Metformin Protects against Podocyte Injury in Diabetic Kidney Disease
Abstract
1. Introduction
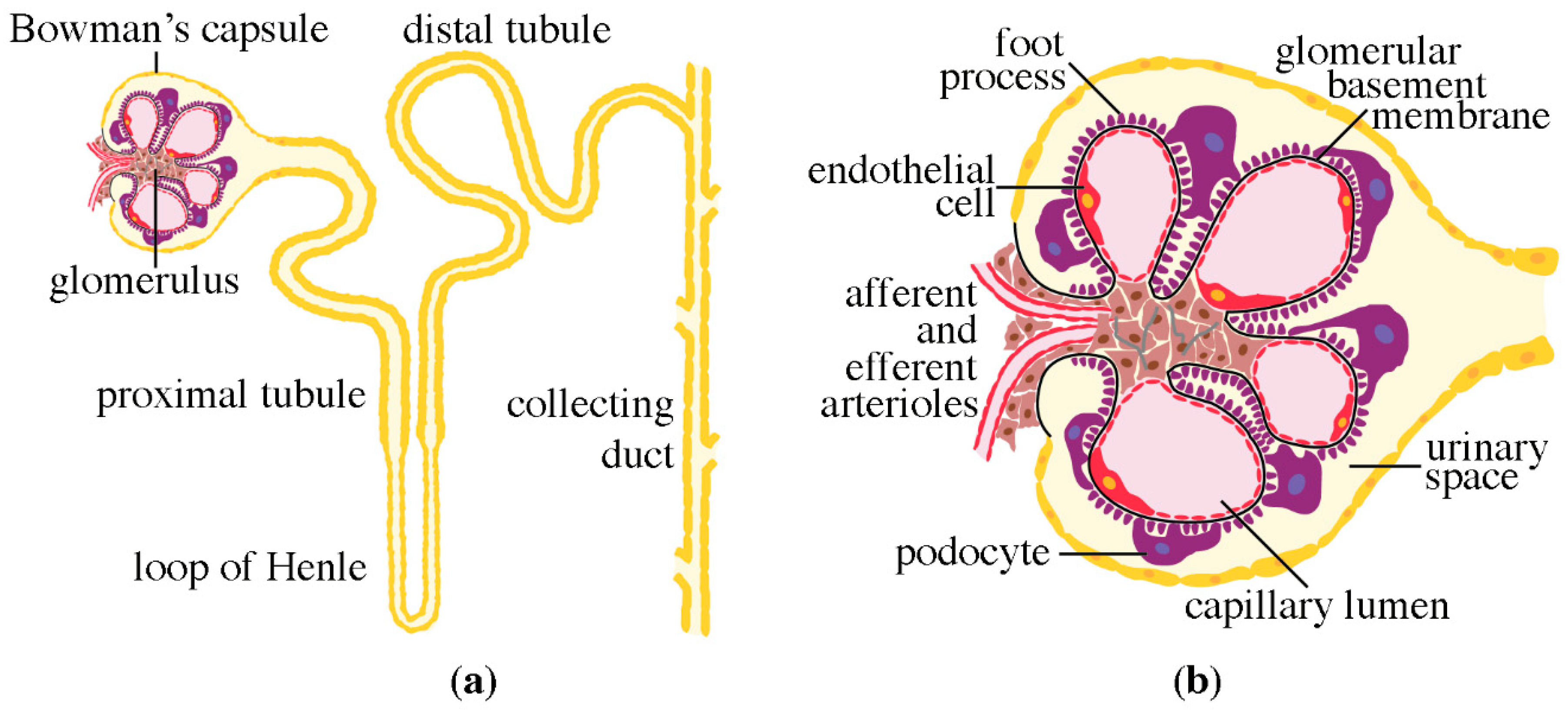
2. Structure of the Nephron
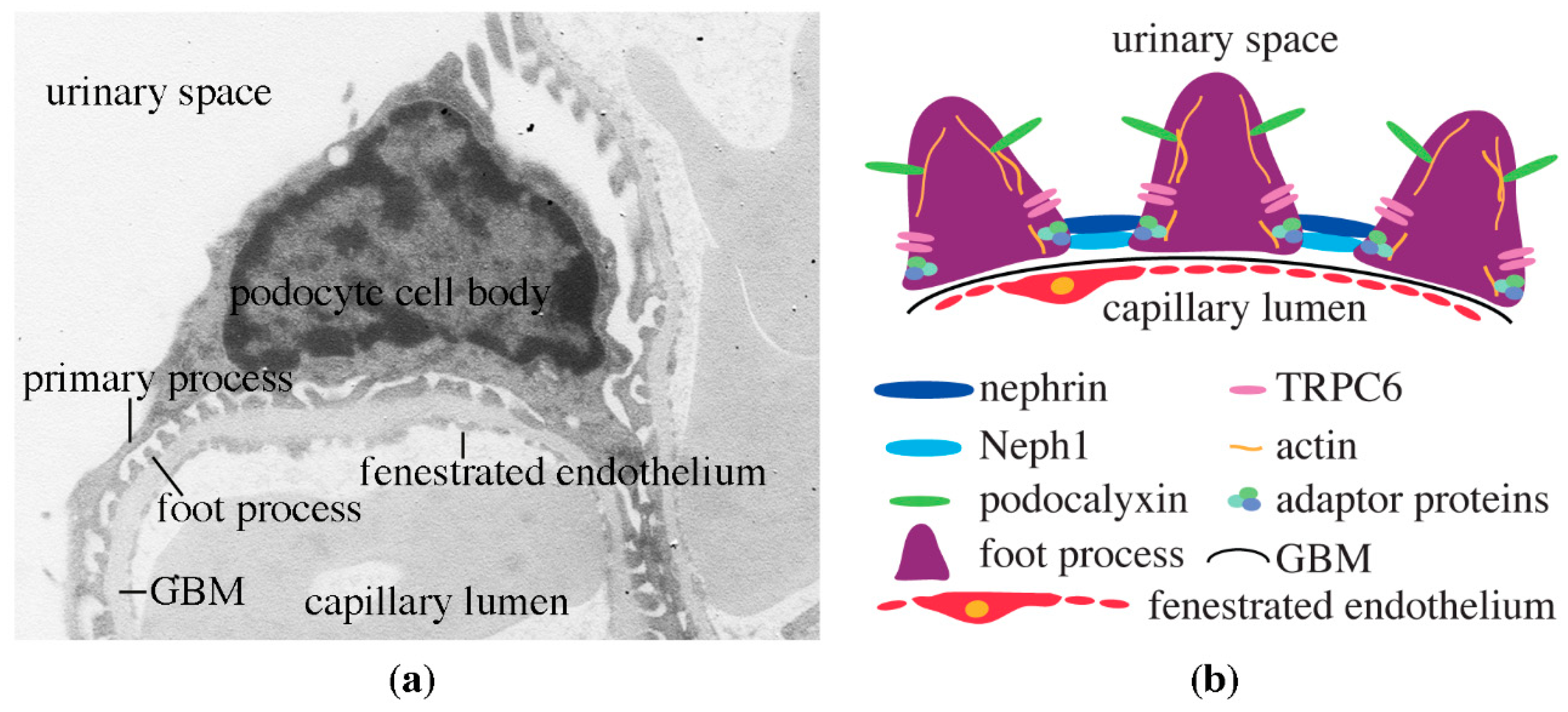
3. Podocytes, the Central Components of the Glomerular Filtration Barrier
4. Diabetic Kidney Disease
4.1. Clinical Definition of DKD
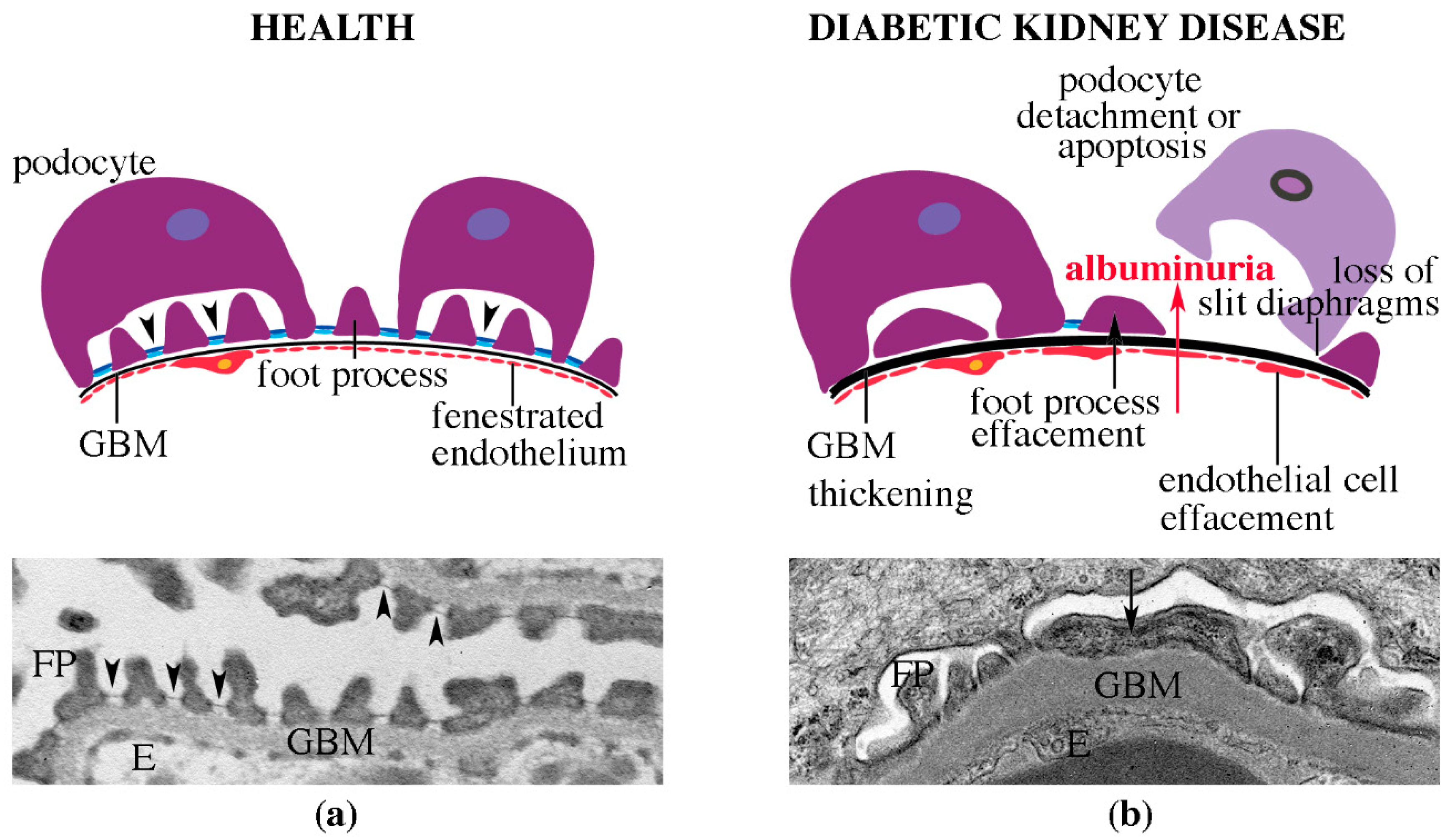
4.2. Pathomorphological Characteristics of DKD
4.3. Risk Factors of DKD
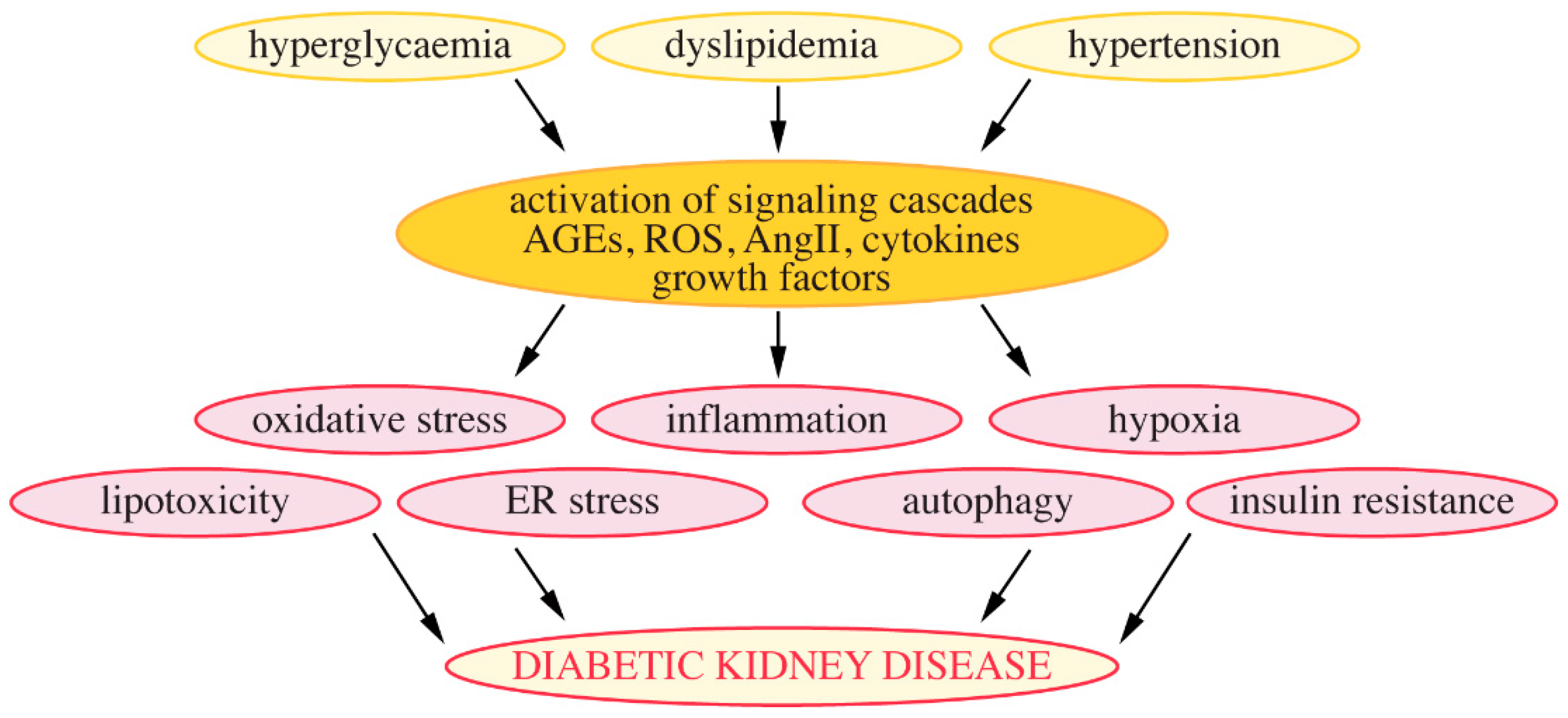
4.4. Mechanisms Leading to the Development of DKD
4.4.1. Hyperglycaemia
4.4.2. Lipotoxicity and Lipid Metabolism-Associated Regulation of Podocytes
4.4.3. Chronic Low-Grade Inflammation
4.4.4. Insulin Resistance
4.4.5. Autophagy
5. Mechanisms Whereby Metformin Reduces Hyperglycaemia
6. Podocyte-Protective Mechanisms of Action of Metformin
6.1. Metformin Restores the Expression of Central Podocyte Proteins
6.2. Metformin Regulates the Dynamics of the Actin Cytoskeleton
6.3. Metformin Reduces Oxidative Stress
6.4. Metformin Ameliorates Dyslipidemia
6.5. Metformin Reduces Inflammation
6.6. Metformin Reduces Insulin Resistance
6.7. Metformin Activates Autophagy
6.8. Effects of Metformin Outside of Podocytes
7. Metformin Monotherapy and Metformin-Based Combination Therapy in T2D and DKD
7.1. Comparison of Metformin and Other Antihyperglycaemic Medications in T2D and DKD
7.2. Use of Metformin When Kidney Function Is Impaired
8. Future Perspectives
8.1. Systemic Versus Podocyte-Specific Effects of Metformin on Dyslipidemia and Inflammation
8.2. Mechanisms Whereby Metformin Reduces Insulin Resistance and Protects the Kidney and Podocytes
8.3. Metformin and Senescence of Podocytes
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Flory, J.; Lipska, K. Metformin in 2019. JAMA 2019, 321, 1926–1927. [Google Scholar] [CrossRef]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Hirst, J.A.; Farmer, A.J.; Ali, R.; Roberts, N.W.; Stevens, R.J. Quantifying the effect of metformin treatment and dose on glycemic control. Diabetes Care 2012, 35, 446–454. [Google Scholar] [CrossRef]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar] [CrossRef]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orhard, T.J.; Bray, G.A.; Schade, D.S.; Temprosa, M.G.; White, N.H.; et al. Long-term metformin use and vitamin B12 deficiency in the diabetes prevention program outcomes study. J. Clin. Endocrinol. Metab. 2016, 101, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.; Fleming, G.A.; Chen, K.; Bicsak, T.A. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metab. Clin. Exp. 2016, 65, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Garrett, M.R. Nephron number, hypertension, and CKD: Physiological and genetic insight from humans and animal models. Physiol. Genom. 2017, 49, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.-S. Genetics of hereditary nephrotic syndrome: A clinical review. Korean J. Pediatr. 2017, 60, 55–63. [Google Scholar] [CrossRef]
- Martin, C.E.; Jones, N. Nephrin signaling in the podocyte: An updated view of signal regulation at the slit diaphragm and beyond. Front. Endocrinol. 2018, 9, 302. [Google Scholar] [CrossRef]
- Grahammer, F.; Wigge, C.; Schell, C.; Kretz, O.; Patrakka, J.; Scheider, S.; Klose, M.; Kind, J.; Arnold, S.J.; Habermann, A.; et al. A flexible, multilayered protein scaffold maintains the slit in between glomerular podocytes. JCI Insight 2016, 1, e86177. [Google Scholar] [CrossRef] [PubMed]
- Kestilä, M.; Lenkkeri, U.; Männikko, M.; Lamerdin, J.; McCready, P.; Putaala, H.; Ruotsalainen, V.; Morita, T.; Nissinen, M.; Herva, R.; et al. Positionally cloned gene for a novel glomerular protein-nephrin-is mutated in congenital nephrotic syndrome. Mol. Cell 1998, 1, 575–582. [Google Scholar] [CrossRef]
- Heikkilä, E.; Ristola, M.; Havana, M.; Jones, N.; Holthöfer, H.; Lehtonen, S. Trans-interaction of nephrin and Neph1/Neph3 induces cell adhesion that associates with decreased tyrosine phosphorylation of nephrin. Biochem. J. 2011, 435, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.; Polu, K.R.; Möller, C.C.; Kenlan, P.; Altintas, M.M.; Wei, C.; Faul, C.; Herbert, S.; Villegas, I.; Avila-Casado, C.; et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Gen. 2005, 37, 739–744. [Google Scholar] [CrossRef]
- Ilatovskaya, D.V.; Staruschenko, A. TRPC6 channel as an emerging determinant of the podocyte susceptibility in kidney diseases. Am. J. Physiol. Ren. Physiol. 2015, 309, F393–F397. [Google Scholar] [CrossRef]
- Dumont, V.; Tolvanen, T.A.; Kuusela, S.; Wang, H.; Nyman, T.A.; Lindfors, S.; Tienari, J.; Nisen, H.; Plomann, M.; Kawachi, H.; et al. PACSIN2 accelerates nephrin trafficking and is upregulated in diabetic kidney disease. FASEB J. 2017, 31, 3978–3990. [Google Scholar] [CrossRef]
- Jones, N.; Blasutig, I.M.; Eremina, V.; Ruston, J.M.; Bladt, F.; Li, H.; Huang, H.; Larose, L.; Li, S.S.-C.; Takano, T.; et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 2006, 440, 818–823. [Google Scholar] [CrossRef]
- Lehtonen, S.; Lehtonen, E.; Kudlicka, K.; Holthöfer, H.; Farquhar, M.G. Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin-Darby Canine Kidney cells expressing nephrin. Am. J. Pathol. 2004, 165, 923–936. [Google Scholar] [CrossRef]
- Lehtonen, S.; Ryan, J.J.; Kudlicka, K.; Iino, N.; Zhou, H.; Farquhar, M.G. Cell-junction associated proteins IQGAP1, MAGI-2, CASK, spectrins, and α-actinin are components of the nephrin multiprotein complex. Proc. Natl. Acad. Sci. USA 2005, 102, 9814–9819. [Google Scholar] [CrossRef]
- Verma, R.; Kovari, I.; Soofi, A.; Nihalani, D.; Patrie, K.; Holzman, L.B. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J. Clin. Investig. 2006, 116, 1346–1359. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Wharram, B.; Kovari, I.; Kunkel, R.; Nihalani, D.; Wary, K.K.; Wiggins, R.C.; Killen, P.; Holzman, L.B. Fyn binds to and phosphorylates the kidney slit diaphragm component nephrin. J. Biol. Chem. 2003, 278, 20716–20723. [Google Scholar] [CrossRef] [PubMed]
- Wasik, A.A.; Polianskyte-Prause, Z.; Dong, M.-Q.; Shaw, A.S.; Yates III, J.R.; Farquhar, M.G.; Lehtonen, S. Septin 7 forms a complex with CD2AP and nephrin and regulates glucose transporter trafficking. Mol. Biol. Cell 2012, 23, 3370–3379. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sun, N.; Aoudjit, L.; Kawachi, H.; Lemay, S.; Takano, T. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 2007, 73, 556–566. [Google Scholar] [CrossRef]
- Tossidou, I.; Teng, B.; Drobot, L.; Meyer-Schwesinger, C.; Worthmann, K.; Haller, H.; Schiffer, M. CIN85/RukL is a novel binding partner of nephrin and podocin and mediates slit diaphragm turnover in podocytes. J. Biol. Chem. 2010, 285, 25285–25295. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Kilpeläinen, P.; Hellman, U.; Sun, Y.; Wartiovaara, J.; Morgunova, E.; Pikkarainen, T.; Yan, K.; Jonsson, A.P.; Tryggvason, K. Characterization of the interactions of the nephrin intracellular domain. FEBS J. 2005, 272, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Venkatareddy, M.; Kalinowski, A.; Patel, S.R.; Salant, D.J.; Garg, P. Shp2 associates with and enhances nephrin tyrosine phosphorylation and is necessary for foot process spreading in mouse models of podocyte injury. Mol. Cell Biol. 2016, 36, 596–614. [Google Scholar] [CrossRef] [PubMed]
- Schell, C.; Huber, T.B. The evolving complexity of the podocyte cytoskeleton. J. Am. Soc. Nephrol. 2017, 28, 3166–3174. [Google Scholar] [CrossRef]
- Lehtonen, S.; Zhao, F.; Lehtonen, E. CD2-associated protein directly interacts with the actin cytoskeleton. Am. J. Physiol. Ren. Physiol. 2002, 283, F734–F743. [Google Scholar] [CrossRef]
- Takeda, T.; McQuistan, T.; Orlando, R.A.; Farquhar, M.G. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J. Clin. Investig. 2001, 108, 289–301. [Google Scholar] [CrossRef]
- Takeda, T.; Go, W.Y.; Orlando, R.A.; Farquhar, M.G. Expression of podocalyxin inhibits cell-cell adhesion and modifies junctional properties in Madin-Darby canine kidney cells. Mol. Biol. Cell. 2000, 11, 3219–3232. [Google Scholar] [CrossRef]
- Refaeli, I.; Hughes, M.R.; Wong, A.K.-W.; Bissonnette, M.L.Z.; Roskelley, C.D.; Vogl, A.W.; Barbour, S.J.; Freedman, B.S.; McNagny, K.M. Distinct functional requirements for podocalyxin in immature and mature podocytes reveal mechanisms of human kidney disease. Sci. Rep. 2020, 10, 9419. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, F.; Cooper, M.E.; Kawachi, H.; Allen, T.J.; NBoner, G.; Cao, Z. Irbesartan normalizes the deficiency in glomerular nephrin expression in a model of diabetes and hypertension. Diabetologia 2001, 44, 874–877. [Google Scholar] [PubMed]
- Hyvönen, M.E.; Dumont, V.; Tienari, J.; Lehtonen, E.; Ustinov, J.; Havana, M.; Jalanko, H.; Otonkoski, T.; Miettinen, P.J.; Lehtonen, S. Early-onset diabetic E1-DN mice develop albuminuria and glomerular injury typical of diabetic nephropathy. BioMed Res. Int. 2015, 102969. [Google Scholar] [CrossRef] [PubMed]
- Wasik, A.A.; Koskelainen, S.; Hyvönen, M.E.; Musante, L.; Lehtonen, E.; Koskenniemi, K.; Tienari, J.; Vaheri, A.; Kerjaschki, D.; Szalay, C.; et al. Ezrin is down-regiulated in diabetic kidney glomeruli and regulates actin organization and glucose uptake via GLUT1 in cultured podocytes. Am. J. Pathol. 2014, 184, 1727–1739. [Google Scholar] [CrossRef]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.M.; Zoungas, S.; Rossing, P.; Groop, P.-H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Primers 2015, 1, 15018. [Google Scholar] [CrossRef] [PubMed]
- Afkarian, M.; Sachs, M.C.; Kestenbaum, B.; Hirsch, I.B.; Tuttke, K.R.; Himmerfarb, J.; de Boer, I.H. Kidney disease and increased mortality risk in type 2 diabetes. J. Am. Soc. Nephrol. 2013, 24, 302–308. [Google Scholar] [CrossRef]
- Groop, P.H.; Thomas, M.C.; Moran, J.L.; Wadèn, J.; Thorn, L.M.; Mäkinen, V.P.; Rosengård-Bärlund, M.; Saraheimo, M.; Hietala, K.; Heikkilä, O.; et al. The presence and severity of chronic disease predicts all-cause mortality in type 1 diabetes. Diabetes 2009, 58, 1651–1658. [Google Scholar] [CrossRef]
- Tervaert, T.W.C.; Mooyaart, A.L.; Amann, K.; Cohen, A.H.; Cook, H.T.; Drachenberg, C.B.; Ferrario, F.; Fogo, A.B.; Haas, M.; de Heer, E.; et al. Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 2010, 21, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Najafian, B.; Fogo, A.B.; Lusco, M.A.; Alpers, C.E. AJKD atlas of renal pathology: Diabetic nephropathy. Am. J. Kidney Dis. 2015, 66, e37–e38. [Google Scholar] [CrossRef] [PubMed]
- Comai, G.; Malvi, D.; Angeletti, A.; Vasuri, F.; Valente, S.; Ambrosi, F.; Capelli, I.; Ravaioli, M.; Pasquinelli, G.; D’Errico, A.; et al. Histological evidence of diabetic kidney doisease precede clinical diagnosis. Am. J. Nephrol. 2020, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Fioretto, P.; Steffes, M.W.; Sutherland, D.E.R.; Mauer, M. Sequential renal biopsies in insulin-dependent diabetic patients: Structural factors associated with clinical progression. Kidney Int. 1995, 48, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Looker, H.C.; Mauer, M.; Saulnier, P.-J.; Harder, J.L.; Nair, V.; Boustany-Kari, C.M.; Guarnieri, P.; Hill, J.; Esplin, C.A.; Kretzler, M.; et al. Changes in albuminuria but not GFR are associated with early changes in kidney structure in type 2 diabetes. J. Am. Soc. Nephrol. 2019, 30, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Harjutsalo, V.; Groop, P.-H. Epidemiology and risk factors for diabetic kidney disease. Adv. Chronic Kidney Dis. 2014, 21, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Kravets, I.; Mallipattu, S.K. The role of podocytes and podocyte-associated biomarkers in diagnosis and treatment of diabetic kidney disease. J. End Soc. 2020, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Brinkoetter, P.T.; Bork, T.; Salou, S.; Liang, W.; Mizi, A.; Özel, C.; Koehler, S.; Hagmann, H.H.; Ising, C.; Kuczkowski, A.; et al. Anaerobic glycolysis maintains the glomerular filtration barrier independent of mitochondrial metabolism and dynamics. Cell Rep. 2019, 27, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Keenan, H.A.; Li, Q.; Ishikado, A.; Kannt, A.; Sadowski, T.; Yorek, M.A.; Wu, I.-H.; Lockhart, S.; Coppey, L.J.; et al. Pyruvate kinase M2 activation may protect against the progresison of diabetic glomerular pathology and mitochondrial dysfunction. Nat. Med. 2017, 23, 753–762. [Google Scholar] [CrossRef]
- Wendt, T.M.; Tanji, N.; Guo, J.; Kislinger, T.R.; Qu, W.; Lu, Y.; Bucciarelli, L.G.; Rong, L.L.; Moser, B.; Markowitz, G.S.; et al. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am. J. Pathol. 2003, 162, 1123–1137. [Google Scholar] [CrossRef]
- Oltean, S.; Qiu, Y.; Ferguson, J.K.; Stevens, M.; Neal, C.; Russell, A.; Kaura, A.; Arkill, K.P.; Harris, K.; Symonds, C.; et al. Vascular endothelial growth factor-A165b is protective and restores endothelial glycocalyx in diabetic nephropathy. J. Am. Soc. Nephrol. 2015, 26, 1889–1904. [Google Scholar] [CrossRef]
- Schiffer, M.; Bitzer, M.; Roberts, I.S.D.; Kopp, J.B.; ten Dijke, P.; Mundel, P.; Böttinger, E.P. Apoptosis in podocytes induced by TGF-β and Smad7. J. Clin. Investig. 2001, 108, 807–816. [Google Scholar] [CrossRef]
- Susztak, K.; Raff, A.C.; Schiffer, M.; Böttinger, E.P. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 2006, 55, 225–233. [Google Scholar] [CrossRef]
- Durvasula, R.V.; Shankland, S.J. Activation of a local renin angiotensin system in podocytes by glucose. Am. J. Physiol. Ren. Physiol. 2008, 294, F830–F839. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.C.; Hunsicker, L.G.; Bain, R.P.; Rohde, R.D. The efect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N. Engl. J. Med. 1993, 329, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; TRitz, E.; Atkins, R.C.; Rohde, R.; Raz, I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-C.; Song, X.; Lu, X.-Y.; Li, D.T.; Eaton, D.C.; Shen, B.-Z.; Li, X.-L.; Ma, H.-P. High glucose induces podocyte apoptosis by stimulating TRPC6 via elevation of reactive oxygen species. Biochim. Biophys. Acta 2013, 1833, 1434–1442. [Google Scholar] [CrossRef]
- Steffes, M.W.; Schmidt, D.; McCrery, R.; Basgen, J.M. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int. 2001, 59, 2104–2113. [Google Scholar] [CrossRef]
- Meyer, T.W.; Bennett, P.H.; Nelson, R.G. Podocyte number predicts long-term urinary albumin excretion in Pima indians with type II diabetes and microalbuminuria. Diabetologia 1999, 42, 1341–1344. [Google Scholar] [CrossRef]
- Herman-Edelstein, M.; Scherzer, P.; Tobar, A.; Levi, M.; Gafter, U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 2014, 55, 561–572. [Google Scholar] [CrossRef]
- Ducasa, G.M.; Mitrofanova, A.; Mallela, S.K.; Liu, X.; Molina, J.; Sloan, A.; Pedigo, C.E.; Ge, M.; Santos, J.V.; Hernandez, Y.; et al. ATP-binding cassette A1 deficiency causes cardiolipin-driven mitochondrial dysfunction in podocytes. J. Clin. Investig. 2019, 129, 3387–3400. [Google Scholar] [CrossRef]
- Mitrofanova, A.; Mallela, S.K.; Ducasa, G.M.; Yoo, T.H.; Rosenfeld-Gur, E.; Zelnik, I.D.; Molina, J.; Santos, J.V.; Ge, M.; Sloan, A.; et al. SMPDL3c modulates insulin receptor signaling in diabetic kidney disease. Nat. Commun. 2019, 10, 2692. [Google Scholar] [CrossRef]
- Ducasa, G.M.; Mitrofanova, A.; Fornoni, A. Crosstalk between lipids and mitochondria in diabetic kidney disease. Curr. Diabetes Rep. 2019, 19, 144. [Google Scholar] [CrossRef]
- Milas, O.; Gadalean, F.; Vlad, A.; Dumitrascu, V.; Velciov, S.; Gluhovschi, C.; Bob, F.; Popescu, R.; Ursoniu, S.; Jianu, D.C.; et al. Pro-inflammatory cytikines are associated with podocyte damage and proximal tubular dysfunction in the early stage of diabetic kidney disease in type 2 diabetes mellitus patients. J. Diabetes Complicat. 2020, 34, 107479. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulou, I.-T.; Stangou, M.; Papagianni, A.; Didangelos, T.; Iliadis, F.; Efstratiadis, G. TNF-a and microalbuminuria in patients with type 2 diabetes. J. Diabetes Res. 2014, 2014, 394206. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.S.; You, H.; Gao, T.; Cooper, T.K.; Nedospasov, S.A.; Vacher, J.; Wilkinson, P.F.; Farrell, F.X.; Reeves, W.B. Macrophage-derived tumor necrosis factor-a mediates diabetic renal injury. Kidney Int. 2015, 88, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Satirapoj, B.; Dispan, R.; Radinahamed, P.; Kitiyakara, C. Urinary epidermal growth factor, monocyte chemoattractant protein-1 or their ratio as predictors for rapid loss of renal function in type 2 diabetic patients with diabetic kiodney disease. BMC Nephrol. 2018, 19, 246. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- Coward, R.J.M.; Welsh, G.I.; Yang, J.; Tasman, C.; Lennon, R.; Koziell, A.; Satchell, S.; Holman, G.D.; Kerjaschki, D.; Tavaré, J.M.; et al. The human glomerular podocyte is a novel target for insulin action. Diabetes 2005, 54, 3095–3102. [Google Scholar] [CrossRef]
- Tejada, T.; Catanuto, P.; Ijaz, A.; Santos, J.V.; Xia, X.; Sanchez, P.; Sanabria, N.; Lenz, O.; Elliot, S.J.; Fornoni, A. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int. 2008, 73, 1385–1393. [Google Scholar] [CrossRef]
- Hyvönen, M.E.; Saurus, P.; Wasik, A.; Heikkilä, E.; Havana, M.; Saleem, M.A.; Holthöfer, H.; Lehtonen, S. Lipid phosphatase SHIP2 downregulates insulin signalling in podocytes. Mol. Cell. Endocrinol. 2010, 328, 70–79. [Google Scholar] [CrossRef]
- Santamaria, B.; Marquez, E.; Lay, A.; Carew, R.M.; Conzáles-Rodríguez, Á.; Welsh, G.; Ni, L.; Hale, L.J.; Ortiz, A.; Saleem, M.A.; et al. IRS2 and PTEN are key molecules controlling insulin sensitivity in podocytes. Biochim. Biophys. Acta 2015, 1853, 3224–3234. [Google Scholar] [CrossRef]
- Lay, A.C.; Coward, R.J.M. The evolving importance of insulin signaling in podocyte health and disease. Front. Endocrinol. 2018, 9, 693. [Google Scholar] [CrossRef]
- Lehtonen, S. SHIPping out diabetes—Metformin, an old friend among new SHIP2 inhibitors. Acta Physiol. 2019, e13349. [Google Scholar] [CrossRef] [PubMed]
- Welsh, G.I.; Hale, L.J.; Eremina, V.; Jeansson, M.; Maezawa, Y.; Lennon, R.; Pons, D.A.; Owen, R.J.; Satchell, S.C.; Miles, M.J.; et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010, 12, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Hartleben, B.; Gödel, M.; Meyer-Schwesinger, C.; Liu, S.; Ulrich, T.; Köbler, S.; Wiech, T.; Grahammer, F.; Arnold, S.J.; Lindenmeyer, M.T.; et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Investig. 2010, 120, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, O.; Jasiek, M.; Hénique, C.; Guyonnet, L.; Hartleben, B.; Bork, T.; Chipont, A.; Flosseau, K.; Bensaada, I.; Schmitt, A.; et al. Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy 2015, 11, 1130–1145. [Google Scholar] [CrossRef]
- Tagawa, A.; Yasuda, M.; Kume, S.; Yamahara, K.; Nakazawa, J.; Chin-Kanasaki, M.; Araki, H.; Araki, S.; Koya, D.; Asanuma, K.; et al. Impaired podocyte autophagy exacerbates proteinuria in diabetic nephropathy. Diabetes 2016, 65, 755–767. [Google Scholar] [CrossRef]
- Moore, F.; Weekes, J.; Hardie, G. Evidence that AMP triggers phosphorylation as well as direct allosteric activation of rat liver AMP-activated protein kinase. Eur. J. Biochem. 1991, 199, 691–697. [Google Scholar] [CrossRef]
- Bork, T.; Liang, W.; Yamahara, K.; Lee, P.; Tian, Z.; Liu, S.; Schell, C.; Thedieck, K.; Hartleben, B.; Patel, K.; et al. Podocytes maintain high basal levels of autophagy independent of mtor signaling. Autophagy 2020, 16, 1932–1948. [Google Scholar] [CrossRef]
- Lee, I.H.; Cao, L.; Mostovslavsky, R.; Lombard, D.B.; Liu, J.; Bruns, N.E.; Tsokos, M.; Alt, F.W.; Finkel, T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. USA 2008, 105, 3374–3379. [Google Scholar] [CrossRef]
- Chuang, P.Y.; Xu, J.; Dai, Y.; Jia, F.; Mallipattu, S.K.; Yacoub, R.; Premsrirut, P.K.; He, J.C. In vivo RNA interference models of inducible and reversible Sirt1 knockdown in kidney cells. Am. J. Pathol. 2014, 184, 1940–1956. [Google Scholar] [CrossRef]
- Packer, M. Role of impaired nutrient and oxygen deprivation signaling and deficient autophagic flux in diabetic CKD development: Implications for understanding the effects of sodium-glucose cotransporter 2-inhibitors. J. Am. Soc. Nephrol. 2020, 31, 907–919. [Google Scholar] [CrossRef]
- Hundal, R.S.; Krssak, M.; Dufour, S.; Laurent, D.; Lebon, V.; Chandramouli, V.; Inzucchi, S.E.; Schumann, W.C.; Petersen, K.F.; Landau, B.R.; et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000, 49, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Cleasby, M.E.; Dzamko, N.; Hegarty, B.D.; Cooney, G.J.; Kraegen, E.W.; Ye, J.-M. metformin prevents the development of acute lipid-induced insulin resistance in the rat through altered hepatic signaling mechanisms. Diabetes 2004, 53, 3258–3266. [Google Scholar] [CrossRef] [PubMed]
- Hundal, H.S.; Ramlal, T.; Reyes, R.; Leiter, L.A.; Klip, A. Cellular mechanism of metformin action involves glucose transporter translocation from an intracellular pool to the plasma membrane in L6 muscle cells. Endocrinology 1992, 131, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Matthaei, S.; Reibold, J.P.; Hamann, A.; Benecke, H.; Häring, H.U.; Greten, H.; Klein, H.H. In vivo metformin treatment ameliorates insulin resistance: Evidence for potentiation of insulin-induced translocation and increased functional activity of glucose transporters in obese (fa/fa) Zucker rat adipocytes. Endocrinology 1993, 133, 304–311. [Google Scholar] [CrossRef]
- Polianskyte-Prause, Z.; Tolvanen, T.A.; Lindfors, S.; Dumont, V.; Van, M.; Wang, H.; Dash, S.N.; Berg, M.; Naams, J.-B.; Hautala, L.C.; et al. Metformin increases glucose uptake and acts renoprotectively by reducing SHIP2 activity. FASEB J. 2019, 33, 2858–2869. [Google Scholar] [CrossRef]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab. Syndr. Obes. 2014, 7, 241–253. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348, 607–614. [Google Scholar] [CrossRef]
- Foretz, M.; Hébrard, S.; Leclerc, J.; Zarrinpashneh, E.; Soty, M.; Mithieux, G.; Sakamoto, K.; Andreelli, F.; Viollet, B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Investig. 2010, 120, 2355–2369. [Google Scholar] [CrossRef]
- Miller, R.A.; Chu, Q.; Xie, J.; Fotetz, M.; Viollet, B.; Birnbaum, M.J. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 2013, 494, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.-M.; Braddock, D.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014, 510, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, A.; Leppert, U. Update on the protective renal efects of metformin in diabetic nephropathy. Curr. Med. Chem. 2017, 24, 3397–3412. [Google Scholar] [CrossRef] [PubMed]
- Kawanami, D.; Takashi, Y.; Tanabe, M. Significance of metformin use in diabetic kidney disease. Int. J. Mol. Sci. 2020, 21, 4239. [Google Scholar] [CrossRef]
- Pan, Q.; Lu, X.-Y.; Zhao, C.; Liao, S.; Chen, X.; Guo, F.; Yang, C.; Liu, H. Metformin: The updated protective property in kidney disease. Aging 2020, 12, 8742–8759. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, A.; Leppert, U. Challenge cardio-renal complications in diabetes-help is coming from an old friend! Austin Diabetes Res. 2017, 2, 1015. [Google Scholar]
- Christensen, M.; Jensen, J.B.; Jakobsen, S.; Jessen, N.; Frokiaer, J.; Kemp, B.E.; Marciszyn, A.L.; Li, H.; Pastor-Soler, N.M.; Hallows, K.R.; et al. Renoprotective effects of metformin are independent of organic cation transporters 1 & 2 and AMP-activated protein kinase in the kidney. Sci. Rep. 2016, 6, 35952. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, X.; Du, M.; Zhao, T.; Wang, J. A preclinical overview of metformin for the treatment of type 2 diabetes. Biomed. Pharmacother. 2018, 106, 1227–1235. [Google Scholar] [CrossRef]
- Liu, L.; Doné, S.C.; Khoshnoodi, J.; Bertorello, A.; Wartiovaara, J.; Berggren, P.-O.; Tryggvason, K. Defective nephrin trafficking caused by missense mutations in the NPHS1 gene: Insight into the mechanisms of congenital nephrotic syndrome. Hum. Mol. Gen. 2001, 10, 2637–2644. [Google Scholar] [CrossRef]
- Patrakka, J.; Kestilä, M.; Wartiovaara, J.; Ruotsalainen, V.; Tissari, P.; Lenkkeri, U.; Mannikko, M.; Visapaa, I.; Holmberg, C.; Rapola, J.; et al. Congenital nephrotic syndrome (NPHS1): Features resulting from different mutations in Finnish patients. Kidney Int. 2000, 58, 972–980. [Google Scholar] [CrossRef]
- Zhai, L.; Gu, J.; Yang, D.; Hu, W.; Wang, W.; Ye, S. Metformin ameliorates podocyte damage by restoring renal tissue nephrin expression in type 2 diabetic rats. J. Diabetes 2017, 9, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Szrejder, M.; Rachubik, P.; Rogacka, D.; Audzeyenka, I.; Rychlowski, M.; Kreft, E.; Angielski, S.; Piwkowska, A. Metformin reduces TRPC6 expression through AMPK activation and modulates cytoskeleton dynamics in podocytes under diabetic conditions. BBA Mol. Basis Dis. 2020, 1866, 165610. [Google Scholar] [CrossRef] [PubMed]
- Ristola, M.; Lehtonen, S. Functions of the podocyte proteins nephrin and Neph3 and the transcriptional regulation of their genes. Clin. Sci. 2014, 126, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Huber, T.B.; Hartleben, B.; Kim, J.; Schmidts, M.; Schermer, B.; Keil, A.; Egger, L.; Lecha, R.L.; Borner, C.; Pavenstädt, H.; et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol. Cell. Biol. 2003, 23, 4917–4928. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Gu, J.; Yang, D.; Wang, W.; Ye, S. Metformin ameliorates podocyte damage by restoring renal tissue podocalyxin expression in type 2 diabetic rats. J. Diabetes Res. 2015, 2015, 231825. [Google Scholar] [CrossRef]
- Spires, D.; Ilatovskaya, D.V.; Levchenko, V.; North, P.E.; Geurts, A.M.; Palygin, O.; Staruschenko, A. Protective role of Trpc6 in the progression of diabetic kidney disease. Am. J. Physiol. Ren. Physiol. 2018, 315, F1091–F1097. [Google Scholar] [CrossRef]
- Falkenberg, C.V.; Azeloglu, E.U.; Stothers, M.; Deerinck, T.J.; Chen, Y.-H.; He, J.C.; Ellisman, M.H.; Hone, J.C.; Iyengar, R.; Loew, L.M. Fragility of foot process morphology in kidney podocytes arises from chaotic spatial propagation of cytoskeletal instability. PLoS Comput. Biol. 2017, 13, e1005433. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, X.; Wang, Y.; Wang, Y.; Li, J.; Zhao, T.; Li, P. Role of transient receptor potential canonical channel 6 (TRPC6) in diabetic kidney disease by regulating podocyte actin cytoskeleton rearrangement. J. Diabetes Res. 2020, 2020, 6897390. [Google Scholar] [CrossRef]
- Ha, T.-S.; Park, H.-Y.; Seong, S.-B.; Aghn, H.-Y. Angiotensin II modulates p130Cas of podocytes by the suppression of AMP-activated protein kinase. J. Korean Med. Sci. 2016, 31, 535–541. [Google Scholar] [CrossRef]
- Alhaider, A.A.; Korashy, H.M.; Sayed-Ahmed, M.M.; Mobark, M.; Kfoury, H.; Mansour, M.A. Metformin attenuates streptozotocin-induced nephropathy in rats throiugh modulation of oxidative stress genes expression. Chem. Biol. Interact. 2011, 192, 233–242. [Google Scholar] [CrossRef]
- Kim, J.; Shon, E.; Kim, C.-S.; Kim, J.S. Renal podocyte injury in a rat model of type 2 diabetes is prevented by metformin. Exp. Diabetes Res. 2012, 2012, 210821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, H.; Yu, X.; Wu, Y.; Sui, D. Metformin ameliorates diabetic nephropathy in a rat model of low-dose streptozotocin-induced diabetes. Exp. Ther. Med. 2017, 14, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Piwkowska, A.; Rogacka, D.; Jankowski, M.; Dominiczak, M.H.; Stepinski, J.K.; Angielski, S. Metformin induces suppression of NAD(P)H oxidase activity in podocytes. Biochem. Biophys. Res. Commun. 2010, 393, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Piwkowska, A.; Rogacka, D.; Jankowski, M.; Angielski, S. Metformin reduces (NAD(P)H oxidase activity in mouse cultured podocytes through purinergic dependent mechanism by increasing extracellular ATP concentration. Acta Biochim. Pol. 2013, 60, 607–612. [Google Scholar] [CrossRef]
- Ren, H.; Shao, Y.; Wu, C.; Ma, X.; Lv, C.; Wang, Q. Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoXO1 pathway. Mol. Cell. Endocrinol. 2020, 500, 110628. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Yang, N.; Gao, X.; Fan, H.; Xu, Y.; Yang, W. MARCH: Comparative assessment of therapeutic effects of acarbose and metformin in newly diagnosed type 2 diabetes patients. PLoS ONE 2014, 9, e105698. [Google Scholar]
- Petrie, J.R.; Chaturvedi, N.; Ford, I.; Brouwers, M.C.G.J.; Greenlaw, N.; Tillin, T.; Hramiak, I.; Hughes, A.D.; Jenkins, A.J.; Klein, B.E.K.; et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): A double-blind, randomized, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 597–609. [Google Scholar] [CrossRef]
- Kim, D.K.; Lee, J.E.; Jung, Y.J.; Lee, A.S.; Lee, S.; Park, S.K.; Kim, S.H.; Park, B.-H.; Kim, W.; Kang, K.P. Metformin decreases high-fat diet-induced renal injury by regulating the expression of adipokines and the renal AMP-activated protein kinase/acetyl-CoA carboxylase pathway in mice. Int. J. Mol. Med. 2013, 32, 1293–1302. [Google Scholar] [CrossRef]
- Sanchez-Nino, M.D.; Sanz, A.B.; Ruiz-Anders, O.; Poveda, J.; Izquierdo, M.C.; Selgas, R.; Egido, J.; Ortiz, A. MIF, CD74 and other partners in kidney disease: Tales of a promiscuous couple. Cytokine Growth Factor Rev. 2013, 24, 23–40. [Google Scholar] [CrossRef]
- Xing, Y.; Ye, S.; Chen, Y.-H.; Fan, A.; Xu, Z.; Jiang, W. MIF/CD74 axis is a target for metformin therapy in diabetic podocytopathy-real world evidence. Endokrynol. Polska 2018, 69, 264–268. [Google Scholar] [CrossRef]
- Kamenova, P. Therapeutic potential of metformin in normal glucose tolerant persons with metabolic syndrome. Biotechnol. Biotechnol. Equip. 2020, 34, 30–37. [Google Scholar] [CrossRef]
- Atabek, M.E.; Pirgon, O. Use of metformin in obese adolescents with hyperinsulinemia: A 6-month, randomized, double-blind, placebo-controlled clinical trial. J. Pediatr. Andocrinol. Metab. 2008, 21, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Kamenova, P.; Atasanova, I.; Kirilov, G. Metformin improves insulin secretion and reduces insulin resistance in people at high risk for development of type 2 diabetes mellitus and cardiovascular disease. Merit Res. J. Med. Med. Sci. 2016, 4, 152–161. [Google Scholar]
- Rogacka, D.; Piwkowska, A.; Audzeyenka, I.; Angielski, S.; Jankowski, M. Involvement of the AMPK-PTEN pathway in insulin resistance induced by high glucose in cultured rat podocytes. Int. J. Biochem. Cell Biol. 2014, 51, 120–130. [Google Scholar] [CrossRef]
- Hori, H.; Sasaoka, T.; Ishihara, H.; Wada, T.; Murakami, S.; Ishiki, M.; Kobayashi, M. Association of SH2-containing inositol phosphatase 2 with the insulin resistance of diabetic db/db mice. Diabetes 2002, 51, 2387–2394. [Google Scholar] [CrossRef]
- Yuan, X.; Ding, L.; Diao, J.; Wen, S.; Xu, C.; Zhou, L.; Du, A. PolyMet-HA nanocomplexs regulates glucose uptake by inhibiting SHIP2 activity. J. Biomater. Appl. 2020, 0, 1–8. [Google Scholar] [CrossRef]
- Saleem, M.A.; O’Hare, M.J.; Reiser, J.; Coward, R.J.; Inward, C.D.; Farren, T.; Xing, C.Y.; Ni, L.; Mathieson, P.W.; Mundel, P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 2002, 13, 630–638. [Google Scholar]
- Wakino, S.; Hasegawa, K.; Itoh, H. Sirtuin and metabolic kidney disease. Kidney Int. 2015, 88, 691–698. [Google Scholar] [CrossRef]
- Rogacka, D.; Audzeyenka, I.; Rychlowski, M.; Rachubik, P.; Szrejder, M.; Angielski, S.; Piwkowska, A. Metformin overcomes high glucose-induced insulin resistance of podocytes by pleiotropic effects on SIRT1 and AMPK. BBA Mol. Basis Dis. 2018, 1864, 115–125. [Google Scholar] [CrossRef]
- Langer, S.; Kreutz, R.; Eisenreich, A. Metformin modulates apoptosis and cell signaling of human podocytes under high glucose conditions. J. Nephrol. 2016, 29, 765–773. [Google Scholar] [CrossRef]
- Yoon, M.-S. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients 2017, 9, 1176. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, L.-Q.; Xu, L.-L.; Xing, Y.; Ye, S. Metformin alleviates renal injury in diabetic rats by inducing Sirt1/FocO1 autophagic signal axis. Clin. Exp. Pharmacol. Physiol. 2020, 47, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Takiyama, Y.; Harumi, T.; Watanabe, J.; Fujita, Y.; Honjo, J.; Shimizu, N.; Makino, Y.; Haneda, M. A possible role of HIF-1a expression and oxygen metabolism. Diabetes 2011, 60, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Schiffer, T.A.; Gustafsson, H.; Krag, S.P.; Norregaard, R.; Palm, F. Metformin attenuates renal medullary hypoxia in diabetic nephropathy through inhibition uncoupling protein-2. Diabetes Metab. Res. Rev. 2018, 35, e3091. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hong, L.; Yang, Y.; GQiao, X.; Cai, W.; Zhong, M.; Wang, M.; Zheng, Z.; Fu, Y. Metformin reduces proteinuria in spontaneously hypertensive rats by activating the HIF-1a-VEGF-A pathway. Eur. J. Pharmacol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ruan, X.; Xue, Y.; Yang, S.; Shi, M.; Wang, L. Metformin reduces the senescence of renal tubular epithelial cells in diabetic nephropathy via the MBNL1/miR-130a-3p/STAT3 pathway. Oxid. Med. Cell. Longev. 2020, 2020, 8708236. [Google Scholar] [CrossRef]
- Roumie, C.L.; Chipman, J.; Min, J.Y.; Hackstadt, A.J.; Hung, A.M.; Greevy, R.A., Jr.; Grijalva, C.G.; Elasy, T.; Griffin, M.R. Association of treatment with metformin vs sulfonylurea with major adverse cardiovascular events among patients with diabetes and reduced kidney function. JAMA 2019, 322, 1167–1177. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Cersosimo, E.; Triplitt, C.; DeFronzo, R.A. Rosiglitazone decreases albuminuria in type 2 diabetic patients. Kidney Int. 2007, 72, 1367–1373. [Google Scholar] [CrossRef]
- Lachin, J.M.; Viberti, G.; Zinman, B.; Haffner, S.M.; Aftring, R.P.; Paul, G.; Kravitz, B.G.; Herman, W.H.; Holman, R.R.; Kahn, S.E.; et al. Renal function in type 2 diabetes with rosiglitazone, metformin, and glyburide monotherapy. Clin. J. Am. Soc. Nephrol. 2011, 6, 1032–1040. [Google Scholar] [CrossRef]
- Amador-Licona, N.; Guízar-Mendoza, J.-M.; Vargas, E.; Sánchez-Camargo, G.; Zamora-Mata, L. The short-term effect of a switch from glibenclamide to metformin on blood pressure and microalbuminuria in patients with type 2 diabetes mellitus. Arch. Med. Res. 2000, 31, 571–575. [Google Scholar] [CrossRef]
- Pan, Q.; Xu, Y.; Yang, N.; Gao, X.; Liu, J.; Yang, W.; Wang, G. Comparison of acarbose and metformin on albumin excretion in patients with newly diagnosed type 2 diabetes. Medicine 2016, 95, e3247. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, Y.C.; Park, J.Y.; Lee, J.; An, J.N.; Kim, C.T.; Oh, S.; Park, S.; Kim, D.K.; Oh, Y.K.; et al. The long-term effects of metformin on patients with type 2 diabetic kidney disease. Diabetes Care 2020, 43, 948–955. [Google Scholar] [CrossRef]
- Kooy, A.; de Jager, J.; Lehert, P.; Bets, D.; Wulffelé, M.G.; Donker, A.J.M.; Stehouwer, C.D.A. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch. Intern. Med. 2009, 169, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Cherney, D.Z.I.; Zinman, B.; Inzucchi, S.E.; Koitka-Weber, A.; Mattheus, M.; von Eynatten, M.; Wanner, C. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: An exploratory analysis from the EMPA-REG OUTCOME randomized, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 610–621. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA Drug Safety Communication: FDA Revises Warnings Regarding Use of the Diabetes Medicine Metformin in Certain Patients with Reduced Kidney Function. 2016. Available online: https://www.fda.gov/media/96771/download (accessed on 5 October 2020).
- European Medicines Agency. Use of Metformin to Treat Diabetes Now Expanded to Patients with Moderately Reduced Kidney Function. 2016. Available online: https://www.ema.europa.eu/en/documents/referral/metformin-article-31-referral-use-metformin-treat-diabetes-now-expanded-patients-moderately-reduced_en.pdf (accessed on 5 October 2020).
- Lalau, J.-D.; Kajbaf, F.; Bennis, Y.; Hurtel-Lemaire, A.-S.; Belpaire, F.; De Broe, M.E. metformin treatment in patients with type 2 diabetes and chronic kidney disease stages 3A, 3B, or 4. Diabetes Care 2018, 41, 547–553. [Google Scholar] [CrossRef]
- Flory, J.H.; Hennessy, S.; Bailey, C.J.; Inzucchi, S.E. Reports of lactic acidosis attributed to metformin, 2015–2018. Diabetes Care 2020, 43, 244–246. [Google Scholar] [CrossRef]
- Chu, P.Y.; Hackstadt, A.J.; Chipman, J.; Griffin, M.R.; Hung, A.M.; Greevy, R.A., Jr.; Grijalva, C.G.; Elasy, T.; Roumie, C.L. Hospitalizations for lactic acidosis among patients with reduced kidney function treated with metformin or sulfonylureas. Diabetes Care 2020, 43, 1462–1470. [Google Scholar] [CrossRef]
- Hung, S.-C.; Chang, Y.-K.; Liu, J.-S.; Kuo, K.-L.; Chen, Y.-H.; Hsu, C.-C.; Tarng, D.-C. Metformin use and mortality in patients with advanced chronic kidney disease: National, retrospective, observational, cohort study. Lancet Diabetes Endocrinol. 2015, 3, 605–614. [Google Scholar] [CrossRef]
- Lazarus, B.; Wu, A.; Shin, J.-I.; Sang, Y.; Alexander, C.; Secora, A.; Inker, L.A.; Coresh, J.; Chang, A.R.; Grams, M.E. Association of metformin use with risk of lactic acidosis across the range of kidney function. JAMA Int. Med. 2018, 178, 903–910. [Google Scholar] [CrossRef]
- Connelly, P.J.; Lonergan, M.; Soto-Pedre, E.; Donnelly, L.; Zhou, K.; Pearson, E.R. Acute kidney injury, plasma lactate concentrations and lactic asidosis in metformin users: A GoDarts study. Diabetes Obes. Metab. 2017, 19, 1579–1586. [Google Scholar] [CrossRef]
- Hussain, S.; Romio, L.; Saleem, M.; Mathieson, P.; Serrano, M.; Moscat, J.; Diaz-Meco, M.; Scambler, P.; Koziell, A. Nephrin deficiency activates NF-kB and promotes glomerular injury. J. Am. Soc. Nephrol. 2009, 20, 1733–1743. [Google Scholar] [CrossRef]
- Ristola, M.; Arpiainen, S.; Saleem, M.A.; Holthöfer, H.; Lehtonen, S. Transcription of nephrin-Neph3 gene pair is synergistically activated by WT1 and NF-kB and silenced by DNA methylation. Nephrol. Dial. Transplant. 2012, 27, 1737–1745. [Google Scholar] [CrossRef]
- Holland, W.; Morrison, T.; Chang, Y.; Wiernsperger, N.; Stith, B.J. Metformin (Glucophage) inhibits tyrosine phosphatase activity to stimulate the insulin receptor tyrosine kinase. Biochem. Pharmacol. 2004, 67, 2081–2091. [Google Scholar] [CrossRef]
- Krishnan, N.; Konidaris, K.F.; Gasser, G.; Tonks, N.K. A potent, selective, and orally bioavailable inhibitor of the protein-tyrosine phosphatase PTP1B improves insulin and leptin signaling in animal models. J. Biol. Chem. 2018, 293, 1517–1525. [Google Scholar] [CrossRef]
- Schmitt, R.; Melk, A. Molecular mechanisms of renal aging. Kidney Int. 2017, 92, 569–579. [Google Scholar] [CrossRef]
- Verzola, D.; Gandolfo, M.T.; Gaetani, G.; Ferraris, A.; Mangerini, R.; Ferrario, F.; Villaggio, B.; Giangiorio, F.; Tosetti, F.; Weiss, U.; et al. Accelerated senescence in the kidneys of patients with tyope 2 diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2008, 295, F1563–F1573. [Google Scholar] [CrossRef]
- Hansen, M.; Rubinsztein, D.C.; Walker, D.W. Autophagy as a promoter of longevity: Insights from model organisms. Nat. Rev. Mol. Cell Biol. 2018, 19, 579–593. [Google Scholar] [CrossRef]

| Model | Metformin Administration | Reference |
|---|---|---|
| spontaneously diabetic Torii rat | 350 mg/kg daily 17 weeks | [111] |
| high-fat diet and single low dose of streptozotocin rat | 150, 300 or 500 mg/kg daily 8 weeks | [112] |
| high-fat diet and single low dose of streptozotocin rat | 70 mg/kg daily 13 weeks | [101] |
| high-fat diet and single low dose of streptozotocin rat | 250 mg/kg daily 8 weeks | [115] |
| high-fat diet and single low dose of streptozotocin rat | 150, 300 or 500 mg/kg daily 8 weeks | [132] |
| streptozotocin rat | 100 or 500 mg/kg daily 8 weeks | [110] |
| high-fat diet mouse | 0.5% w/w in diet 12 weeks | [118] |
| db/db mouse | 200 mg/kg daily 16 weeks | [136] |
| Study/Cohort | Outcome | Reference |
|---|---|---|
| MARCH/newly diagnosed, drug-naïve individuals with T2D (n = 762) | Reduced albuminuria | [140] |
| Individuals with T2D with incipient nephropathy (switched from glybenclamide; n = 51) | Reduced albuminuria | [141] |
| Individuals with uncontrolled T2D (add-on to sulfonylurea; n = 202) | Reduced albuminuria | [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehtonen, S. Metformin Protects against Podocyte Injury in Diabetic Kidney Disease. Pharmaceuticals 2020, 13, 452. https://doi.org/10.3390/ph13120452
Lehtonen S. Metformin Protects against Podocyte Injury in Diabetic Kidney Disease. Pharmaceuticals. 2020; 13(12):452. https://doi.org/10.3390/ph13120452
Chicago/Turabian StyleLehtonen, Sanna. 2020. "Metformin Protects against Podocyte Injury in Diabetic Kidney Disease" Pharmaceuticals 13, no. 12: 452. https://doi.org/10.3390/ph13120452
APA StyleLehtonen, S. (2020). Metformin Protects against Podocyte Injury in Diabetic Kidney Disease. Pharmaceuticals, 13(12), 452. https://doi.org/10.3390/ph13120452





