | - | - | - | - |
| QT interval prolongation | - | - | - | - | - | - |
| Alkaline phosphatase increased | - | 7 | - | - | - | - |
| Neuropathy | 1 | - | - | - | - | - |
| Neutropenia | 11 | 1 | - | 0 | - | - |
| Anemia | 0 | 2 | 5 | 0 | - | - |
| Stomatitis | 1 | - | - | - | - | - |
| Treatment discontinuation due to AEs | 12 | 5 | 11 | 12 | 0 | 5.2 |
| Interstitial lung disease | - | 0 | - | - | - | - |
| Pruritus | - | - | - | 1 | 0 | 5 |
| Cognitive effects | - | - | - | - | 2 | - |
| Hypertriglyceridemia | - | - | - | - | 0 | - |
| Hypercholesterolemia | - | - | - | - | - | - |
| Hypertension | - | - | - | 10 | - | - |
| ALK-Independent Resistance Mechanism | ALK-Dependent Resistance Mechanism | |
|---|---|---|
| Crizotinib | EGFR overexpression and IGF-1R activation | Amplification of the ALK fusion gene; L1196M, G1269A/S, I1151Tins, L1152P/R, C1156Y/T, I1171T/N/S, F1174C/L/V, V1180L, G1202R, S1206C/Y, E1210K mutation acquisition |
| Ceritinib | c-Met gene amplification; activating mutation of MEK and PIK3CA mutations | G1202R, F1174C/L/V, G1202del, I1151Tins, L1152P/R, C1156Y/T |
| Alectinib | c-Met gene amplification and PIK3CA mutations | G1202R, I1171T/N/S, V1180L, L1196M |
| Brigatinib | Not reported | E1210K + S1206C, E1210K + D1203N, G1202Ra |
| Lorlatinib | NF2 loss of function mutations | L1198F + C1156Yc, L1196M/D1203N, F1174L/G1202R, C1156Y/G1269A [79] |
| Clinical Trial Identifier | Study Title | Drug | Phase | Status |
|---|---|---|---|---|
| NCT02393625 | Study of Safety and Efficacy of Ceritinib in Combination with Nivolumab in Patients With ALK-positive Non-small Cell Lung Cancer | Ceritinib + Nivolumab | I | Active, not recruiting |
| NCT03087448 | Ceritinib + Trametinib in Patients with Advanced ALK-Positive Non-Small Cell Lung Cancer | Ceritinib + Trametinib | I/II | Recruiting |
| NCT04227028 | Brigatinib and Bevacizumab for the Treatment of ALK-Rearranged Locally Advanced, Metastatic, or Recurrent Non-small Cell Lung Cancer | Brigatinib + Bevacizumab | I | Recruiting |
| NCT03202940 | A Phase IB/II Study of Alectinib Combined with Cobimetinib in Advanced ALK-Rearranged (ALK+) NSCLC | Alectinib + Cobimetinib | IB/II | Recruiting |
| NCT02521051 | Phase I/II Trial of Alectinib and Bevacizumab in Patients with Advanced, Anaplastic Lymphoma Kinase (ALK)-Positive, Non-Small Cell Lung Cancer | Alectinib + Bevacizumab | I/II | Recruiting |
| NCT01998126 | Combination Checkpoint Inhibitor Plus Erlotinib or Crizotinib for EGFR or ALK Mutated Stage IV Non-Small Cell Lung Cancer | ICI + Erlotinib/Crizotinib | I | Recruiting |
| NCT04292119 | Lorlatinib Combinations in Lung Cancer | Lorlatinib Crizotinib Binimetinib | I/II | Recruiting |
| NCT03611738 | Ceritinib Plus Docetaxel in ALK-Negative, EGFR WT Advanced NSCLC | Ceritinib + Docetaxel | I | Recruiting |
| NCT04005144 | Brigatinib and Binimetinib in Treating Patients with Stage IIIB-IV ALK or ROS1-Rearranged Non-small Cell Lung Cancer | Brigatinib + Binimetinib | I | Recruiting |
| NCT03202940 | A Phase IB/II Study of Alectinib Combined with Cobimetinib in Advanced ALK-Rearranged (ALK+) NSCLC | Alectinib + Cobimetinib | IB/II | Recruiting |
| NCT02521051 | Phase I/II Trial of Alectinib and Bevacizumab in Patients with Advanced, Anaplastic Lymphoma Kinase (ALK)-Positive, Non-Small Cell Lung Cancer | Alectinib + Bevacizumab | I/II | Recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gristina, V.; La Mantia, M.; Iacono, F.; Galvano, A.; Russo, A.; Bazan, V. The Emerging Therapeutic Landscape of ALK Inhibitors in Non-Small Cell Lung Cancer. Pharmaceuticals 2020, 13, 474. https://doi.org/10.3390/ph13120474
Gristina V, La Mantia M, Iacono F, Galvano A, Russo A, Bazan V. The Emerging Therapeutic Landscape of ALK Inhibitors in Non-Small Cell Lung Cancer. Pharmaceuticals. 2020; 13(12):474. https://doi.org/10.3390/ph13120474
Chicago/Turabian StyleGristina, Valerio, Maria La Mantia, Federica Iacono, Antonio Galvano, Antonio Russo, and Viviana Bazan. 2020. "The Emerging Therapeutic Landscape of ALK Inhibitors in Non-Small Cell Lung Cancer" Pharmaceuticals 13, no. 12: 474. https://doi.org/10.3390/ph13120474
APA StyleGristina, V., La Mantia, M., Iacono, F., Galvano, A., Russo, A., & Bazan, V. (2020). The Emerging Therapeutic Landscape of ALK Inhibitors in Non-Small Cell Lung Cancer. Pharmaceuticals, 13(12), 474. https://doi.org/10.3390/ph13120474








