1. Introduction
Osteoporosis is characterized by a weak bone structure and a decrease in bone density; it has become a major health problem [
1]. Estrogen deficiency, which is observed in the postmenopausal state and as a consequence of aging, is a major contributor to osteoporosis by inducing excessive osteoclast differentiation [
2]. Bone homeostasis is maintained by the balance of osteoclastic bone resorption and osteoblastic bone formation [
3]. Osteoclasts are multinucleated cells derived from monocyte–macrophage lineage precursor cells [
4,
5]. Macrophage colony-stimulating factor (M-CSF) and receptor activator nuclear factor-κB (NF-κB) ligand (RANKL) are the two major factors involved in osteoclast differentiation. M-CSF induces the proliferation and survival of osteoclast precursor cells, whereas RANKL stimulates osteoclast differentiation and activation. RANKL expressed in activated osteoblasts binds its receptor, RANK, which is expressed on the surface of osteoclast precursors, and then activates multiple downstream signaling pathways [
6]. RANKL induces the recruitment of adaptor molecules, such as TNF receptor-associated factor 6 (TRAF6). TRAF6 activates downstream signaling pathways, including NF-κB, mitogen-associated protein kinase (MAPK) extracellular-regulated kinase (ERK), Jun N-terminal kinase (JNK), and P38, Akt, activator protein 1 (AP-1) and nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1) [
6,
7,
8,
9]. In addition, one of the major downstream signals involved in osteoclast differentiation is calcium signaling, which is mediated by immunoreceptor tyrosine-based activation motif (ITAM)-bearing adaptor molecules, such as Fc receptor common γ subunit (FcRγ) and DNAX-activating protein 12 (DAP12) [
8,
9,
10,
11]. ITAM phosphorylation activates Syk, which then promotes the PLCγ. Subsequently, inositol-1,4,5-triphosphate (IP
3) is produced, and Ca
2+ is released from the endoplasmic reticulum (ER) via IP
3 binding to the inositol triphosphate receptor (IP
3R), leading to calcium mobilization [
10,
12]. Calcium signaling is critical for the activation of Ca
2+/calmodulin-dependent protein kinase IV (CaMKIV) and cAMP response element-binding protein (CREB), which are required for the activation of NFATc1 [
9,
13,
14]. CREB and NFATc1 induce the expression of osteoclast-specific genes such as tartrate-resistant acid phosphatase (TRAP), MMP9, NFATc1 and Cstk [
13,
15].
Natural-plant-derived extracts and compounds have become the focus of studies developing therapeutic agents for human disease due to the potentially untenable side effects of pharmaceutical agents or hormone treatments. As shown in many recent reports, compounds derived from natural products are considered new therapeutic agents for bone diseases, such as rheumatoid arthritis, periodontal disease and osteoporosis [
16,
17,
18]. According to our previous study, a
Salvia plebeia extract inhibited RANKL-induced osteoclastogenesis and prevented ovariectomized (OVX)-induced bone loss [
19]. Eudebeiolide B, which was used in the present study, is a eudesmane-type sesquiterpenoid compound isolated from a
Salvia plebeia R. Br. extract [
20]. Our previous studies were reported that various eudesmane-type sesquiterpenoid lactones, eudebeiolides A−K [
20,
21] were isolated from the aerial part of
Salvia plebeia. Among these isolates, eudebeiolide B has a 3-oxoeudesman-1(2),7(11)-dien-8,12-olide skeleton with conjugated carbonyl groups at C-3 and C-7. Although eudebeiolide B showed a mild anti-inflammatory effect based on previous results, no additional biological reports were observed in the recent literature (the effects of eudebeiolide B on osteoclastogenesis have not yet been investigated). Therefore, we attempted to investigate the effect of eudebeiolide B on RANKL-induced osteoclast differentiation and elucidate the underlying molecular mechanisms. In addition, the therapeutic effects of eudebeiolide B on OVX-induced bone loss were evaluated. The inhibition of calcium signaling in osteoporosis by targeting RANKL may be a useful approach for osteoporosis-specific chemotherapy.
3. Discussion
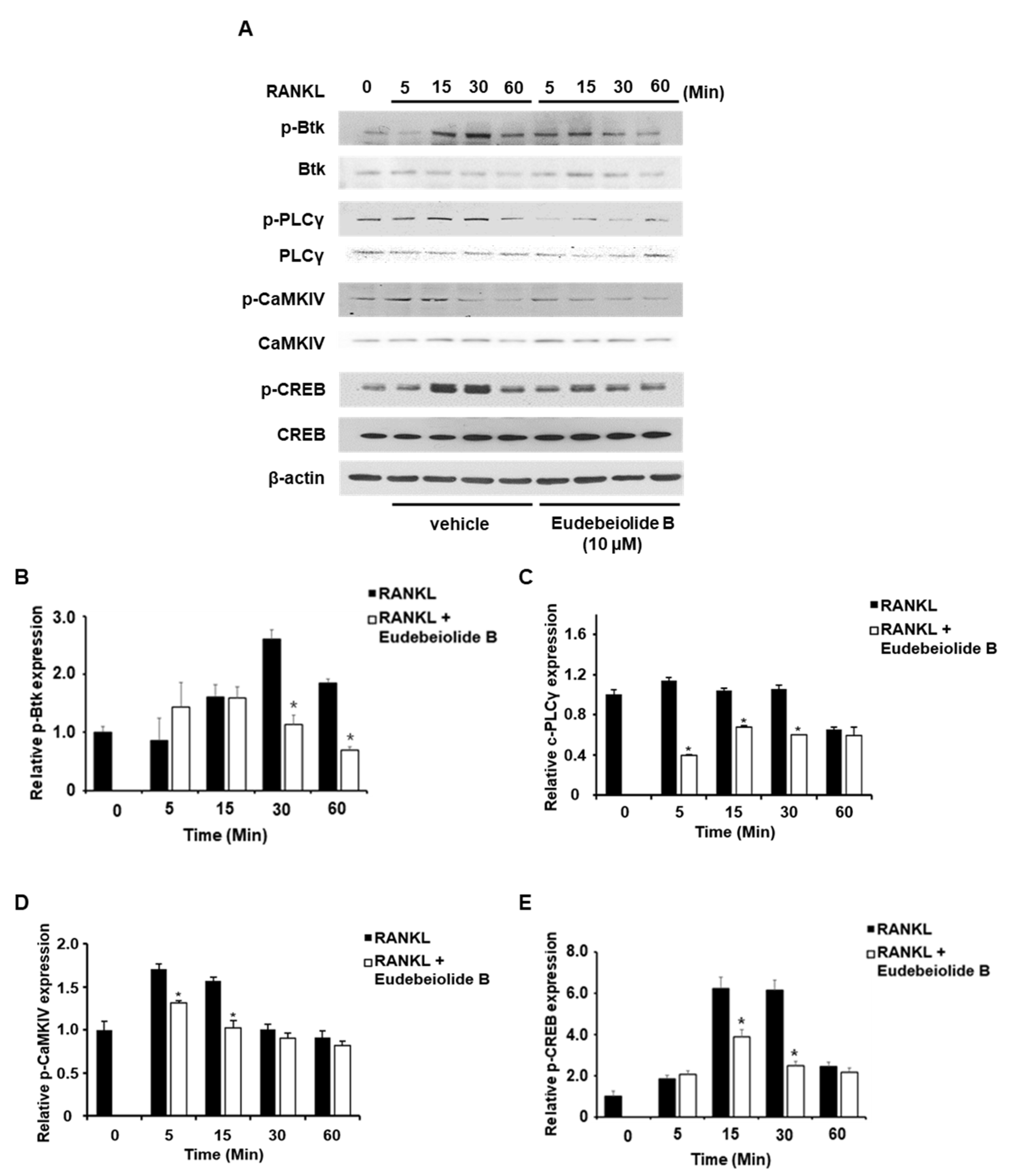
New therapeutic agents for osteoporosis are required to effectively regulate osteoclast and osteoblast differentiation and activity with no undesirable side effects [
22,
23,
24,
25]. Thus, the ideal strategy is the development of an antiosteoporotic agent to inhibit bone resorption and enhance bone formation. To develop this strategy, we examined natural-product-derived compounds as new phytomedicine antiosteoporotic candidates in this study. Eudebeiolide B is a eudesmane-type sesquiterpenoid compound that was isolated from
Salvia plebeian [
20]. We examined the effects of eudebeiolide B on osteoblast formation. Eudebiolide B promoted osteoblast differentiation and calcium accumulations. We investigated the effect of eudebeiolide B on osteoclastogenesis and OVX-induced bone loss. Eudebeiolide B inhibited RANKL-induced osteoclast differentiation and bone resorption through the suppression of PLCγ2, NFATc1 and c-Fos. Calcium signaling activates FcRγ and DAP12 by interacting with ITAM sequences and subsequently phosphorylating PLCγ [
13,
14,
15]. PLCγ2 plays an important role in the adhesion, spreading and migration of preosteoclasts [
26]. Additionally, targeted deletion of PLCγ2 resulted in an osteopetrotic phenotype in vivo and decreased RANKL-mediated NFATc1 expression in vitro [
25,
27]. CaMKIV/CREB induce c-Fos expression and NFATc1 amplification, resulting in the transcription of osteoclast-specific genes [
13,
28]. CaMKIV knockout mice exhibited increased bone mineral density and decreased bone resorption [
13]. Binding of RANKL to RANK activates MAPKs and NF-κB signaling through and calcium signaling. If eudebeiolide B inhibited the receptor directly, downstream signaling pathways including MAPKs, NF-κB and calcium signaling were inhibited by eudebeiolide B treatment. MAPK’s signaling was not affected by eudebeiolide B treatment. These results imply eudebeiolide B exerts its activity by directly inhibiting specific signaling molecules. NFATc1 regulates the expression of a number of osteoclast-specific genes, such as TRAP, MMP9, cathepsin K and DC-STAMP, which are important for osteoclast differentiation and function [
7,
8,
29,
30,
31]. These genes are upregulated during RANKL-induced osteoclastogenesis and attenuated by eudebeiolide B treatment, suggesting that eudebeiolide B not only affects the expression of NFATc1 but also the expression of its downstream genes.
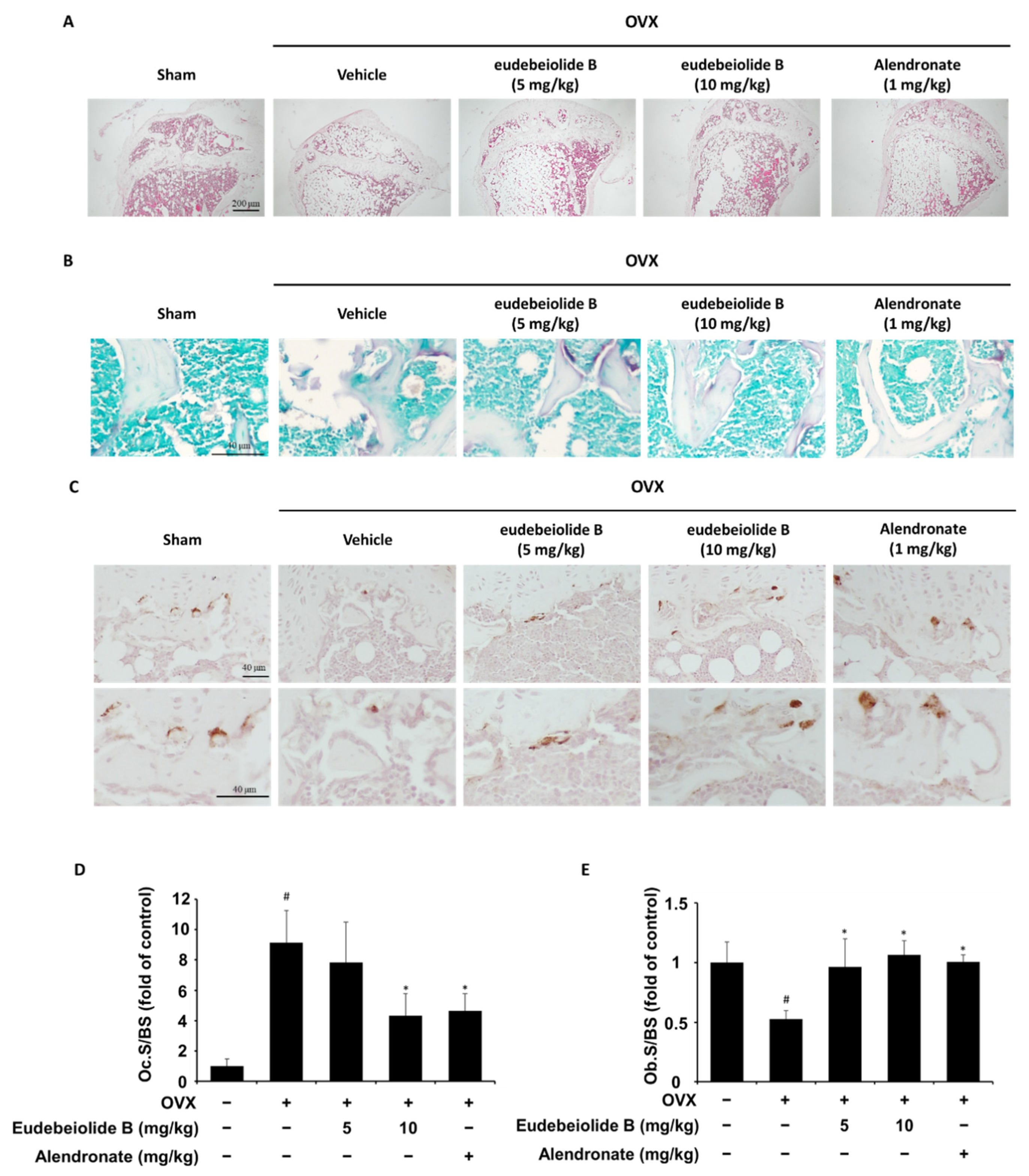
Estrogen deficiency is related to the increase in bone turnover, which accelerates bone loss and eventually increases the risk of osteoporosis [
2]. Therefore, a postmenopausal mouse model established by ovariectomy has been widely used for in vivo screens of new drug candidates [
32]. OVX increased bone turnover, as shown by the decrease in serum ALP and OPG and the increased osteocalcin, CTX and RANKL levels, but the serum levels of these markers were recovered by the administration of eudebeiolide B in OVX mice, indicating that eudebeiolide B prevents bone loss by inhibiting bone turnover.
4. Materials and Methods
4.1. Reagents
All cell culture reagents, including media, antibiotics and fetal bovine serum, were purchased from Gibco BRL (Grand Island, NY, USA). Recombinant human M-CSF and human RANKL were purchased from Peprotech (London, UK). Specific antibodies against p-ERK (Tyr202/204), p-P38, p-JNK (Tyr1007/1008), p-Akt, p-CREB, p-PLCγ2 (Tyr759), p-Btk (Tyr223), NFATc1 and c-Fos were obtained from Cell Signaling Technology (Boston, MA, USA). The monoclonal β-actin antibody was purchased from Sigma (St. Louis, MO, USA), and secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
4.2. Isolation of Eudebeiolide B
After preparing the ethanolic Salvia plebeia R. Br. extract, the residue (2.0 kg) was suspended in distilled water (2 L) and then progressively partitioned using n-hexane (30 L) and ethyl acetate (EtOAc) (30 L) to yield the n-hexane- (779 g) and EtOAc-soluble fractions (541 g), respectively. The EtOAc-soluble fraction was subjected to silica gel column chromatography and eluted with a stepwise gradient of increasing concentrations of CH3OH in CHCl3 (100:0, 50:1, 25:1, 10:1, 5:1, 2:1, 1:1 and 0:1) to obtain 14 subfractions (SPE1–SPE14) that were combined for thin-layer chromatography (TLC) analysis. SPE6 (9.5 g) was loaded onto a silica gel column to yield 15 additional subfractions (SPE6A–SPE6O) using a medium-pressure liquid chromatography (MPLC) gradient solvent system (CHCl3:CH3OH, 1:0/0:1, v/v), and SPE6D (1.39 g) was applied to the C18 MPLC column to generate 19 fractions (SPE6D1–SPE6D19) with a gradient solvent system composed of H2O:MeOH (9:1/0:1, v/v). Eudebeiolide B (24.5 mg) was obtained from SPE6D6 (0.12 g) by semipreparative HPLC (CH3CN:H2O, 25:75, v/v, 6 mL/min). For in vitro analysis, eudebeiolide B was dissolved in dimethyl sulfoxide (DMSO) (50 μM) and treated with 1 μL of serially diluted eudebeiolide B. For in vivo analysis, eudebeiolide B was suspended in 2% carboxymethyl cellulose (CMC) and administered.
4.3. Determination of Eudebeiolide B Content in Salvia Plebeia EtOH Extract
For the HPLC-DAD analysis, the Agilent 1200 HPLC system (Agilent Technologies, Wilmington, DE, USA), consisting of a quaternary pump (G1311A), a degasser (G1322A), an autosampler (G1329A), a column oven (G1316A) and a diode array detector (G1315D) at a 190–400 nm wavelength, was employed. A reverse-phase column (J′sphere ODS-H80, 4 μm, 4.6 × 150 mm, YMC, Kyoto, Japan) maintained at 25 °C was used, and elution was performed with a gradient of water (A, H2O) and acetonitrile (B, CH3CN) at a flow rate of 1 mL/min. The following solvent gradient was used: 15% B within 5 min; from 15% to 40% B within 35 min; from 40% to 100% B within 5 min. The quantification of eudebeiolide B was performed using a calibration curve at six concentrations over the linear range (0.5–100.0 μg/mL).
4.4. Cell Culture
Mouse osteoblastic MC3T3-E1 cells were purchased from ATCC (Rockville, MD, USA) and cultured in α-MEM containing 10% FBS, 100 U/mL penicillin and 100 mg/mL streptomycin (Gibco). For differentiation, the medium was added to 50 mg/mL ascorbic acid and 10 mM β–glycerophosphate (Sigma-Aldrich, St. Louis, MO, USA). Mouse bone marrow cells were obtained from 5-week-old ICR mice and differentiated to bone marrow macrophages (BMMs) in α-MEM containing 10% FBS, 30 ng/mL M-CSF, 100 U/mL penicillin and 100 mg/mL streptomycin.
4.5. In Vitro Osteoclastogenesis Assay
Mouse bone marrow cells obtained from 5-week-old ICR mice were cultured as previously described [
19]. Briefly, BMMs were plated on 48-well culture plates at a density of 2 × 10
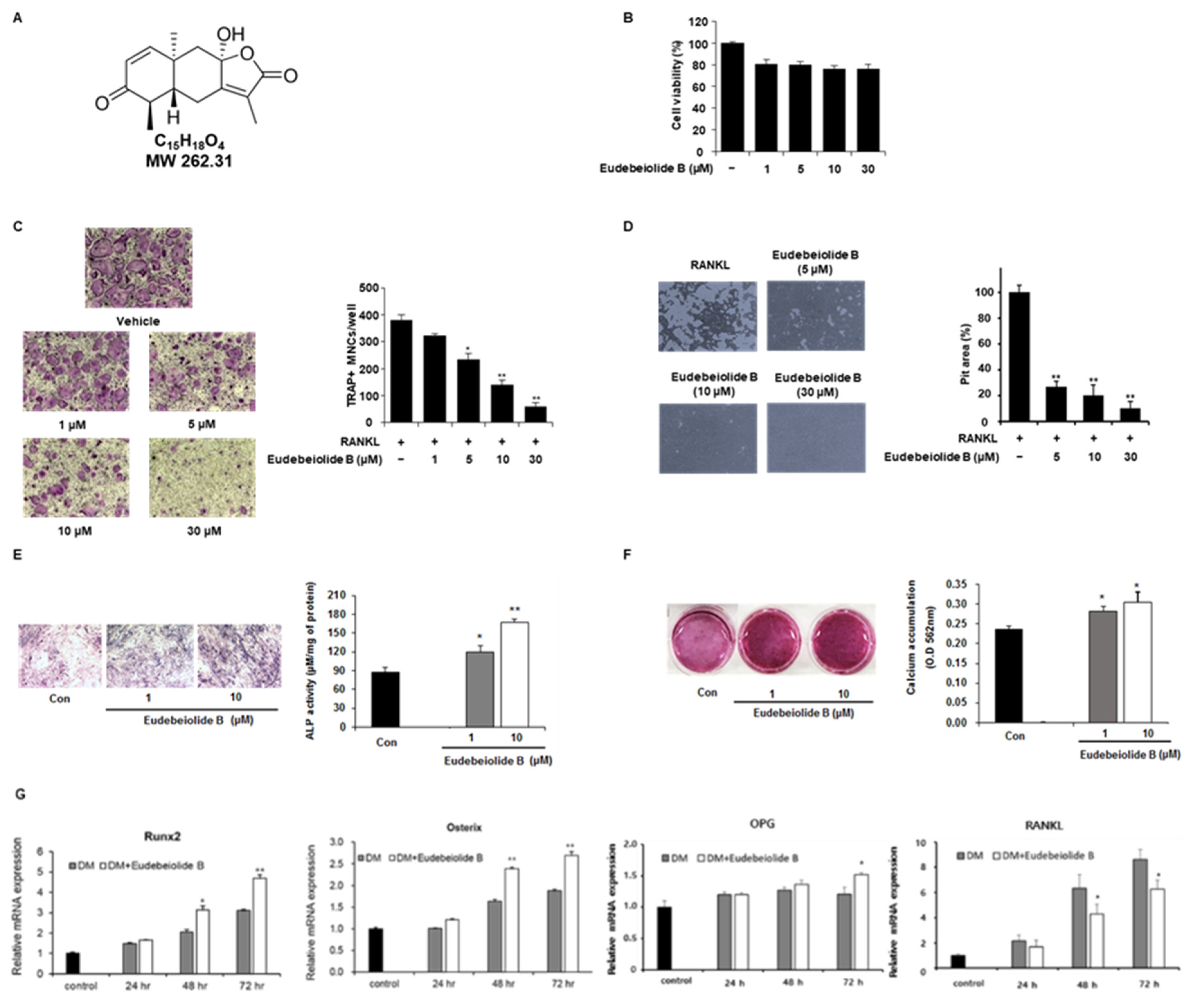
4 cells/well and treated with M-CSF (30 ng/mL) and RANKL (100 ng/mL) after pretreatment with various concentrations of eudebeiolide B. DMSO and PBS were used as vehicles of eudebeiolide B and M-CSF, RANKL. After 5 days, the cells were fixed and stained with TRAP according to the manufacturer’s instructions (Sigma-Aldrich). In brief, cells were fixed with 10% formaldehyde and washed with deionized water. The sample was then stained with a TRAP solution for 1 h. TRAP solution was prepared following the manufacturer’s instructions. After staining, cells were washed and TRAP-positive multinucleated osteoclasts were counted.
4.6. Cell Viability Assay
BMMs were seeded in 96-well plates at a density of 1 × 104 cells/well. The cells were treated with various concentrations of eudebeiolide B in the presence of M-CSF (30 ng/mL) for 72 h. DMSO and PBS were used as vehicles of eudebeiolide B and M-CSF. A 50 µL volume of XTT solution (Cell Signaling Technology) was then added to the culture plate and incubated for 3 h. The absorbance was measured at 450 nm using a microplate ELISA reader (Molecular Devices, Sunnyvale, CA, USA).
4.7. Bone Resorption Assay
BMMs were seeded on hydroxyapatite-coated plates (Corning, NY, USA) at a density of 2 × 104 cells/well and cultured with M-CSF (30 ng/mL) and RANKL (50 ng/mL) for 3 days. Mature multinucleated osteoclasts were treated with or without eudebeiolide B for 48 h. DMSO and PBS were used as vehicles of eudebeiolide B and M-CSF, RANKL. After incubation, a 1.2% sodium hypochlorite solution was added to each well to promote cell detachment. The plate was rinsed with distilled water, and the resorption pits were analyzed using the ImageJ program.
4.8. Alkaline Phosphatease (ALP) Staining and Determination of ALP Activity
MC3T3-E1 cells were seeded at 1 × 104 cells/well in a 12-well plate and incubated for 2 days. The media was changed with differentiation media, and cells were treated with eudebeiolide B for 7 days. For the ALP staining, cells were fixed with 10% formaldehyde and stained with BCIP/NBT substrate solution (Sigma-Aldrich). For the measuring ALP activity, cells were lysed with lysis buffer and centrifuged. The supernatants were analyzed by an alkaline phosphatase assay kit (Abcam, Cambridge, MA, USA) following the manufacturer’s instructions. The ALP activity was normalized by total protein concentrations.
4.9. Alizarin Red Staining
MC3T3-E1 cells were seeded at 1 × 104 cells/well in a 12-well plate and incubated for 2 days. The media was changed with differentiation media, and cells were treated with eudebeiolide B for 21 days. The medium was changed every 3 days. The cells were then washed with PBS and fixed with 10% formalin for 30 min at room temperature. After fixation, the cells were washed with PBS and stained with 2% Alizarin red S staining solution (Sigma-Aldrich) for 45 min at room temperature in the dark. The cells were washed with distilled water and dissolved with 10% (w/v) cetylpyridinium chloride in 10 mM sodium phosphate. The absorbance was measured at 562 nm.
4.10. Western Blot Analysis
BMMs were pretreated with eudebeiolide B for 1 h and then stimulated with RANKL (100 ng/mL) for the indicated times. DMSO and PBS were used as vehicles of eudebeiolide B and M-CSF, RANKL. Total protein was extracted using a cell lysis buffer (Cell Signaling Technology) containing a protease and phosphatase inhibitor cocktail (Thermo Scientific, Cheshire, UK). Protein concentrations were measured using a bicinchoninic acid protein assay kit (Sigma-Aldrich Co., St. Louis, MO, USA). Equal amounts of protein were separated on 4–12% SDS-PAGE gels and transferred onto PVDF membranes (Amersham Bioscience, Freiburg, Germany). The membranes were blocked with 1× TBS containing 5% skim milk and washed with 1× TBS containing 0.1% Tween-20 (TBST). After blocking, the membranes were incubated with the appropriate primary antibodies and washed with TBST. After washing. The membranes were incubated with the appropriate secondary antibodies and washed with TBST. The antibodies were diluted to 1:1000 in TBS containing 5% BSA. Finally, target-specific signals were detected using an enhanced chemiluminescence solution (Intron Biotechnology, Seongnam, Korea).
4.11. Quantitative Real-Time RT-PCR
Total RNA was extracted from the total cell lysates with a PureLink RNA Mini kit (Invitrogen, San Diego, CA, USA), according to the manufacturer’s instructions. The complementary DNA was synthesized from 1 µg/mL of total RNA using SuperScript III First-Strand Synthesis System for RT-PCR (Thermo Scientific). Real-time PCR was performed using the StepOnePlus Real-Time PCR System using the TaqMan probe with the TaqMan Real-Time PCR Master Mixes (Applied Biosystems, Foster City, CA, USA). The relative mRNA expression levels were quantified using the threshold cycle (Ct) value, and the Ct value was normalized using the levels of the mouse GAPDH gene as an endogenous reference.
4.12. In Vivo Models
Six-week-old female C57BL/6 mice were purchased from OrientBio (Seongnam, Korea). The mice were randomly divided into 5 groups (n = 6 mice per group): sham-operated mice (SHAM), OVX mice treated with vehicle (OVX), OVX mice treated with 1 mg/kg alendronate (ALN) and OVX mice treated with eudebeiolide B (5 mg/kg or 10 mg/kg). Alendronate was used as a positive control. After stabilization for one week, the mice received a sham operation or a bilateral ovariectomy (OVX) under anesthesia. The mice were maintained without any treatment for 6 weeks after surgery until bone loss was apparent. After 6 weeks, eudebeiolide B and alendronate were suspended in 2% CMC and intragastrically injected into the appropriate groups once daily for 6 weeks. The animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Korea Research Institute of Bioscience and Biotechnology (permission number # KRIBB-AEC-17059) and all mice were handled in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
4.13. Dual-Energy X-Ray Absorptiometry (DEXA)
At the end of the experiment, the mice were euthanized, and the bone mineral density (BMD) and bone mineral content (BMC) of the total body area were analyzed using GE Lunar PIXImus2 Dual-energy X-ray absorptiometry (GE Healthcare, Madison, WI, USA), according to the manufacturer’s instructions. The instrument was calibrated using a phantom provided by the manufacturer.
4.14. Microcomputed Tomography (CT) Measurements
For the micro-CT analysis, the femurs were removed from the mice, cleaned of soft tissue, fixed in 10% formalin and stored in PBS at 4 °C. The bone morphometric parameters and microarchitectural properties of the femurs were analyzed using a SkyScan 1076 micro-CT scanner (Bruker microCT, Kontich, Belgium). The femurs were prepared in polystyrene tubes filled with PBS to prevent drying during scanning. The specimen was mounted on the 1076 scanner sample chamber for micro-CT imaging and the sample was rotated automatically along the axis (angular step: 0.42°, reconstruction angular range: 360.36°). Scanning was performed using a 0.5 mm Al filter, an 88 kV source voltage and a 112 μA source current with a 9 μm resolution. The region of interest was defined between 0.5 mm and 2.5 mm below the growth plate of the proximal femur. The bone volume per tissue volume (BV/TV), trabecular number (Tb.N.), trabecular thickness (Tb.Th.) and three-dimensional images of the femur were analyzed using the Nrecon®, CTAn® and CTVol® software programs.
4.15. Biochemical Analysis of Serum
Blood samples were collected from all mice by cardiac puncture before sacrifice. Serum was obtained by centrifugation and then stored at −80 °C for the analysis of biochemical parameters. The serum levels of ALP, CTX, OPG and RANKL were measured by commercial ELISA kits following the manufacturer’s instructions. The ELISA kits of ALP, OPG and RANKL were obtained from Abcam and CTX was obtained from immunodiagnostic systems (Boldon, UK).
4.16. Histological Analysis
For histological analysis, the tibiae were removed from the mice, cleaned of soft tissue, fixed in 10% formalin for a day and decalcified in 10% ethylenediaminetetraacetic acid (EDTA) at 4 °C for a month. The decalcified sample was embedded in paraffin, sectioned at 5 μm and deparaffinized. H&E staining and histochemical staining of TRAP were then performed using an acid phosphatase staining kit (Sigma-Aldrich) according to the manufacturer’s instructions. Immunohistochemical staining for ALP was performed with the following procedures. In brief, deparaffinized slide was subjected to heat-induced antigen retrieval in 1 mM EDTA (pH8) for 15 min and incubated for 10 min in 3% hydrogen peroxide and blocked with TBST containing 5% normal goat serum for 1 h at room temperature. After blocking, the anti-ALP primary antibody (Abcam, Cambridge, UK) was bound overnight at 4 °C and washed with TBST, incubated on a slide with HRP-conjugated secondary antibody, developed with diaminobenzidine (DAB) substrate and counter-stained with hematoxylin. The osteoclast surface/bone surface and osteoblast surface/bone surface were measured using HistoMorph software and ImageJ software.
4.17. Statistical Analysis
Statistical analyses were performed on data collected in triplicate for all the experiments. All quantitative results are presented as means ± standard deviations (SD). Statistical analyses were performed using Prism 5 software (GraphPad Software, San Diego, CA, USA), and statistical significance was determined by one-way ANOVA followed by Dunnett’s test or the unpaired Student’s t-test.