Comprehensive Study of the Risk Factors for Medication-Related Osteonecrosis of the Jaw Based on the Japanese Adverse Drug Event Report Database
Abstract
1. Introduction
2. Results
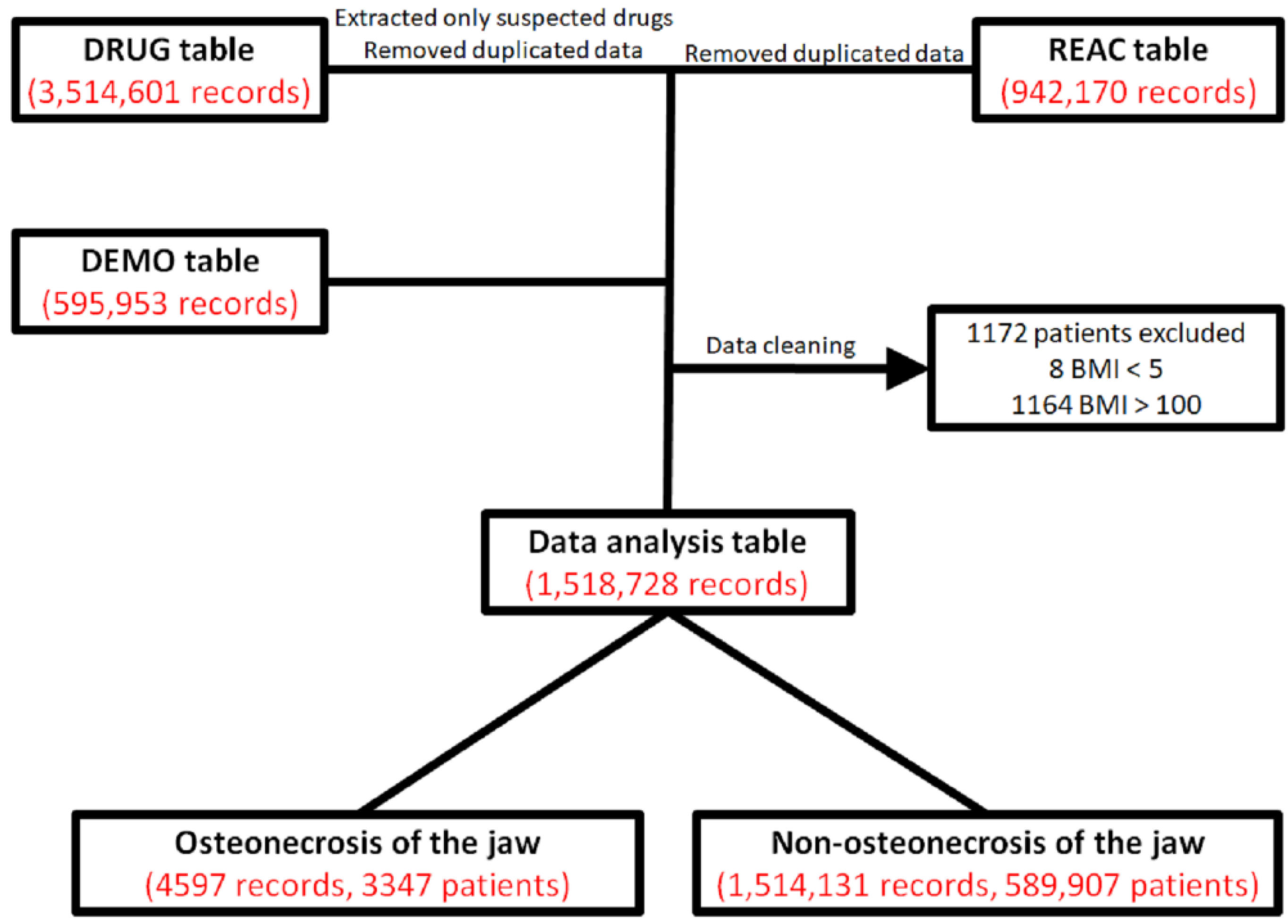
2.1. Presentation of Data
2.2. Patient Background and MRONJ
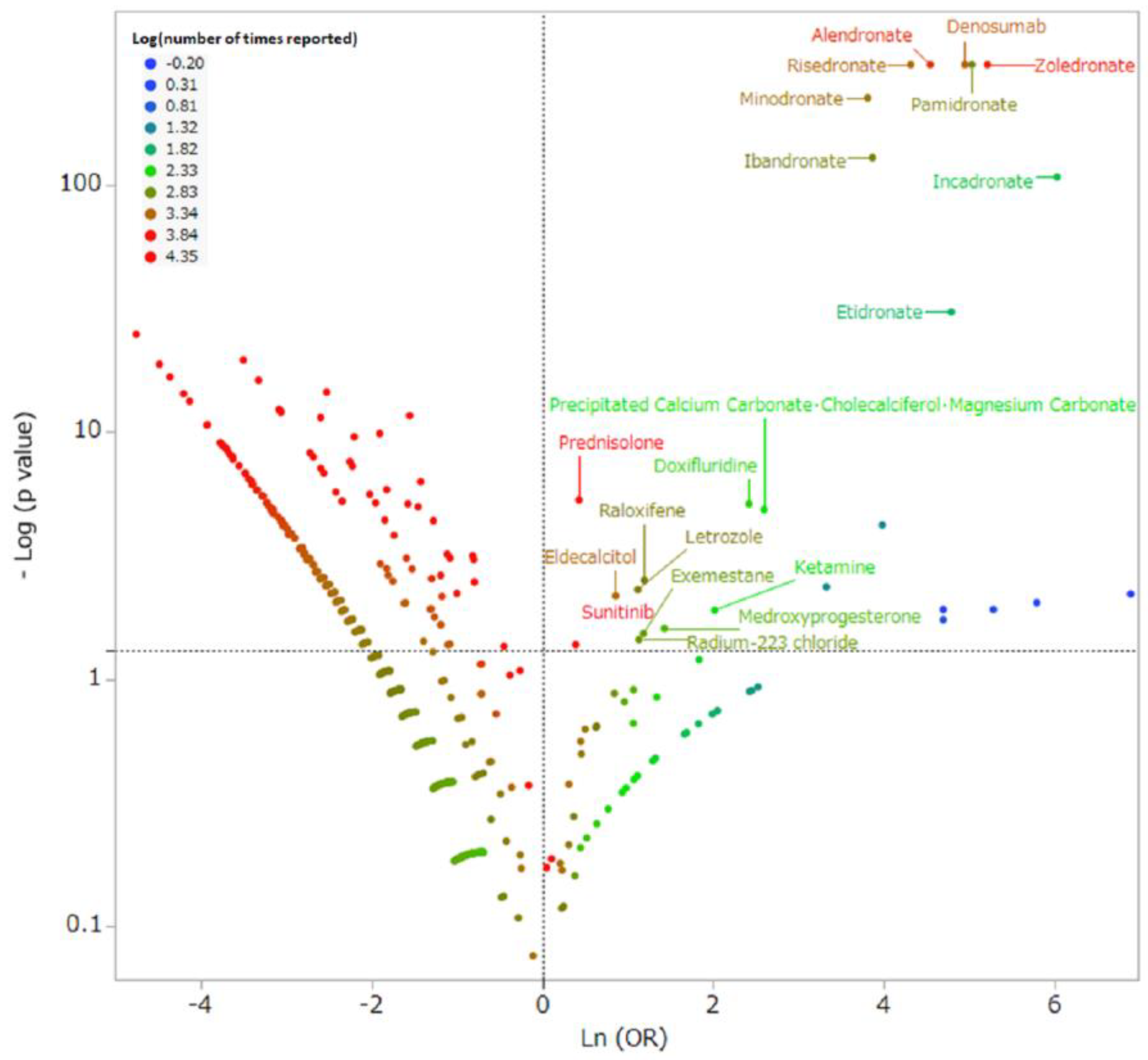
2.3. Medicines and MRONJ
2.4. Analysis of MRONJ Using Multivariate Analysis
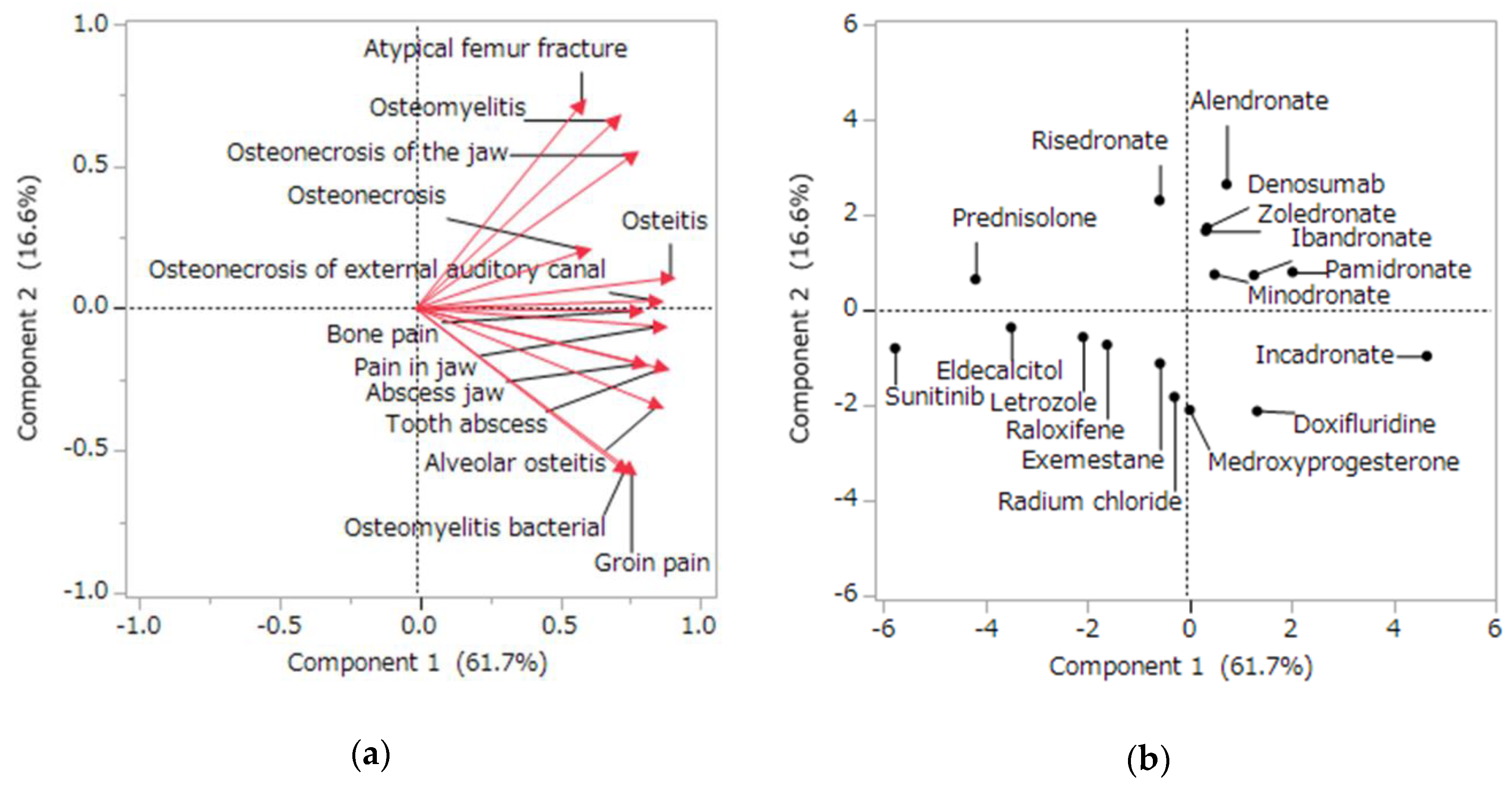
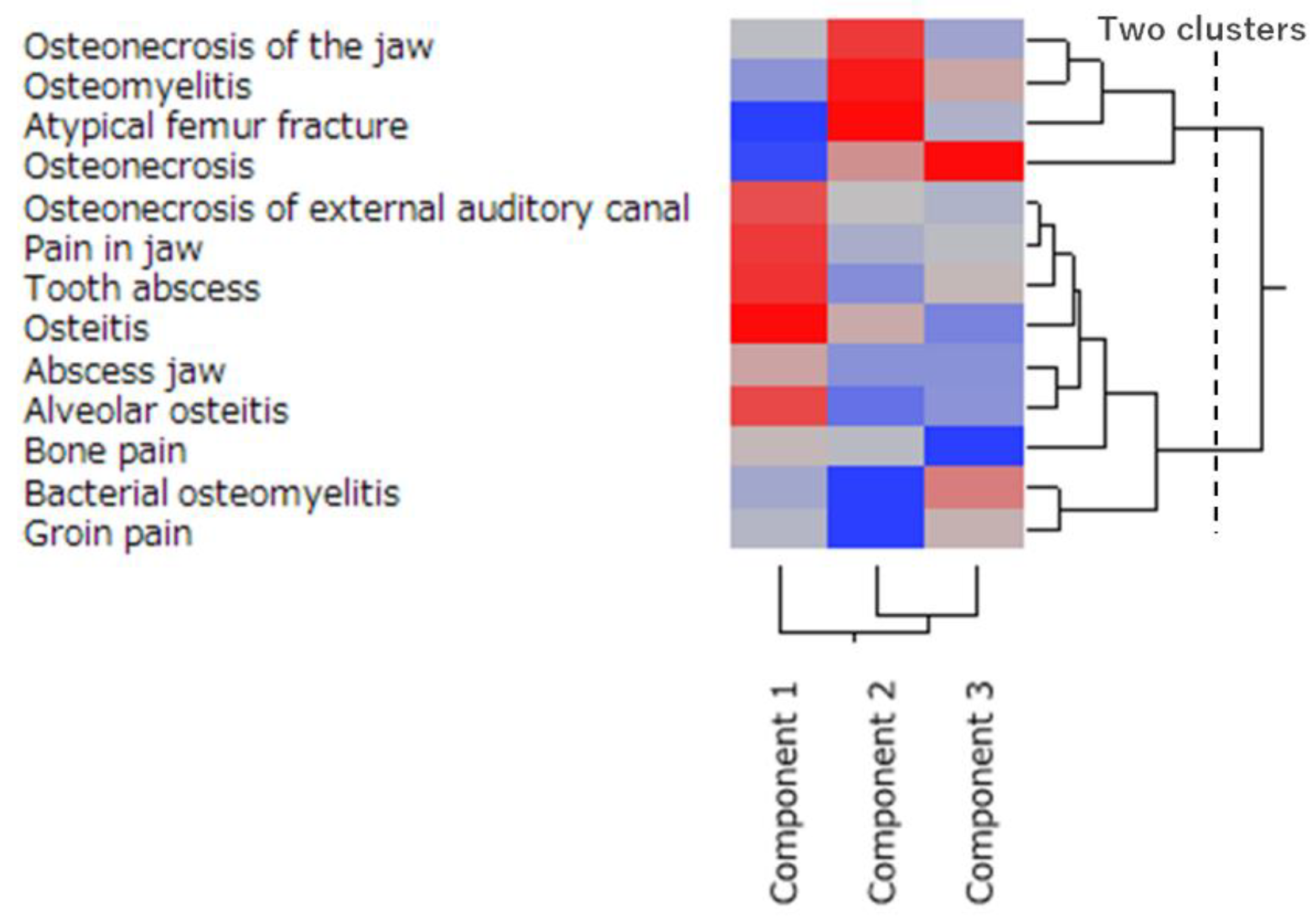
2.5. Characteristics of the Side Effects Related to Osteonecrosis Using Principal Component and Cluster Analyses
3. Discussion
3.1. Risk Factor of MRONJ
3.2. Relationship between MRONJ and Related Diseases
3.3. Limitations
4. Materials and Methods
4.1. JADER and Production of the Data Analysis Table
4.2. Relationship between Patient Information and MRONJ
4.3. Relationship between Medicines and MRONJ
4.4. Multivariate Analysis
4.5. Analysis of the Side Effects Related to Osteonecrosis Using Principal Component and Cluster Analyses
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the jaw-2014 Update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- Di Fede, O.; Panzarella, V.; Mauceri, R.; Fusco, V.; Bedogni, A.; Muzio, L.L.; Board, S.O.; Campisi, G. The Dental Management of Patients at Risk of Medication-Related Osteonecrosis of the Jaw: New Paradigm of Primary Prevention. Biomed. Res. Int. 2018, 2684924. [Google Scholar] [CrossRef] [PubMed]
- Schiodt, M.; Otto, S.; Fedele, S.; Bedogni, A.; Nicolatou-Galitis, O.; Guggenberger, R.; Herlofson, B.B.; Ristow, O.; Kofod, T. Workshop of European task force on medication-related osteonecrosis of the jaw-Current challenges. Oral Dis. 2019, 25, 1815–1821. [Google Scholar] [CrossRef]
- Campisi, G.; Mauceri, R.; Bertoldo, F.; Bettini, G.; Biasotto, M.; Colella, G.; Consolo, U.; Fede, D.O.; Favia, G.; Fusco, V.; et al. Medication-Related Osteonecrosis of Jaws (MRONJ) Prevention and Diagnosis: Italian Consensus Update 2020. Int. J. Environ. Res. Public Health. 2020, 17, 5998. [Google Scholar] [CrossRef]
- Saad, F.; Brown, S.F.; Poznak, C.V.; Ibrahim, T.; Stemmer, S.M.; Stopeck, A.T.; Diel, I.J.; Takahashi, S.; Shore, N.; Henry, D.H.; et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: Integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann. Oncol. 2012, 23, 1341–1347. [Google Scholar] [CrossRef]
- Eguia, A.; Bagán-Debón, L.; Cardona, F. Review and update on drugs related to the development of osteonecrosis of the jaw. Med. Oral Patol. Oral Cir. Bucal. 2020, 25, e71–e83. [Google Scholar] [CrossRef]
- Ng, Y.H.; Gino, P.D.; Lingaraj, K.; De, S.D. Femoral shaft fractures in the elderly--role of prior bisphosphonate therapy. Injury 2011, 42, 702–706. [Google Scholar] [CrossRef]
- Thorsteinsson, A.L.; Vestergaard, P.; Eiken, P. External auditory canal and middle ear cholesteatoma and osteonecrosis in bisphosphonate-treated osteoporosis patients: A Danish national register-based cohort study and literature review. Osteoporos. Int. 2014, 25, 1937–1944. [Google Scholar] [CrossRef]
- Miksad, R.A.; Lai, K.C.; Dodson, T.B.; Woo, S.B.; Treister, N.S.; Akinyemi, O.; Treister, N.S.; Akinyemi, O.; Bihrle, M.; Maytal, G.; et al. Quality of life implications of bisphosphonate-associated osteonecrosis of the jaw. Oncologist 2011, 16, 121–132. [Google Scholar] [CrossRef]
- Capocci, M.; Romeo, U.; Guerra, F.; Mannocci, A.; Tenore, G.; Annibali, S.; Ottolenghi, L. Medication-related osteonecrosis of the jaws (MRONJ) and quality of life evaluation: A pilot study. Clin. Ter. 2017, 168, e253–e257. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S. International Task Force on Osteonecrosis of the Jaw. Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, T.; Hagino, H.; Sugimoto, T.; Ohta, H.; Takahashi, S.; Soen, S.; Taguchi, A.; Nagata, T.; Urade, M.; Shibahara, T.; et al. Antiresorptive agent-related osteonecrosis of the jaw: Position Paper 2017 of the Japanese Allied Committee on Osteonecrosis of the Jaw. J. Bone Miner. Metab. 2017, 35, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, T.; Yamada, M. Evaluation of AEs risk using the “Japanese Adverse Drug Event Report database” of PMDA. In Proceedings of the SAS User General Assembly, Tokyo, Japan, 1–3 December 2012; pp. 263–270. [Google Scholar]
- Vahtsevanos, K.; Kyrgidis, A.; Verrou, E.; Katodritou, E.; Triaridis, S.; Andreadis, C.G.; Boukovinas, I.; Koloutsos, G.E.; Teleioudis, Z.; Kitikidou, K.; et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J. Clin. Oncol. 2009, 27, 5356–5362. [Google Scholar] [CrossRef]
- Ruza, I.; Mirfakhraee, S.; Orwoll, E.; Gruntmanis, U. Clinical Experience with Intravenous Zoledronic Acid in the Treatment of Male Osteoporosis: Evidence and Opinions. Ther. Adv. Musculoskelet. Dis. 2013, 5, 182–198. [Google Scholar] [CrossRef]
- Shibahara, T.; Morikawa, T.; Yago, K.; Kishimoto, H.; Imai, Y.; Kurita, K. National Survey on Bisphosphonate-Related Osteonecrosis of the Jaws in Japan. J. Oral Maxillofac. Surg. 2018, 76, 2105–2112. [Google Scholar] [CrossRef]
- Graharm, R.; Russell, G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef]
- Urade, M.; Tanaka, N.; Furusawa, K.; Shimada, J.; Shibata, T.; Kirita, T.; Yamamoto, T.; Ikebe, T.; Kitagawa, Y.; Fukuta, J. Nationwide survey for bisphosphonate-related osteonecrosis of the jaws in Japan. J. Oral Maxillofac. Surg. 2011, 69, e364–e371. [Google Scholar] [CrossRef]
- Cranney, A.; Guyatt, G.; Griffith, L.; Wells, G.; Tugwell, P.; Rosen, C. Meta-analyses of therapies for postmenopausal osteoporosis. IX: Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr. Rev. 2002, 23, 570–578. [Google Scholar] [CrossRef]
- Crépin, S.; Laroche, M.L.; Sarry, B.; Merle, L. Osteonecrosis of the jaw induced by clodronate, an alkylbiphosphonate: Case report and literature review. Eur. J. Clin. Pharmacol. 2010, 66, 547–554. [Google Scholar] [CrossRef]
- Cummings, S.R.; Martin, J.S.; Mc Clung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009, 20, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 5, 813–822. [Google Scholar] [CrossRef]
- Baron, R.; Ferrari, S.; Russell, R.G. Denosumab and bisphosphonates: Different mechanisms of action and effects. Bone 2011, 48, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Brunello, A.; Saia, G.; Bedogni, A.; Scaglione, D.; Basso, U. Worsening of osteonecrosis of the jaw during treatment with sunitinib in a patient with metastatic renal cell carcinoma. Bone 2009, 44, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Pimolbutr, K.; Porter, S.; Fedele, S. Osteonecrosis of the Jaw Associated with Antiangiogenics in Antiresorptive-Naïve Patient: A Comprehensive Review of the Literature. Biomed Res. Int. 2018, 8071579. [Google Scholar] [CrossRef]
- Powell, C.; Chang, C.; Gershwin, M.E. Current concepts on the pathogenesis and natural history of steroid-induced osteonecrosis. Clin. Rev. Allergy Immunol. 2011, 41, 102–113. [Google Scholar] [CrossRef]
- Di Fede, O.; Bedogni, A.; Giancola, F.; Saia, G.; Bettini, G.; Toia, F.; D’Alessandro, N.; Firenze, A.; Matranga, D.; Fedele, S.; et al. BRONJ in patients with rheumatoid arthritis: A multicenter case series. Oral Dis. 2016, 22, 543–548. [Google Scholar] [CrossRef]
- Tsao, C.; Darby, I.; Ebeling, P.R.; Walsh, K.; O’Brien-Simpson, N.; Reynolds, E.; Borromeo, G. Oral health risk factors for bisphosphonate-associated jaw osteonecrosis. J. Oral Maxillofac. Surg. 2013, 71, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.K.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef]
- Suominen, M.I.; Fagerlund, K.M.; Rissanen, J.P.; Konkol, Y.M.; Morko, J.P.; Peng, Z.; Alhoniemi, E.J.; Laine, S.K.; Corey, E.; Mumberg, D.; et al. Radium-223 Inhibits Osseous Prostate Cancer Growth by Dual Targeting of Cancer Cells and Bone Microenvironment in Mouse Models. Clin. Cancer Res. 2017, 23, 4335–4346. [Google Scholar] [CrossRef]
- Costa, R.P.; Tripoli, V.; Princiotta, A.; Murabito, A.; Licari, M.; Mauceri, R.; Campisi, G.; Pinto, A. Can radium 223 be a conservative non-surgical management of medication-related osteonecrosis of the jaw? World J. Nucl. Med. 2019, 18, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 978-0-387-95442-4. [Google Scholar]
- Odvina, C.V.; Zerwekh, J.E.; Rao, D.S.; Maalouf, N.; Gottschalk, F.A.; Pak, C.Y. Severely suppressed bone turnover: A potential complication of alendronate therapy. J. Clin. Endocrinol. Metab. 2005, 90, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, H.; Noguchi, K.; Takaoka, K. Novel insight into the management of bisphosphonate-related osteonecrosis of the jaw (BRONJ). Jpn. Dent. Sci. Rev. 2019, 55, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Everitt, B.S.; Landau, S.; Leese, M.; Stahl, D. Cluster Analysis, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-0-470-74991-3. [Google Scholar]
- Shane, E.; Burr, D.; Ebeling, P.R.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; et al. Atypical subtrochanteric and diaphyseal femoral fractures: Report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2010, 25, 2267–2294. [Google Scholar] [CrossRef]
- Won, Y.; Lim, J.R.; Kim, Y.H.; Song, H.K.; Yang, K.H. Atypical femoral fracture combined with osteonecrosis of jaw during osteoporosis treatment with bisphosphonate. J. Bone Metab. 2014, 21, 155–159. [Google Scholar] [CrossRef][Green Version]
- Sánchez, A.; Blanco, R. Osteonecrosis of the jaw (ONJ) and atypical femoral fracture (AFF) in an osteoporotic patient chronically treated with bisphosphonates. Osteoporos. Int. 2017, 28, 1145–1147. [Google Scholar] [CrossRef]
- Pariente, A.; Avillach, P.; Salvo, F.; Thiessard, F.; Miremont-Salamé, G.; Fourrier-Reglat, A.; Haramburu, F.; Bégaud, B.; Moore, N. Effect of Competition Bias in safety singnal generation. Drug Saf. 2012, 35, 855–864. [Google Scholar] [CrossRef]
- Avillach, P.; Salvo, F.; Thiessard, F.; Miremont-Salamé, G.; Fourrier-Reglat, A.; Haramburu, F.; Bégaud, B.; Moore, N.; Pariente, A. Pilot evaluation of an automated method to decrease false-positive signals induced by co-prescriptions in spontaneous reporting databases. Pharmacoepidemiol. Drug Saf. 2014, 23, 186–194. [Google Scholar] [CrossRef]
- Pharmaceutical and Medical Devices Agency. Available online: https://www.pmda.go.jp/safety/info-services/drugs/adr-info/suspected-adr/0005.html (accessed on 11 September 2019).
- MedDRA Japanese Maintenance Organization. Available online: https://www.pmrj.jp/jmo/php/indexj.php (accessed on 11 September 2019).
- Nagai, J.; Uesawa, Y.; Kagaya, H. Analyses of oxycodoneinduced AEs based on the Japanese Adverse Drug Event Report Database. Palliat. Care Res. 2015, 10, 161–168. [Google Scholar] [CrossRef][Green Version]
- Watanabe, H.; Matsushita, Y.; Watanabe, A.; Maeda, T.; Nukui, K.; Ogawa, Y.; Sawa, J.; Maeda, H. Early detection of important safety information. Jpn. J. Biomet. 2004, 25, 37–60. [Google Scholar] [CrossRef]
- Ohyama, K.; Sugiura, M. Evaluation of the association between topical prostaglandin F2α analogs and asthma using the JADER database: Comparison with β-blockers. Yakugaku Zasshi. 2018, 138, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Van Puijenbroek, E.P.; Bate, A.; Leufkens, H.G.M.; Lindquist, M.; Orre, R.; Egberts, A.C.G. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug Saf. 2002, 11, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Wang, S.J.; Tsai, C.A.; Lin, C.J. Selection of differentially expressed genes in microarray data analysis. Pharm. J. 2007, 7, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, H.; Uchida, M.; Suzuki, S.; Suga, Y.; Uesawa, Y.; Nakagawa, T.; Takase, H. Analyses of Respiratory Depression Associated with Opioids in Cancer Patients Based on the Japanese Adverse Drug Event Report Database. Biol. Pharm. Bull. 2019, 42, 1185–1191. [Google Scholar] [CrossRef]
- Hosoya, R.; Uesawa, Y.; Ishii-Nozawa, R.; Kagaya, H. Analysis of factors associated with hiccups based on the Japanese Adverse Drug Event Report database. PLoS ONE 2017, 12, e0172057. [Google Scholar] [CrossRef]
- MedDRA Maintenance and Support Services Organization (MSSO). Standardised MedDRA Queries (SMQs). Available online: https://www.meddra.org/standardised-meddra-queries (accessed on 11 September 2019).
- Nagai, J.; Uesawa, Y.; Shimamura, R.; Kagaya, H. Characterization of the Adverse Effects Induced by Acetaminophen and Nonsteroidal Anti-Inflammatory Drugs Based on the Analysis of the Japanese Adverse Drug Event Report Database. Clin. J. Pain. 2017, 33, 667–675. [Google Scholar] [CrossRef]
- Umetsu, R.; Abe, J.; Ueda, N.; Kato, Y.; Nakayama, Y.; Kinosada, Y.; Nakamura, M. Adverse Event Trends Associated with Over-the-counter Drugs: Data Mining of the Japanese Adverse Drug Event Report Database. Yakugaku Zasshi. 2015, 135, 991–1000. [Google Scholar] [CrossRef][Green Version]

| Patients | MRONJ (4597) | Non-MRONJ (1,517,553) | p-Value |
|---|---|---|---|
| Gender # (male/female) | 1270/3159 (4429) | 754,933/711,784 (1,466,717) | <0.0001 ## |
| Age † | 71.4 ± 11.5 (4230) | 59.1 ± 21.7 (1,416,925) | <0.0001 ** |
| Height (cm) † | 154.1 ± 10.0 (1477) | 156.8 ± 18.9 (650,399) | <0.0001 ** |
| Weight (kg) † | 51.7 ± 12.1 (1567) | 54.3 ± 16.5 (761,613) | <0.0001 ** |
| BMI † | 21.8 ± 4.2 (1451) | 21.9 ± 4.5 (628,976) | 0.4802 |
| Medicine | Drug Class | Reporting Times | Reporting Ratio (%) | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|---|---|---|
| Zoledronate | BP | 1352 | 29.41 | 184.40 | 171.63–198.12 | <0.0001 ** |
| Alendronate | BP | 780 | 16.97 | 94.06 | 86.46–102.33 | <0.0001 ** |
| Denosumab | RANKL inhibitor | 674 | 14.66 | 141.22 | 128.60–155.08 | <0.0001 ** |
| Risedronate | BP | 353 | 7.68 | 74.90 | 66.52–84.33 | <0.0001 ** |
| Pamidronate | BP | 252 | 5.48 | 153.50 | 131.98–178.53 | <0.0001 ** |
| Minodronate | BP | 188 | 4.09 | 45.27 | 38.78–52.84 | <0.0001 ** |
| Prednisolone | Corticosteroid | 137 | 2.98 | 1.53 | 1.29–1.81 | <0.0001 ** |
| Ibandronate | BP | 105 | 2.28 | 47.83 | 38.92–58.77 | <0.0001 ** |
| Incadronate | BP | 54 | 1.17 | 417.19 | 279.77–622.09 | <0.0001 ** |
| Sunitinib | Tyrosine kinase inhibitor | 32 | 0.70 | 1.48 | 1.04–2.09 | 0.0416 * |
| Etidronate | BP | 19 | 0.41 | 120.44 | 71.66–202.44 | <0.0001 ** |
| Eldecalcitol | Vitamin D | 14 | 0.30 | 2.36 | 1.40–3.95 | 0.0067 * |
| Raloxifene | SERM | 9 | 0.20 | 3.29 | 1.74–6.24 | 0.0031 * |
| Letrozole | Aromatase inhibitor | 9 | 0.20 | 3.05 | 1.61–5.79 | 0.0049 * |
| Doxifluridine | Pyrimidine fluoride drug | 7 | 0.15 | 11.26 | 5.44–23.33 | <0.0001** |
| Precipitated Calcium Carbonate・ Cholecalciferol・ Magnesium Carbonate | Calcium・ vitamin D・ magnesium | 6 | 0.13 | 13.43 | 6.13–29.43 | <0.0001 ** |
| Exemestane | Aromatase inhibitor | 5 | 0.11 | 3.26 | 1.41–7.56 | 0.0294 * |
| Radium chloride | Radiopharmaceutical | 5 | 0.11 | 3.09 | 1.33–7.15 | 0.0357 * |
| Medroxyprogesterone | Progestogen | 4 | 0.09 | 4.17 | 1.64–10.57 | 0.0248 * |
| Ketamine | Anesthetic | 3 | 0.07 | 7.51 | 2.60–21.67 | 0.0124 * |
| Risk Factor | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Etidronate | 1609.16 | 680.33–3806.08 | <0.0001 ** |
| Incadronate | 1258.09 | 613.01–2582.01 | <0.0001 ** |
| Pamidronate | 963.56 | 713.45–1301.35 | <0.0001 ** |
| Denosumab | 775.18 | 640.35–938.41 | <0.0001 ** |
| Zoledronate | 733.31 | 610.87–880.28 | <0.0001 ** |
| Alendronate | 404.38 | 328.08–498.42 | <0.0001 ** |
| Risedronate | 288.97 | 218.07–382.91 | <0.0001 ** |
| Minodronate | 264.79 | 191.03–367.03 | <0.0001 ** |
| Ibandronate | 216.06 | 136.45–342.12 | <0.0001 ** |
| Medroxyprogesterone | 42.10 | 10.33–171.72 | <0.0001 ** |
| Exemestane | 37.30 | 11.80–117.91 | <0.0001 ** |
| Letrozole | 34.65 | 12.76–94.08 | <0.0001 ** |
| Doxifluridine | 33.07 | 4.58–238.87 | 0.0005 * |
| Raloxifene | 19.91 | 7.35–53.97 | <0.0001 ** |
| Prednisolone | 19.75 | 14.98–26.05 | <0.0001 ** |
| Eldecalcitol | 16.11 | 7.10–36.58 | <0.0001 ** |
| Radium chloride | 13.13 | 1.83–94.25 | 0.0104 * |
| Sunitinib | 9.76 | 5.45–17.50 | <0.0001 ** |
| Female | 1.28 | 1.10–1.48 | 0.0012 * |
| Unit Odds Ratio | |||
| Risk Factor | Odds Ratio | 95% Confidence Interval | p-Value |
| Age | 1.02 | 1.01–1.02 | <0.0001 ** |
| Height | 0.99 | 0.985–0.998 | 0.0111 * |
| Weight | 1.00 | 0.997–1.008 | 0.4120 |
| Range Odds Ratio | |||
| Risk Factor | Odds Ratio | 95% Confidence Interval | p-Value |
| Age | 6.24 | 4.08–9.55 | <0.0001 ** |
| Height | 0.23 | 0.07–0.71 | 0.0111 * |
| Weight | 1.61 | 0.52–5.03 | 0.4120 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toriumi, S.; Kobayashi, A.; Uesawa, Y. Comprehensive Study of the Risk Factors for Medication-Related Osteonecrosis of the Jaw Based on the Japanese Adverse Drug Event Report Database. Pharmaceuticals 2020, 13, 467. https://doi.org/10.3390/ph13120467
Toriumi S, Kobayashi A, Uesawa Y. Comprehensive Study of the Risk Factors for Medication-Related Osteonecrosis of the Jaw Based on the Japanese Adverse Drug Event Report Database. Pharmaceuticals. 2020; 13(12):467. https://doi.org/10.3390/ph13120467
Chicago/Turabian StyleToriumi, Shinya, Akinobu Kobayashi, and Yoshihiro Uesawa. 2020. "Comprehensive Study of the Risk Factors for Medication-Related Osteonecrosis of the Jaw Based on the Japanese Adverse Drug Event Report Database" Pharmaceuticals 13, no. 12: 467. https://doi.org/10.3390/ph13120467
APA StyleToriumi, S., Kobayashi, A., & Uesawa, Y. (2020). Comprehensive Study of the Risk Factors for Medication-Related Osteonecrosis of the Jaw Based on the Japanese Adverse Drug Event Report Database. Pharmaceuticals, 13(12), 467. https://doi.org/10.3390/ph13120467






