Abstract
The wide spread of pathogens resistance requires the development of new antimicrobial agents capable of overcoming drug resistance. The main objective of the study is to elucidate the effect of substitutions in tris(1H-indol-3-yl)methylium derivatives on their antibacterial activity and toxicity to human cells. A series of new compounds were synthesized and tested. Their antibacterial activity in vitro was performed on 12 bacterial strains, including drug resistant strains, that were clinical isolates or collection strains. The cytotoxic effect of the compounds was determined using an test with HPF-hTERT (human postnatal fibroblasts, immortalized with hTERT) cells. The activity of the obtained compounds depended on the carbon chain length. Derivatives with C5–C6 chains were more active. The minimum inhibitory concentration (MIC) of the most active compound on Gram-positive bacteria, including MRSA, was 0.5 μg/mL. Compounds with C5–C6 chains also revealed high activity against Staphylococcus epidermidis (1.0 and 0.5 μg/mL, respectively) and moderate activity against Gram-negative bacteria Escherichia coli (8 μg/mL) and Klebsiella pneumonia (2 and 8 μg/mL, respectively). However, they have no activity against Salmonella cholerasuis and Pseudomonas aeruginosa. The most active compounds revealed higher antibacterial activity on MRSA than the reference drug levofloxacin, and their ratio between antibacterial and cytotoxic activity exceeded 10 times. The data obtained provide a basis for further study of this promising group of substances.
1. Introduction
In recent years, the situation in the field of therapy of infectious diseases has been significantly complicated due to the wide spread of pathogens resistant to known antibiotic drugs [1,2,3]. To solve this problem, it is proposed to try and prevent the drug resistance by rational use of antibiotics, and by development of new antimicrobial agents capable of overcoming drug resistance. The latter direction is one of the most important ones of modern medicinal chemistry [4]. The most promising compounds are the ones that have low toxicity for human cells and retain high activity on resistant strains of pathogens. The main objective of this study was to elucidate the effect of substituents on the activity of compounds that contain tris(1-alkylindol-3-yl)methylium core with respect to various test microorganisms, as well as their toxicity for human cells.
Recently, compounds containing triphenylmethyl or triindolylmethyl fragments have attracted the interest of researchers. This is due to the fact that some compounds of this type exhibit useful biological properties, such as antimicrobial and antiproliferative [5,6,7,8,9,10,11,12,13].
Earlier, we studied the structure-antimicrobial and cytotoxic activity relationship among a new class of compounds—tris(1-alkylindol-3-yl)methylium salts, structurally similar to the natural antibiotic turbomycin A [5]. Among them, a number of substances with a high (submicromolar) activity, even on multidrug-resistant strains of Staphylococcus aureus, were found. The first symmetrical alkyl derivatives we obtained were highly active against bacteria, but their toxicity was higher than the antibacterial activity [11,12]. Their structure needed to be improved. One of the successful attempts were chimeric structures [14] combining fragments of tris(1-alkylindol-3-yl)methylium and 3,4-disubstituted pyrrole-2,5-diones (maleinimides) [15,16]. They had a relatively low toxicity to human cells, and at the same time, a high activity on resistant strains of Gram-positive bacteria [14], acting by disrupting the functioning of their membranes [17].
When analyzing the structure-activity relationship of these compounds, we observed that the activity of these substances is closely related to their lipophilicity. The most promising substances had a LogPow (partition between octanol and water) in the region of 2–5, and if the LogPow was lower than that, the substance had neither pronounced antibacterial activity nor cytotoxicity. If the LogPow was higher then 5, the cytotoxicity was higher than the antibacterial activity. Thus, the assumption was made about the optimal region of LogPow, where one can expect to find substances with a good ratio of antibacterial activity to cytotoxicity. Symmetrical N-(hydroxyalkyl) derivatives of tris(1H-indol-3-yl)methylium seem very promising in this regard, since by changing the length of the hydrocarbon part of the substituent, it is possible to adjust the lipophilicity of the molecule. In addition, the presence of hydroxyl groups improves the solubility of substances in aqueous media. This paper presents the synthesis and study of antibacterial and cytotoxic activity in the homologous series of N-(hydroxyalkyl) derivatives of tris(1H-indol-3-yl)methylium with a hydrocarbon chain length from C2 to C6.
2. Results and Discussion
2.1. Chemistry
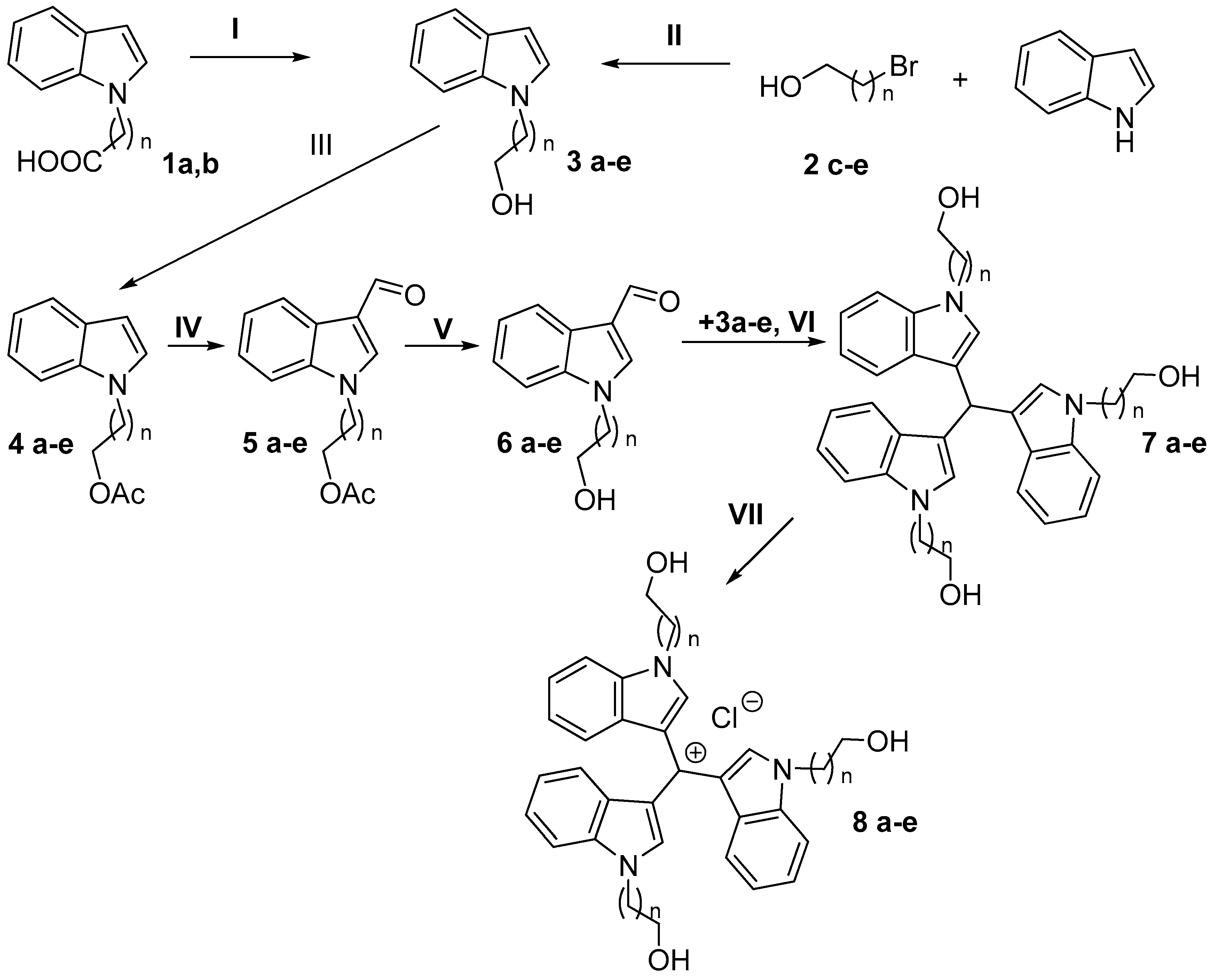
The title compounds were synthesized according to Scheme 1. To start, reagents 2-(1H-indol-1-yl)acetic acid (1a) and 3-(1H-indol-1-yl)propanoic acid (1b) were used, which were converted to the corresponding (1H-indol-1-yl)alkanols 3a and 3b by LiAlH4 reduction. To obtain (1H-indol-1-yl)alkanols 3c–e, another synthesis method was used—alkylation of indole by ω-bromoalkanols 2c–e. The hydroxyl group of compounds 3a–e was then protected by acetylation to obtain substances 4a–e, which were then subjected to formylation by the Wilsmeier–Haack method, with further deacetylation to obtain 1-(ω-hydroxyalkyl)-1H-indole-3-carbaldehydes 6a–e. Without the protection of the hydroxyl group, formylation of 3a–e is not possible, the reaction leading to formation of a complex mixture of products. To obtain symmetric tris(1-[ω-hydroxyalkyl]-1H-indol-3-yl)methanes 7a–e, we condensed (1H-indol-1-yl)alkanoles 3c–e with the corresponding aldehydes 6a–e in 2:1 molar ratio in boiling methanol using dysprosium triflate Dy(OTf)3 as a catalyst. As a final stage, compounds 7a–e were oxidized by DDQ (2,3-dichloro-5,6-dicyano-1,4-benzoquinone) in THF with subsequent HCl treatment to yield the target tris(1-[ω-hydroxyalkyl]-1H-indol-3-yl)methylium salts 8a–e. All synthesized compounds were characterized by NMR spectroscopy, high-resolution mass spectrometry (HRMS), and HPLC.

Scheme 1.
Synthesis of all compounds. For compounds: 1a: n = 1; 1b: n = 2; 2–8a: n = 1; 2–8b: n = 2; 2–8c: n = 3; 2–8d: n = 4; 2–8e: n = 5. Reagents and conditions: (I) LiAlH4, THF, reflux; (II) KOH, DMSO, rt; (III) Ac2O, pyridine, DMAP, rt; (IV) POCl3, DMF, rt; (V) NaOMe, MeOH, rt; (VI) MeOH, Dy(OTf)3, reflux; (VII) DDQ, THF, HCl rt.
2.2. Biological Evaluation
2.2.1. Antibacterial Activity
As reference compounds, we used levofloxacin, a common broad-spectrum antibiotic, and Brilliant Green, a common antiseptic with a structure somewhat similar to substances 8a–e, being triarylmethylium salt. The compounds 7a–e were insoluble in water, so for a test for antibacterial activity, a solubilizer had to be used. Addition of Kolliphor EL (5× by weight) provided enough solubility for the compounds. The compounds 8a–e were soluble enough in water by themselves.
Trisidolylmethanes 7a–e showed practically no antibacterial activity (MICs >64 μg/mL). This is in good agreement with our earlier data that triindolylmethanes are biologically inactive until they are oxidized to trisindolylmethylium salts [11,12,14]. Trisindolylmethylium salts 8a–e exhibited significant antibacterial activity, as shown in Table 1. The data obtained show that 8a–b substituted with C2 and C3 hydroxyalkyl substituents have practically no antibacterial activity, however, with further growth of the chain length starting from C4, pronounced activity appears, reaching 0.5 µg/mL in the C6 derivative (compound 8e). The compounds which are active on bacteria but are low-toxic should balance between pore-forming activity on the lipid bilayers and non-selective detergent activity [17,18].

Table 1.
Antibacterial and cytotoxic activity of 8a–e.
Compounds 8d and 8e were highly active against Gram-positive bacteria, including strains with antibiotic resistance. For example, activity of compound 8e against S. aureus ATCC 25,923 and clinical isolate S. aureus 10, that were sensitive to all antibiotics, was 0.5 μg/mL. At the same time, activity of this compound against two methicillin resistant strains (MRSA) (S. aureus 5 and S. aureus 100 KC) was just the same high (0.5 μg/mL). The same activity (0.5 μg/mL) was revealed against S. aureus ATCC 3798, which was resistant not only to ampicillin, oxacillin, cefuroxime, and carbenicillin (antibiotics of penicillin and cefalosporine group), but also to clindamycin, erythromycin, rifampicin, ciprofloxacin, and levofloxacin. Compounds 8d and 8e were active against S. aureus ATCC 700699, which possess resistance to levofloxacin.
Compounds 8d and 8e were also active (1, 0.5 μg/mL) against Staphylococcus epidermidis 533, which is resistant to gentamicin, but, in contrast to 8d (8 μg/mL), compound 8e was almost inactive (>64 μg/mL) to Enterococcus faecium 569, which possess resistance to cefuroxime, clindamycin, gentamycin, vancomycin, and doxycycline.
A moderate level of activity of 8d and 8e was found against Escherichia coli ATCC 25,922 and Klebsiella pneumoniae ATCC 13883. Against K. pneumoniae compound 8d was slightly more active (2 μg/mL) than compound 8e (8 μg/mL). However, it should be noted that these two compounds, in general, were significantly less active against Gram-negative bacteria than against Gram-positive ones.
2.2.2. Cytotoxic Activity
Compounds 7a–e all showed similar cytotoxicity with IC50 higher than 50 μg/mL. Cytotoxicity of compounds 8a–e (Table 1) depended on the length of the hydroxyalkyl chain and for C2–C4 substituents IC50, were higher than 50 µg/mL. For C5 compound 8d, it was 13 µg/mL, and then in C6, there is a sharp increase in cytotoxicity, which reached 2 µg/mL. Apparently, this rapid increase in cytotoxicity is associated with an increase in the total detergent activity of the molecule, which leads to a decrease in the selectivity of the action on the lipid layers of the membrane [17,18].
2.3. Study of the Relationship between Lipophility and Biological Activity
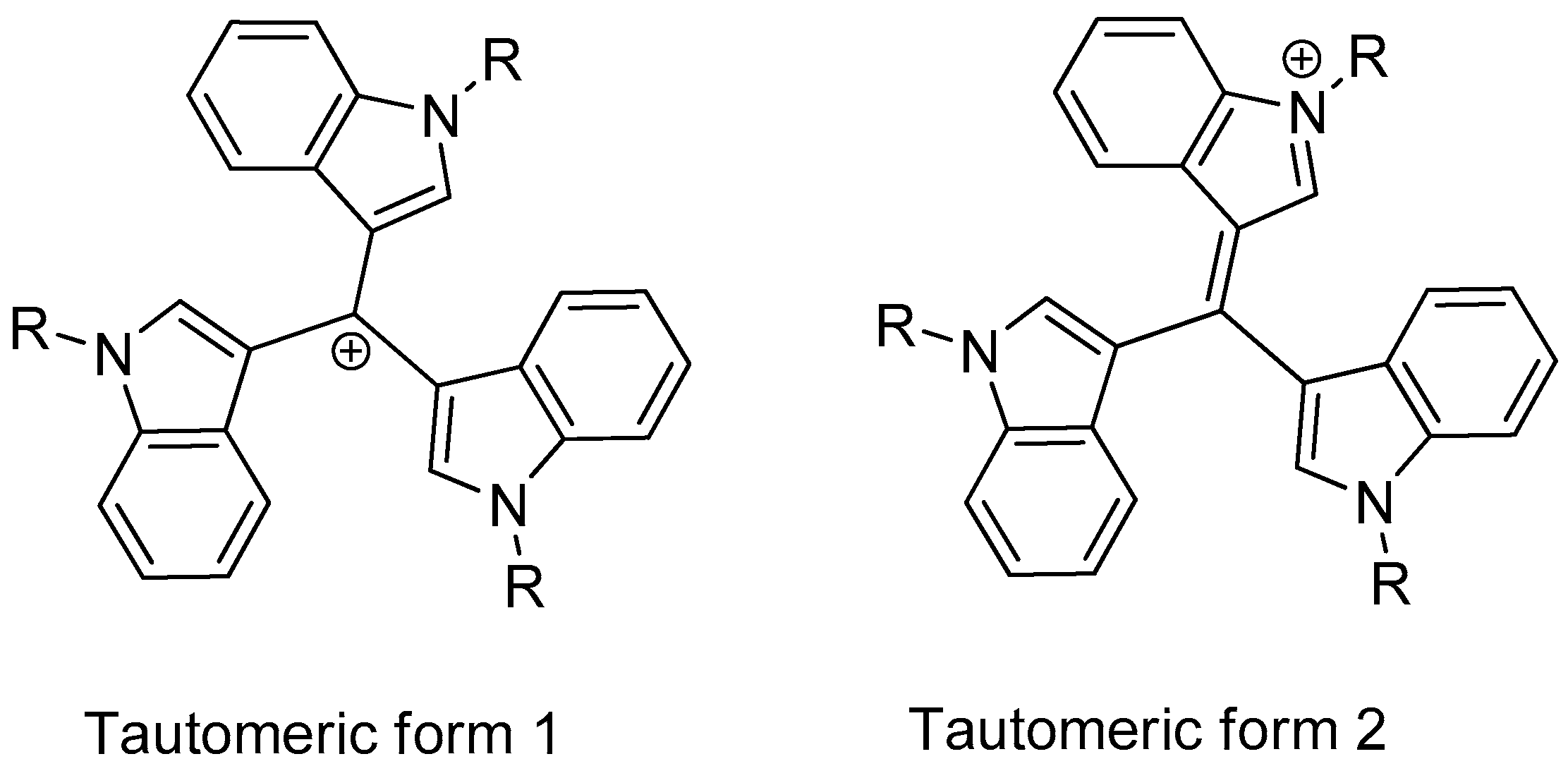
Analyzing the structure-activity relationship for compounds 8a–e, it was observed that the activity of these substances is closely related to their lipophilicity. There are two possible tautomeric variations of methylium (Figure 1): form 1, where the positive charge is on the central carbon atom, and closer to the real form 2, where the positive charge is on one of the nitrogen atoms of the indole cycles.

Figure 1.
Tautomeric forms of compounds 8a–e.
It was shown that the calculated values in this case are very close to the real ones when the contribution of both forms is taken into account. In other words, the arithmetic mean (miLogPow form1 + miLogPow form2)/2 will be closer to the real LogPow obtained experimentally (Table 2). Data analysis shows that molecules with a good ratio of antibacterial activity to cytotoxicity are more likely to be in the LogP range from 2 to 5. Above 5, a sharp increase in cytotoxicity begins, and below 2, there is an almost complete absence of antimicrobial activity. The best result is likely to be expected with a LogP in the region of 4. Computer calculations of lipophilicity in the Molinspiration package fairly adequately predict LogP for molecules and can be used to select potentially promising compounds of this group.

Table 2.
Experimental and calculated values LogPow for 8a–e.
3. Materials and Methods
3.1. Chemistry
All the reagents were obtained commercially and used without further purification. Indole and all ω-bromoalkanols, all solvents, LiAlH4, Dy(OTf)3, DMAP, DDQ were purchased from Sigma-Aldrich; 2-(1H-indol-1-yl)acetic acid and 3-(1H-indol-1-yl)propanoic acid were purchased from Alinda company (www.alinda.ru). Purity of the compounds was checked by thin layer chromatography using silica-gel 60 F254-coated Al plates (Merck) and spots were observed under UV light (254 nm). Column chromatography was performed on Kieselgel 60 (Merck). Proton nuclear magnetic resonance (1H NMR) and carbon-13 nuclear magnetic resonance (13C NMR) spectra (in DMSO-d6) were recorded on a Varian VXR-400 spectrometer at 400 and 100 MHz respectively, the chemical shift values are expressed in ppm (δ scale) using DMSO as an internal standard, the coupling constants expressed in Hz. The NMR spectra of the compounds 8a–e were recorded at 80 °C to avoid peaks broadening. The mass spectral measurements were carried out by ESI method on microTOF-QII (Brucker Daltonics GmbH). Analytical high-performance liquid chromatography (HPLC) was performed on a Shimadzu LC-20AD system using Kromasil-100-5-C18 (Akzo-Nobel) column, 4.6 × 250 mm, 20 °C temperature, UV detection, mobile phase A—0.2% HCOONH4), mobile phase B-MeCN, (pH 7.4), fl-1 mL/min., loop 20 mkl. The NMR spectra of the compounds 3–10 are presented in Supplementary file NMR_spectra.pdf.
3.2. Antibacterial Activity
Compounds were tested against Gram-positive and Gram-negative bacteria, including sensitive or drug resistant strains from American Type Culture Collection (ATCC), as well as resistant clinical isolates from the culture collection of the Laboratory for Control of Hospital Infections (Sechenov University, Moscow, Russia). Collection cultures of Gram-positive bacteria: Staphylococcus aureus ATCC 25923, Staphylococcus aureus ATCC 3798, Staphylococcus aureus ATCC 700,699 and clinical isolates of Gram-positive bacteria: Staphylococcus aureus 5, Staphylococcus aureus 10, Staphylococcus aureus 100 KS, Staphylococcus epidermidis 533, Enterococcus faecium 569 and collection cultures of Gram-negative bacteria: Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 13883, Salmonella cholerasuis ATCC 14,028 were used.
For the cultivation of the strains, various nutrient media were used: Trypticase Soy Agar BBL for Staphylococcus sp., E. coli, K. pneumoniae, S. cholerasuis and Columbia Agar Base BBL for the cultivation of Enterococcus sp. Cultures grown on appropriate nutrient media at 35 °C for 1 day were used to set up experiments. For determination of the antibacterial action Mueller-Hinton (Acumedia, Baltimore, MD, USA) liquid medium was used. The minimum inhibitory concentrations (MIC) were determined by the microdilution method in 96 well sterile plates in a cation-adjusted Müller-Hinton medium in accordance with the requirements of the Institute of Clinical and Laboratory Standards (CLSI/NCCLS) [19]. MIC was defined as the minimum drug concentration that completely prevents the growth of the test organism.
3.3. Cytotoxic Activity
The cytotoxic properties of the compounds obtained were tested using the MTT assay as described previously [11] on the healthy donor (postnatal) human fibroblasts immortalized by transfection of the hTERT gene of the catalytic component of telomerase (hereinafter, FB).
Cells were grown at 37 °C and 5% CO2. Human donor fibroblasts were cultivated in DMEM medium (Paneko, Russia) with addition of 10% FBS (Hyclone, Austria), 2 mM L-glutamine (Paneko, Russia), and 1% penicillin-streptomycin (Paneko, Russia). Cells were seeded at concentration 2500 cells/well in 96 well plates (Corning, NY, USA), and left overnight to attach. The next day, the cells were treated with compounds, with indicated concentrations (ten two-fold dilutions, starting from 50 uM) for 72 h. After incubation, MTT reagent (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) (Sigma-Aldrich, Saint-Louis, MO, USA) was added to a final concentration of 0.5 ug/mL and the cells were incubated for 2 h at 37 °C and 5% CO2. After incubation, the medium was discarded, and 100 uL of DMSO was added. The optical densities were read at 570 nm wavelength on Multiskan FC (ThermoFisher, Waltham, MA, USA). The OD values for controls were taken as 100%. The IC50 values were calculated in GraphPadRrism 6.0.
3.4. Determination of Lipophilicity
We used the partition coefficient as an indicator of lipophilicity. The partition coefficient (P) is defined as the ratio of the equilibrium solute concentrations in a two-phase system of immiscible solvents. The most common in practice is the octanol-water (Pow) system. The partition coefficient is usually represented as a decimal logarithm (LogPow). It can be measured in several ways. A most advanced, accurate, and less time-consuming is the HPLC method for determining Pow using high-performance liquid chromatography [20]. HPLC is performed on an analytical column with a solid phase containing long hydrocarbon chains chemically bound to silica gel. The retention time on such a column (Rt) is directly related to the partition coefficient Pow. The most informative in our case was the partition coefficient at a physiological pH value of 7.4. It was at this pH that the main biological experiments with the studied substances were carried out, namely tests of antimicrobial activity and cytotoxicity. HPLC was performed on a Shimadzu LC-20AD system using Kromasil-100-5-C18 (Akzo-Nobel) column, 4.6 × 250 mm, 20 °C temperature, UV detection, mobile phase A—0.2% HCOONH4), mobile phase B-MeCN, (pH 7.4), fl-1 mL/min., loop 20 mkl. After calibration using substances with a known LogPow, it is possible to recalculate Rt to LogPow. For calibration, we used aniline (LogPow 0.9), p-chloroaniline (LogPow 1.8), diphenylamine (LogPow 3.4), triphenylamine (LogPow 5.7).
To study the possibility of using computer models to calculate LogPow [21], miLogPow values were calculated for the same substances by the Molinspiration package [22], which is an online tool available at www.molinspiration.com. The method for logP prediction developed at Molinspiration (miLogPow) is based on group contributions. These have been obtained by fitting calculated logP with experimental logP for a training set more than twelve thousand, mostly drug-like molecules. In this way, hydrophobicity values for 35 small simple “basic” fragments were obtained, as well as values for 185 larger fragments, characterizing intramolecular hydrogen bonding contribution to logP and charge interactions. Molinspiration methodology for logP calculation is very robust and is able to process practically all organic molecules. For 50.5% of molecules, logP is predicted with error <0.25, for 80.2% with error <0.5 and for 96.5% with error <1.0. Only for 3.5% of structures, logP is predicted with error >1.0. The statistical parameters listed above rank Molinspiration miLogP as one of the best methods available for logP prediction. MiLogP is used due to its robustness and good prediction quality in the popular ZINC database for virtual screening. A report by the National Institute of Standards documenting excellent agreement between experimental logP and Molinspiration calculated logP for some industrial chemicals [23].
3.5. Chemical Experimental Data
2-(1H-Indol-1-yl)-ethanol (3a).
To the boiling suspension of LiAlH4 (15.2 g, 0.4 mol) in THF (500 mL), the solution of 2-(1H-indol-1-yl)acetic acid 1a (17.56 g, 0.1 mol) in THF (100 mL) was gradually added, then the reaction mixture was refluxed for 5 h. After cooling to RT, the reaction mixture was quenched with KOH (20% aqueous solution), then was filtered and diluted with EtOAc (300 mL) and aqueous solution of citric acid (10.0 g in 100 mL) was added. The organic layer was separated, washed with water and brine, and evaporated in vacuo. The residue was purified by flash chromatography (50 g of silica gel) using EtOAc-hexane (1:10 to 1:1) as an eluent, to give 3a (13.2 g, 82%) as a colorless oil.
1H NMR: δ 7.52 (dt, 1H, J = 7.8, 1.1 Hz), 7.48–7.41 (m, 1H), 7.33 (d, 1H, J = 3.1 Hz), 7.10 (ddd, 1H, J = 8.2, 7.0, 1.3 Hz), 6.99 (ddd, 1H, J = 8.0, 7.0, 1.0 Hz), 6.40 (dd, 1H, J = 3.1, 0.9 Hz), 4.93 (t, 1H, J = 5.3 Hz), 4.19 (t, 2H, J = 5.7 Hz), 3.70 (q, 2H, J = 5.5 Hz). 13C NMR: δ 136.33, 129.59, 128.53, 121.26, 120.73, 119.24, 110.32, 100.67, 60.76, 48.66, 48.64. HRMS (EI) m/z [M + H]+ Calcd for C10H12NO+ 162.0913; Found 162.0923.
3-(1H-Indol-1-yl)propan-1-ol (3b).
The same procedure as above was carried out using 3-(1H-indol-1-yl)propanoic acid (18.9 g, 0.1 mol), to give 3b (13.8 g, 79%) as a colorless oil.
1H NMR: δ 7.53 (dt, 1H, J = 7.8, 1.0 Hz), 7.44 (dd, 1H, J = 8.3, 1.0 Hz), 7.32 (d, 1H, J = 3.1 Hz), 7.14–7.07 (m, 1H), 7.04–6.97 (m, 1H), 6.41 (dd, 1H, J = 3.2, 0.9 Hz), 4.69 (s, 1H), 4.21 (t, 2H, J = 6.9 Hz), 3.37 (d, 2H, J = 5.2 Hz), 1.88 (t, 2H, J = 6.6 Hz). 13C NMR: δ 136.08, 129.12, 128.53, 121.38, 120.85, 119.27, 110.17, 100.82, 58.27, 42.84, 33.43. HRMS (EI) m/z [M + H]+ Calcd for C11H14NO+ 176.1070; Found 176.1073.
4-(1H-Indol-1-yl)butan-1-ol (3c).
To the suspension of KOH (20 g, 0.35 mol) in DMSO (100 mL), indole (11.7 g, 0.1 mol) and 4-bromobutan-1-ol (16.8 g, 0.11 mol) were added. After intensive stirring at RT for 5 h, the reaction mixture was filtered, diluted with EtOAc (300 mL), and washed with an aqueous solution of citric acid (10.0 g, 100 mL). The organic layer was separated, washed with water and brine, and evaporated in vacuo. The residue was purified by flash chromatography (50 g of silica gel) using EtOAc-Hexane (1:10 to 1:1) as an eluent, to give 3c (17.5 g, 93%) as a colorless oil.
1H NMR: δ 7.53 (dt, 1H, J = 7.8, 1.0 Hz), 7.44 (dd, 1H, J = 8.3, 1.1 Hz), 7.33 (d, 1H, J = 3.1 Hz), 7.11 (ddd, 1H, J = 8.2, 6.9, 1.2 Hz), 7.00 (ddd, 1H, J = 8.0, 7.0, 1.0 Hz), 6.41 (dd, 1H, J = 3.1, 0.9 Hz), 4.47 (t, 1H, J = 5.1 Hz), 4.15 (t, 2H, J = 7.1 Hz), 3.39 (td, 2H, J = 6.4, 4.9 Hz), 1.77 (dq, 2H, J = 9.6, 7.2 Hz), 1.43–1.32 (m, 2H). 13C NMR: δ 136.11, 129.00, 128.57, 121.34, 120.85, 119.23, 110.20, 100.79, 60.80, 45.84, 30.21, 27.09. HRMS (EI) m/z [M + H]+ Calcd for C12H18NO+ 190.1226; Found 190.1228.
5-(1H-Indol-1-yl)pentan-1-ol (3d).
The same procedure as above was carried out using indole (11.7 g, 0.1 mol) and 5-bromopentan-1-ol (18.3 g, 0.11 mol), to give 3d (18.1 g, 89%) as a colorless oil.
1H NMR: δ 7.54 (dt, 1H, J = 7.9, 1.0 Hz), 7.42 (dd, 1H, J = 8.2, 1.1 Hz), 7.31 (d, 1H, J = 3.2 Hz), 7.12 (ddd, 1H, J = 8.2, 7.0, 1.3 Hz), 7.01 (ddd, 1H, J = 8.0, 6.9, 1.0 Hz), 6.41 (dd, 1H, J = 3.1, 0.9 Hz), 4.43 (s, 1H), 4.11 (t, 2H, J = 7.0 Hz), 3.37 (t, 2H, J = 6.5 Hz), 1.73 (p, 2H, J = 7.2 Hz), 1.49–1.37 (m, 2H), 1.33–1.19 (m, 2H). 13C NMR: δ 136.10, 128.97, 128.57, 121.35, 120.86, 119.23, 110.14, 100.80, 61.06, 45.95, 32.54, 30.23, 23.37. HRMS (EI) m/z [M + H]+ Calcd for C13H18NO+ 204.1383; Found 204.1381.
6-(1H-Indol-1-yl)hexan-1-ol (3e).
The same procedure as above was carried out using indole (11.7 g, 0.1 mol) and 6-bromohexan-1-ol (20.0 g, 0.11 mol), to give 3e (19.5 g, 90%) as a colorless oil.
1H NMR: δ 7.22–7.15 (m, 1H), 7.09–6.98 (m, 3H), 3.52 (t, 1H, J = 6.8 Hz), 2.85 (t, 1H, J = 7.2 Hz), 2.20 (d, 3H, J = 11.3 Hz), 1.77 (p, 1H, J = 6.9 Hz). 13C NMR: δ 170.04, 165.67, 151.95, 136.37, 135.88, 134.80, 132.56, 130.15, 129.63, 125.82, 42.19, 37.21, 30.97, 28.14, 20.96, 20.84. HRMS (EI) m/z [M + H]+ Calcd for C14H20NO+ 218.1539; Found 218.1540.
2-(1H-Indol-1-yl)ethyl acetate (4a).
To the solution of 2-indol-1-yl-ethanol 3a (12 g, 75 mmol) in pyridine (100 mL), Ac2O (8 mL, 10 mmol) and DMAP (100 mg, 0.08 mmol) were added. After stirring at rt for 5 h, the reaction mixture was evaporated in vacuo, diluted with EtOAc (300 mL) and washed with an aqueous solution of citric acid (1.0 g in 100 mL). The organic layer was separated, washed with water and brine and evaporated in vacuo. The residue was purified by flash chromatography (100 g of silica gel) using EtOAc-Hexane (1:10 to 1:3) as an eluent, to give 4a (14.7 g, 97%) as a colorless oil. 1H NMR: δ 7.63 (d, 1H, J = 7.8 Hz), 7.50 (dd, 1H, J = 8.3, 0.6 Hz), 7.35 (d, 1H, J = 3.2 Hz), 7.26–7.16 (m, 1H), 7.11 (td, 1H, J = 7.5, 0.9 Hz), 6.52 (dd, 1H, J = 3.1, 0.7 Hz), 4.37 (qd, 4H, J = 6.2, 1.5 Hz), 1.93 (s, 3H). 13C NMR: δ 170.53, 136.43, 129.16, 128.75, 121.63, 120.96, 119.58, 110.09, 101.50, 63.40, 44.93, 20.86.
3-(1H-Indol-1-yl)propyl acetate (4b).
The same procedure as above was carried out using 3-indol-1-yl-propan-1-ol 3b (7.0 g, 40 mmol), to give 4b (8.1 g, 94%) as a colorless oil. 1H NMR: δ 7.62 (d, 1H, J = 7.8 Hz), 7.46 (d, 1H, J = 8.2 Hz), 7.31 (d, 1H, J = 3.1 Hz), 7.24–7.15 (m, 1H), 7.15–7.06 (m, 1H), 6.55 – 6.47 (m, 1H), 4.22 (t, 2H, J = 6.8 Hz), 3.96 (t, 2H, J = 6.4 Hz), 2.14–1.96 (m, 5H). 13C NMR: δ 170.75, 136.20, 128.81, 128.74, 121.54, 120.97, 119.43, 110.00, 101.23, 61.71, 42.73, 29.29, 20.92.
4-(1H-Indol-1-yl)butyl acetate (4c).
The same procedure as above was carried out using 4-(1H-indol-1-yl)butan-1-ol 3c (10.0 g, 53 mmol), to give 4c (11.7 g, 96%) as a colorless oil. 1H NMR: δ 7.53 (d, 1H, J = 7.8 Hz), 7.47–7.39 (m, 1H), 7.32 (d, 1H, J = 3.1 Hz), 7.15–7.05 (m, 1H), 7.04–6.96 (m, 1H), 6.41 (dd, 1H, J = 3.1, 0.7 Hz), 4.15 (t, 2H, J = 7.0 Hz), 3.95 (t, 2H, J = 6.6 Hz), 1.94 (s, 3H), 1.92–1.69 (m, 2H), 1.69–1.42 (m, 2H). 13C NMR: δ 170.85, 136.06, 128.93, 128.55, 121.40, 120.86, 119.28, 110.12, 100.92, 100.89, 85.49, 63.84, 45.44, 26.84, 25.99, 21.06, 21.05.
5-(1H-Indol-1-yl)pentyl acetate (4d).
The same procedure as above was carried out using 5-(1H-indol-1-yl)pentan-1-ol 3d (10.0 g, 49 mmol), to give 4d (11.4 g, 95%) as a colorless oil. 1H NMR: δ 7.52 (d, J = 7.8 Hz, 2H), 7.47–7.38 (m, 2H), 7.32 (d, J = 3.1 Hz, 2H), 7.15–7.05 (m, 2H), 7.04–6.94 (m, 2H), 6.40 (dd, J = 3.0, 0.6 Hz, 2H), 4.12 (t, J = 7.0 Hz, 4H), 3.92 (t, J = 6.6 Hz, 4H), 2.48 (s, 1H), 1.94 (s, 6H), 1.82–1.64 (m, 4H), 1.62–1.47 (m, 4H), 1.23 (dd, J = 9.2, 6.2 Hz, 4H). 13C NMR: δ 170.84, 136.03, 128.97, 128.51, 121.33, 120.83, 119.22, 110.14, 100.77, 64.07, 45.69, 29.86, 28.10, 23.15, 21.10.
6-(1H-Indol-1-yl)hexyl acetate (4e).
The same procedure as above was carried out using 6-(1H-indol-1-yl)hexan-1-ol 3e (10.0 g, 46 mmol), to give 4e (10.97 g, 93%) as a colorless amorphous solid. 1H NMR: δ 7.51 (d, 2H, J = 7.8 Hz), 7.42 (d, 2H, J = 8.2 Hz), 7.31 (d, 2H, J = 3.0 Hz), 7.09 (d, 2H, J = 7.3 Hz), 6.99 (d, 2H, J = 7.3 Hz), 6.39 (d, 2H, J = 2.7 Hz), 4.12 (t, 4H, J = 7.0 Hz), 3.92 (t, 4H, J = 6.6 Hz), 1.95 (s, 6H), 1.72 (d, 3H, J = 7.2 Hz), 1.69–1.25 (m, 10H), 1.25–0.98 (m, 5H). 13C NMR: δ 170.86, 136.05, 128.95, 128.51, 121.31, 120.82, 119.20, 110.11, 100.75, 64.16, 45.76, 30.15, 28.44, 26.34, 25.45, 21.11.
2-(3-Formyl-1H-indol-1-yl)ethyl acetate (5a).
2-(1H-Indol-1-yl)ethyl acetate 4a (14.7 g, 72 mmol) was dissolved in the solution of POCl3 (0.9 mL, 10 mmol) in DMF (50 mL) and intensively stirred at 5 °C for 5 h. The reaction mixture was quenched with Na2CO3 (10% aqueous solution), diluted with EtOAc (100 mL) and water (200 mL). The organic layer was separated and the water layer was re-extracted with EtOAc (100 mL). The combined extracts were washed with water and brine and evaporated in vacuo. The residue was purified by flash chromatography (100 g of silica gel) using EtOAc-Hexane (1:5 to 1:1) as an eluent to give 5a (11.9 g, 71%) as a colorless oil. 1H NMR: δ 9.94 (s, 1H), 8.27 (s, 1H), 8.16 (d, 1H, J = 7.3 Hz), 7.61 (d, 1H, J = 7.9 Hz), 7.36–7.21 (m, 2H), 4.51 (t, 2H, J = 5.0 Hz), 4.38 (t, 2H, J = 5.1 Hz), 1.88 (s, 3H). 13C NMR: δ 185.18, 170.48, 141.46, 137.60, 125.09, 124.04, 122.98, 121.56, 117.98, 111.35, 62.73, 45.81, 20.84.
3-(3-Formyl-1H-indol-1-yl)propyl acetate (5b).
The same procedure as above was carried out using 3-(1H-indol-1-yl)propylacetate 4b (8.0 g, 36.8 mmol), to give 5b (6.1 g, 68%) as a colorless amorphous solid. 1H NMR δ 9.91 (s, 1H), 8.27 (s, 1H), 8.19–8.11 (m, 1H), 7.58 (d, J = 8.0 Hz, 1H), 7.33–7.21 (m, 2H), 4.32 (t, J = 6.9 Hz, 2H), 3.96 (t, J = 6.2 Hz, 2H), 3.53 (s, 2H), 2.18–2.02 (m, 2H), 1.91 (s, 3H). 13C NMR: δ 184.98, 170.77, 170.76, 141.11, 137.43, 125.13, 124.00, 122.92, 121.56, 117.73, 111.29, 61.61, 43.80, 28.75, 20.91.
4-(3-Formyl-1H-indol-1-yl)butyl acetate (5c).
The same procedure as above was carried out using 4-(1H-indol-1-yl)butyl acetate 4c (11.7 g, 50 mmol), to give 5c (9.7 g, 74%) as a colorless amorphous solid. 1H NMR: δ 9.89 (s, 1H), 8.30 (s, 1H), 8.10 (d, 1H, J = 7.5 Hz), 7.61 (d, 1H, J = 8.1 Hz), 7.33–7.20 (m, 2H), 4.28 (t, 2H, J = 7.1 Hz), 3.98 (t, 2H, J = 6.6 Hz), 1.94 (s, 3H), 1.91–1.75 (m, 2H), 1.75–1.47 (m, 2H). 13C NMR: δ 184.99, 170.84, 141.12, 137.41, 125.11, 123.97, 122.91, 121.51, 117.55, 111.46, 85.48, 63.71, 46.32, 26.39, 25.83, 21.08.
5-(3-Formyl-1H-indol-1-yl)pentyl acetate (5d).
The same procedure as above was carried out using 5-(1H-indol-1-yl)pentyl acetate 4d (11.4 g, 46 mmol), to give 5d (9.78 g, 77%) as a colorless amorphous solid. 1H NMR: δ 9.90 (s, 1H), 8.30 (s, 1H), 8.12 (d, 1H, J = 7.4 Hz), 7.61 (d, 1H, J = 8.1 Hz), 7.34–7.22 (m, 2H), 4.26 (t, 2H, J = 7.1 Hz), 3.95 (t, 2H, J = 6.6 Hz), 1.94 (s, 3H), 1.88–1.73 (m, 2H), 1.64–1.51 (m, 2H), 1.34–1.06 (m, 2H). 13C NMR: δ 184.57, 170.46, 140.76, 137.06, 124.74, 123.57, 122.50, 121.13, 117.13, 111.09, 63.61, 46.18, 28.94, 27.63, 22.60, 20.70.
6-(3-Formyl-1H-indol-1-yl)hexyl acetate (5e).
The same procedure as above was carried out using 6-(1H-indol-1-yl)hexyl acetate 4e (10.9 g, 42 mmol), to give 5e (8.8 g, 73%) as a colorless amorphous solid. 1H NMR: δ 9.87 (s, 1H), 8.30 (s, 1H), 8.09 (d, 1H, J = 7.6 Hz), 7.61 (d, 1H, J = 8.2 Hz), 7.29 (dt, 2H, J = 8.2, 1.3 Hz), 7.23 (dt, 2H, J = 7.0, 1.0 Hz), 4.29 (t, 2H, J = 7.1 Hz), 3.92 (t, 2H, J = 7.0 Hz), 1.94 (s, 3H), 1.82–1.75 (m, 2H), 1.53–1.46 (m, 2H) 1.34–1.22 (m, 4H). 13C NMR: δ 184.89, 170.55, 140.86, 137.15, 124.88, 123.53, 122.59, 121.19, 117.20, 111.01, 64.14, 46.64, 29.59, 28.37, 26.14, 25.38, 21.13.
1-(2-Hydroxyethyl)-1H-indole-3-carbaldehyde (6a).
2-(3-Formyl-1H-indol-1-yl)ethyl acetate 5a (11.9 g, 51 mmol) was dissolved in the solution Na (200 mg, 0.8 mmol) in MeOH (50 mL) and stirred at 10 min. Then, the reaction mixture was evaporated in vacuo, quenched with an aqueous solution of citric acid (0.5 g, 50 mL), and EtOAc (200 mL). The organic layer was separated and the water layer was re-extracted with EtOAc (100 mL). The extracts were combined, washed with water and brine and evaporated in vacuo. The residue was purified by flash chromatography (50 g of silica gel) using EtOAc-Hexane (1:5 to 1:0) as an eluent, to give 6a (8.8 g, 91%) as a colorless amorphous solid. 1H NMR: δ 9.90 (s, 1H), 8.24 (s, 1H), 8.14–8.08 (m, 1H), 7.60 (d, 1H, J = 8.0 Hz), 7.33–7.20 (m, 2H), 5.03 (t, 1H, J = 5.2 Hz), 4.30 (t, 2H, J = 5.3 Hz), 3.76 (q, 2H, J = 5.2 Hz). 13C NMR: δ 185.08, 141.97, 137.70, 125.14, 123.83, 122.84, 121.42, 117.41, 111.63, 60.04, 49.51. HRMS (EI) m/z [M + H]+ Calcd for C11H12NO2+ 190.0863; Found 190.0872.
1-(3-Hydroxypropyl)-1H-indole-3-carbaldehyde (6b).
The same procedure as above was carried out using 3-(3-Formyl-1H-indol-1-yl)propyl acetate (5b) (6.0 g, 24 mmol), to give 6b (4.5 g, 92%) as a colorless amorphous solid. 1H NMR: δ 9.89 (s, 1H), 8.26 (s, 1H), 8.10 (d, 1H, J = 7.5 Hz), 7.59 (d, 1H, J = 8.1 Hz), 7.35–7.21 (m, 2H), 4.73 (t, 1H, J = 5.0), 4.32 (t, 2H, J = 7.0 Hz), 3.39 (dd, 2H, J = 11.2, 5.9 Hz), 1.94 (p, 2H, J = 6.5 Hz). 13C NMR: δ 184.99, 141.29, 137.45, 125.11, 123.96, 122.90, 121.50, 117.48, 111.44, 57.99, 43.82, 32.79. HRMS (EI) m/z [M + H]+ Calcd for C12H14NO2+ 204.1019; Found 204.1030.
1-(4-Hydroxybutyl)-1H-indole-3-carbaldehyde (6c).
The same procedure as above was carried out using 4-(3-formyl-1H-indol-1-yl)butyl acetate 5c (5.0 g, 19 mmol), to give 6c (3.7 g, 90%) as a colorless amorphous solid. 1H NMR: δ 9.89 (s, 1H), 8.29 (s, 1H), 8.10 (d, 1H, J = 7.6 Hz), 7.60 (d, 1H, J = 8.1 Hz), 7.33–7.21 (m, 2H), 4.47 (t, 1H, J = 5.1 Hz), 4.27 (t, 2H, J = 7.1 Hz), 1.90–1.76 (m, 2H), 1.47–1.33 (m, 2H). 13C NMR: δ 184.93, 141.11, 137.46, 125.14, 123.93, 122.86, 121.49, 117.49, 111.49, 60.64, 46.69, 29.94, 26.60. HRMS (EI) m/z [M + H]+ Calcd for C13H16NO2+ 218.1176; Found 218.1186.
1-(5-Hydroxypentyl)-1H-indole-3-carbaldehyde (6d).
The same procedure as above was carried out using 5-(3-formyl-1H-indol-1-yl)pentyl acetate 5d (5.0 g, 18 mmol), to give 6d (3.9 g, 93%) as a colorless amorphous solid. 1H NMR: δ 9.88 (s, 1H), 8.29 (s, 1H), 8.13–8.06 (m, 1H), 7.59 (d, 1H, J = 8.2 Hz), 7.32–7.20 (m, 2H), 4.41 (br, 1H), 4.24 (t, 2H, J = 7.0 Hz), 3.34 (t, 2H, J = 6.4 Hz), 1.78 (p, 2H, J = 7.2 Hz), 1.41 (p, 2H, J = 6.7 Hz), 1.31–1.20 (m, 2H). 13C NMR (100 MHz, dmso) δ 185.00, 141.21, 137.46, 125.12, 123.96, 122.89, 121.51, 117.47, 111.49, 106.48, 60.90, 46.79, 32.36, 29.66, 23.18. HRMS (EI) m/z [M + H]+ Calcd for C14H18NO2+ 232.1332; Found 232.1338.
1-(6-Hydroxyhexyl)-1H-indole-3-carbaldehyde (6e).
The same procedure as above was carried out using 6-(3-formyl-1H-indol-1-yl)hexyl acetate 5e (6.0 g, 20 mmol), to give 6e (4.8 g, 94%) as a colorless amorphous solid. 1H NMR: δ 9.88 (s, 1H), 8.29 (s, 1H), 8.09 (d, 1H, J = 7.7 Hz), 7.59 (d, 1H, J = 8.1 Hz), 7.33–7.19 (m, 2H), 4.38 (s, 1H), 4.24 (t, 2H J = 7.1 Hz), 3.33 (t, 2H, J = 6.3 Hz), 1.77 (p, 2H, J = 7.1 Hz), 1.41–1.19 (m, 6H). 13C NMR: δ 185.11, 140.95, 137.09, 125.33, 123.99, 122.77, 121.82, 117.40, 111.85, 61.02, 45.91, 32.65, 30.34, 26.75, 26.77. HRMS (EI) m/z [M + H]+ Calcd for C15H20NO2+ 246.1489; Found 246.1483.
tris(1-[2-Hydroxyethyl]-1H-indol-3-yl)methane (7a).
To the solution of 2-indol-1-yl-ethanol 3a (3.0 g, 18.7 mmol) in MeOH (100 mL), 1-(2-hydroxyethyl)-1H-indole-3-carbaldehyde 6a (1.7 g, 9.1 mmol), AcOH (1 mL), and Dy(OTf)3 (10 mg, 16.4 μmol) were added. After refluxing for 12 h, the reaction mixture was cooled to RT. The resulting suspension was filtered, the precipitate washed with MeOH (2 × 50 mL) and Et2O (50 mL). The residue was dried in vacuo, to give 7a (3.9 g, 89%) as a colorless amorphous solid.
1H NMR: δ 7.52–7.32 (m, 6H), 7.05 (t, 3H, J = 7.5 Hz), 7.01 (s, 3H), 6.88 (t, 3H, J = 7.3 Hz), 6.03 (s, 1H), 4.80 (t, 3H, J = 5.0 Hz), 4.11 (t, 6H, J = 5.1 Hz), 3.64 (d, 6H, J = 5.3 Hz). 13C NMR: δ 136.48, 127.25, 127.11, 120.59, 119.39, 118.0, 117.33, 109.77, 60.36, 48.12. HRMS (EI) m/z [M − H]+ Calcd for C34H37N3O3+ 493.2365; Found 492.2282.
tris(1-[3-Hydroxypropyl]-1H-indol-3-yl)methane (7b).
The same procedure as above was carried out using 3-indol-1-yl-propan-1-ol 3b (3.0 g, 17.1 mmol) and 1-(3-hydroxypropyl)-1H-indole-3-carbaldehyde 6b (1.7 g, 8.5 mmol), to give 7b (3.8 g, 85%) as a colorless amorphous solid. 1H NMR: δ 7.39 (t, 6H, J = 7.5 Hz), 7.05 (d, 3H, J = 7.9 Hz), 6.98 (s, 3H), 6.88 (d, 3H, J = 7.7 Hz), 6.03 (s, 1H), 4.52 (t, 3H, J = 5.0 Hz), 4.13 (t, 6H, J = 6.8 Hz), 3.42–3.23 (m, 6H), 1.89–1.67 (m, 6H). 13C NMR: δ 136.2, 127.05, 126.69, 120.71, 119.55, 117.99, 117.24, 109.63, 57.79, 42.2, 33.03. HRMS (EI) m/z [M − H] + Calcd for C34H37N3O3+ 535.2835; Found 534.2747.
tris(1-[4-Hydroxybutyl]-1H-indol-3-yl)methane (7c).
The same procedure as above was carried out using 4-indol-1-yl-butan-1-ol 3c (3.0 g, 15.8 mmol) and 1-(4-Hydroxybutyl)-1H-indole-3-carbaldehyde 6c (1.6 g, 7.8 mmol), to give 7c (3.7 g, 82%) as a colorless amorphous solid. 1H NMR: δ 7.40 (d, J = 2.8 Hz, 3H), 7.38 (d, J = 3.3 Hz, 3H), 7.05 (t, J = 7.5 Hz, 3H), 6.96 (s, 3H), 6.86 (t, J = 7.4 Hz, 3H), 6.03 (s, 1H), 4.44 (t, J = 5.0 Hz, 3H), 4.07 (t, J = 6.8 Hz, 6H), 3.35 (q, J = 6.1 Hz, 6H), 1.90–1.48 (m, 6H), 1.48–1.11 (m, 6H). 13C NMR: δ 157.88, 144.03, 138.55, 124.44, 123.13, 120.55, 112.03, 59.90, 46.90, 29.14, 25.85. HRMS (EI) m/z [M − H] + Calcd for C37H43N3O3+ 577.3304; Found 576.3213.
tris(1-[5-Hydroxypentyl]-1H-indol-3-yl)methane (7d).
The same procedure as above was carried out using 5-indol-1-yl-pentan-1-ol 3d (3.0 g, 15.8 mmol) and 1-(5-hydroxypentyl)-1H-indole-3-carbaldehyde 5d (1.6 g, 7.8 mmol), to give 7d (4.2 g, 88%) as a colorless amorphous solid. 1H NMR: δ 7.38 (d, 6H, J = 5.3 Hz), 7.05 (t, 3H, J = 7.3 Hz), 6.95 (s, 3H), 6.86 (t, 3H, J = 7.3 Hz), 6.02 (s, 1H), 4.33 (t, 3H, J = 4.5 Hz), 4.06 (br, 6H), 1.65 (t, 6H, J = 6.8 Hz), 1.39 (t, 6H, J = 6.7 Hz), 1.37–0.95 (m, 6H). 13C NMR: δ 136.2, 127.05, 126.67, 120.68, 119.59, 117.91, 117.14, 109.64, 60.56, 45.24, 32.07, 29.69, 22.76. HRMS (EI) m/z [M] + Calcd for C40H49N3O3+ 619.3774; Found 618.3677.
tris(1-[6-Hydroxyhexyl]-1H-indol-3-yl)methane (7e).
The same procedure as above was carried out using 6-indol-1-yl-hexan-1-ol 3e (3.0 g, 13.8 mmol) and 1-(6-hydroxyhexyl)-1H-indole-3-carbaldehyde 6e (1.7 g, 6.9 mmol), to give 7e (3.7 g, 83%) as a colorless amorphous solid. 1H NMR: δ 7.38 (d, 6H, J = 8.6 Hz), 7.05 (t, 3H, J = 7.6 Hz), 6.93 (s, 3H), 6.85 (t, 3H, J = 7.7 Hz), 6.02 (s, 1H), 4.30 (t, 3H, J = 5.1 Hz), 4.06 (t, 6H, J = 6.7 Hz), 3.40–3.20 (m, 6H), 1.73–1.52 (m, 6H), 1.40–1.05 (m, 18H). 13C NMR (100 MHz, DMSO) δ = 136.18, 127.03, 126.67, 120.67, 119.56, 117.89, 117.09, 109.63, 60.54, 45.15, 32.43, 29.80, 26.07, 25.08. HRMS (EI) m/z [M]+ Calcd for C43H55N3O3+ 661.4243; Found 660.4160.
tris(1-(2-Hydroxyethyl)-1H-indol-3-yl)methylium chloride (8a).
To the solution of tris(1-[2-hydroxyethyl]-1H-indol-3-yl)methane 7a (2.0 g, 4.0 mmol) in THF (50 mL), DDQ (0.9 g, 4.0 mmol) was added. After stirring at rt for 1 h, the reaction mixture was quenched with conc. HCl (0.4 mL, 5 mmol) and evaporated in vacuo. The residue was purified by flash chromatography (100 g of silica gel) using CH2Cl2-MeOH (100:1 to 10:1) as an eluent to give 8a (1.6 g, 78%) as a red amorphous solid. Rt = 3.39 min. 1H NMR: δ 8.35 (s, 3H), 7.84 (d, 3H, J = 8.3 Hz), 7.40 (t, 3H, J = 7.9 Hz), 7.15–7.05 (m, 6H), 4.92 (s, 3H), 4.51 (t, 6H, J = 5.2 Hz), 3.93 (t, 6H). 13C NMR: δ 144.8, 138.69, 126.74, 124.22, 122.98, 120.60, 117.42, 112.00, 59.12, 49.57. HRMS (EI) m/z [M]+ Calcd for C31H30N3O3+ 492.2282; Found 492.2270.
tris(1-(3-Hydroxypropyl)-1H-indol-3-yl)methylium chloride (8b).
The same procedure as above was carried out using tris(1-[3-hydroxypropyl]-1H-indol-3-yl)methane 7b (2.0 g, 3.7 mmol), to give 8b (1.6 g, 73%) as a red amorphous solid. Rt = 3.55 min. 1H NMR: δ 8.38 (s, 3H), 7.83 (d, 3H, J = 8.3 Hz), 7.41 (t, 3H, J = 7.6 Hz), 7.13 (t, 3H, J = 7.6 Hz), 7.02 (s, 3H), 4.52 (t, 6H, J = 6.9 Hz), 4.46 (br, 3H), 3.56 (t, 6H, J = 5.9 Hz), 2.32–1.92 (m, 6H). 13C NMR: δ 157.77, 144.25, 138.49, 126.7, 124.35, 123.06, 120.49, 111.89, 57.47, 44.22, 31.89. HRMS (EI) m/z [M]+ Calcd for C34H36N3O3+ 534.2751; Found 534.2745.
tris(1-(4-Hydroxybutyl)-1H-indol-3-yl)methylium chloride (8c).
The same procedure as above was carried out using tris(1-[4-hydroxybutyl]-1H-indol-3- yl)methane 7c (2.0 g, 3.4 mmol), to give 8c (1.7 g, 81%) as a red amorphous solid. Rt = 8.76 min. 1H NMR: δ 8.42 (s, 3H), 7.83 (d, J = 8.3 Hz, 3H), 7.40 (t, J = 7.6 Hz, 3H), 7.12 (t, J = 7.5 Hz, 3H), 6.99 (d, J = 7.4 Hz, 3H), 4.49 (t, J = 7.1 Hz, 6H), 4.37 (s, 3H), 3.49 (t, J = 6.2 Hz, 6H), 2.15–1.90 (m, 6H), 1.68–1.47 (m, 6H). 13C NMR: δ 157.88, 144.03, 138.55, 126.78, 124.44, 123.13, 120.55, 117.4, 112.03, 59.90, 46.90, 29.14, 25.85. HRMS (EI) m/z [M]+ Calcd for C37H42N3O3+ 576.3221; Found 576.3227.
tris(1-(5-Hydroxypentyl)-1H-indol-3-yl)methylium chloride (8d).
The same procedure as above was carried out using tris(1-[5-hydroxypentyl]-1H-indol-3-yl)methane 7d (2.0 g, 3.2 mmol), to give 8d (1.8 g, 86%) as a red amorphous solid. Rt = 11.94 min. 1H NMR: δ 8.42 (s, 3H), 7.84 (d, 3H, J = 8.3 Hz), 7.41 (t, 3H, J = 7.6 Hz), 7.13 (t, 3H, J = 7.5 Hz), 7.01 (s, 3H), 4.46 (t, 6H, J = 7.0 Hz), 4.17 (s, 3H), 3.44 (br, 6H), 2.20–1.76 (m, 6H), 1.57–1.40 (m, 12H). 13C NMR: δ 138.47, 124.35, 123.06, 120.48, 111.94, 60.12, 46.93, 31.52, 28.73, 22.38. HRMS (EI) m/z [M]+ Calcd for C40H48N3O3+ 618.3690; Found 618.3682.
tris(1-(6-hydroxyhexyl)-1H-indol-3-yl)methylium chloride (8e).
The same procedure as above was carried out using tris(1-[6-hydroxyhexyl]-1H-indol-3- yl)methane 7e (2.0 g, 3.0 mmol), to give 8e (1.7 g, 81%) as a red amorphous solid. Rt = 16.08 min. 1H NMR: δ 8.42 (s, 3H), 7.84 (d, J = 8.3 Hz, 3H), 7.41 (t, J = 7.7 Hz, 3H), 7.12 (t, J = 7.5 Hz, 3H), 7.00 (d, J = 7.1 Hz, 3H), 4.45 (t, J = 7.1 Hz, 6H), 4.09 (br, 3H), 3.41 (t, J = 6.1 Hz, 6H), 2.17–1.75 (m, 6H), 1.50–1.35 (m, 18H). 13C NMR: δ 157.82, 143.94, 138.49, 126.76, 124.36, 123.03, 120.44, 111.93, 60.24, 46.89, 31.87, 28.87, 25.61, 24.68. HRMS (EI) m/z [M]+ Calcd for C43H54N3O3+ 660.4160; Found 660.4150.
4. Conclusions
The first 5 representatives of N-(hydroxyalkyl) derivatives of tris(1H-indol-3-yl)methylium salts were synthesized and tested. Substances 8d and 8e showed high activity on Gram-positive bacteria, including resistant strains, and slightly less on Gram-negative ones. At the same time, the cytotoxicity of 8d was 13 times lower than the antibacterial activity, which indicates the possible prospects for further search among this group of substances. Despite the fact that the exact target of these substances has not yet been established, it is known that the mechanism of their action is associated with a disruption of the membrane. Analysis of the structure-activity relationship showed an empirical dependence of the ratio of antibacterial/cytotoxic activity on the lipophilicity of the molecule. It is found that the best ratio is most likely achieved with LogPow close to 4. The possibility of theoretical calculation of LogPow for predicting the activity of new molecules using the Molinspiration package is shown.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-8247/13/12/469/s1, Supplementary file manuscript-supplementary. pdf: NMR spectra of compounds 3–8.
Author Contributions
Conceptualization, S.N.L.; methodology, S.N.L.; investigation, S.N.L., E.B.I., A.A.P., A.Y.S., V.V.T.; data curation, S.N.L., E.B.I., A.A.P., A.Y.S., V.V.T.; writing—original draft preparation, S.N.L.; writing—review and editing, S.N.L., A.A.P., A.Y.S., A.S.T.; supervision, S.N.L., A.S.T.; project administration, S.N.L., A.S.T.; All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by a grant from the Russian Science Foundation (project no. 16-15-10300P).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Shallcross, L.J.; Davies, S.C. The World Health Assembly resolution on antimicrobial resistance. J. Antimicrob. Chemother. 2014, 69, 2883–2885. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.A.; Murray, M.D. Antibiotic-resistant bugs in the 21st century-a clinical super-challenge. N. Engl. J. Med. 2009, 360, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Tsakou, F.; Jersie-Christensen, R.; Jenssen, H.; Mojsoska, B. The Role of Proteomics in Bacterial Response to Antibiotics. Pharmaceuticals 2020, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, F.M.; Struelens, M.J.; Towner, K.J.; Gould, I.M. Report of the Consensus Conference on Antibiotic Resistance; Prevention and Control (ARPAC). Clin. Microbiol. Infect. 2005, 11, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, D.E.; Brady, S.F.; Bettermann, A.D.; Cianciotto, N.P.; Liles, M.R.; Rondon, M.R.; Clardy, J.; Goodman, R.M.; Handelsman, J. Isolation of Antibiotics Turbomycin A and B from a Metagenomic Library of Soil Microbial DNA. Appl. Environ. Microbiol. 2002, 8, 4301–4306. [Google Scholar] [CrossRef] [PubMed]
- Dothager, R.S.; Putt, K.S.; Allen, B.J.; Leslie, B.J.; Nesterenko, V.; Hergenrother, P.J. Synthesis and Identification of Small Molecules that Potently Induce Apoptosis in Melanoma Cells through G1 Cell Cycle Arrest. J. Am. Chem. Soc. 2005, 127, 8686. [Google Scholar] [CrossRef] [PubMed]
- Palchaudhuri, R.; Nesterenko, V.; Hergenrother, P.J. The Complex Role of the Triphenylmethyl Motif in Anticancer Compounds. J. Am. Chem. Soc. 2008, 130, 10274. [Google Scholar] [CrossRef] [PubMed]
- Palchaudhuri, R.; Hergenrother, P.J. Triphenylmethylamides (TPMAs): Structure–activity relationship of compounds that induce apoptosis in melanoma cells. Bioorg. Med. Chem. Lett. 2008, 18, 5888. [Google Scholar] [CrossRef] [PubMed]
- Lavrenov, S.N.; Bychkova, O.P.; Dezhenkova, L.G.; Mkrtchyan, A.S.; Tatarskiy, V.V.; Tsvigun, E.A.; Trenin, A.S. Synthesis and study of cytotoxic activity of novel 3,3-bis(indol-3-yl)-1,3-dihydroindol-2-ones. Chem. Heterocycl. Compd. 2020, 56, 741–746. [Google Scholar] [CrossRef]
- Al-Qawasmeh, R.A.; Lee, Y.; Cao, M.-Y.; Gu, X.; Vassilakos, A.; Wright, J.A.; Young, A. Triaryl methane derivatives as antiproliferative agents. Bioorg. Med. Chem. Lett. 2004, 14, 347. [Google Scholar] [CrossRef] [PubMed]
- Lavrenov, S.N.; Luzikov, Y.N.; Bykov, E.E.; Reznikova, M.I.; Stepanova, E.V.; Glazunova, V.A.; Volodina, Y.L.; Tatarsky, V.V., Jr.; Shtil, A.A.; Preobrazhenskaya, M.N. Synthesis and cytotoxic potency of novel tris(1-alkylindol-3-yl)methylium salts: Role of N-alkyl substituents. Bioorg. Med. Chem. 2010, 18, 6905. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, E.V.; Shtil, A.A.; Lavrenov, S.N.; Bukhman, V.M.; Inshakov, A.N.; Mirchink, E.P.; Trenin, A.S.; Galatenko, O.A.; Isakova, E.B.; Glazunova, V.A.; et al. Tris(1-alkylindol-3-yl)methylium salts as a novel class of antitumor agents. Russ. Chem. Bull. Int. Ed. 2010, 59, 2259. [Google Scholar] [CrossRef]
- Durandin, N.A.; Tsvetkov, V.B.; Bykov, E.E.; Kaluzhny, D.N.; Lavrenov, S.N.; Tevyashova, A.N.; Preobrazhenskaya, M.N. Quantitative parameters of complexes of tris(1-alkylindol-3-yl)methylium salts with serum albumin: Relevance for the design of drug candidates J. Photochem. Photobiol. B Biol. 2016, 162, 570–576. [Google Scholar] [CrossRef]
- Lavrenov, S.N.; Simonov, A.Y.; Panov, A.A.; Lakatosh, S.A.; Isakova, E.B.; Tsvigun, E.A.; Bychkova, O.P.; Tatarskiy, V.V.; Ivanova, E.S.; Mirchink, E.P.; et al. Synthesis and biological activity of new antimicrobial agents-Hybrid derivatives of maleimides and triindolylmethanes. Antibiot. Chemother. 2018, 63, 4–10. (In Russian) [Google Scholar]
- Panov, A.A.; Simonov, A.Y.; Lavrenov, S.N.; Lakatosh, S.A.; Trenin, A.S. 3,4-Disubstituted maleimides: Synthesis and biological activity (Review). Chem. Heterocycl. Compd. 2018, 54, 103–113. [Google Scholar] [CrossRef]
- Panov, A.; Lavrenov, S.; Simonov, A.; Mirchink, E.; Isakova, E.; Trenin, A. Synthesis and antimicrobial activity of 3,4-bis(arylthio)maleimides. J. Antibiot. 2019, 72, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Efimova, S.S.; Tertychnaya, T.E.; Lavrenov, S.N.; Ostroumova, O.S. The mechanisms of action of triindolylmethane derivatives on lipid membranes. Acta Nat. 2019, 11, 38–45. [Google Scholar] [CrossRef]
- Seddon, A.M.; Curnow, P.; Booth, P.J. Membrane proteins, lipids and detergents: Not just a soap opera. Biochim. Biophys. Acta Rev. Biomembr. 2004, 1666, 105–117. [Google Scholar] [CrossRef]
- NCCLS. Reference Method for Broth Dilution Antibacterial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PS, USA, 2000. [Google Scholar]
- OECD. Test Guideline, Test № 117; OECD iLibrary is the Online Library of the Organisation for Economic Cooperation and Development (OECD). Available online: https://www.oecd-ilibrary.org/ (accessed on 15 December 2020).
- Ciura, K.; Fedorowicz, J.; Andri, F.; Greber, K.E.; Gurgielewicz, A.; Sawicki, W.; Saczewski, J. Lipophilicity Determination of Quaternary (Fluoro)Quinolones by Chromatographic and Theoretical Approaches. Int. J. Mol. Sci. 2019, 20, 5288. [Google Scholar] [CrossRef]
- Online Version v 2018 10. Available online: https://www.molinspiration.com (accessed on 15 December 2020).
- logP–Octanol-Water Partition Coefficient Calculation. Available online: https://www.molinspiration.com/services/logp.pdf (accessed on 15 December 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).