Repurposing of Plasminogen: An Orphan Medicinal Product Suitable for SARS-CoV-2 Inhalable Therapeutics
Abstract
1. Introduction
2. Results
2.1. Physical Characterization
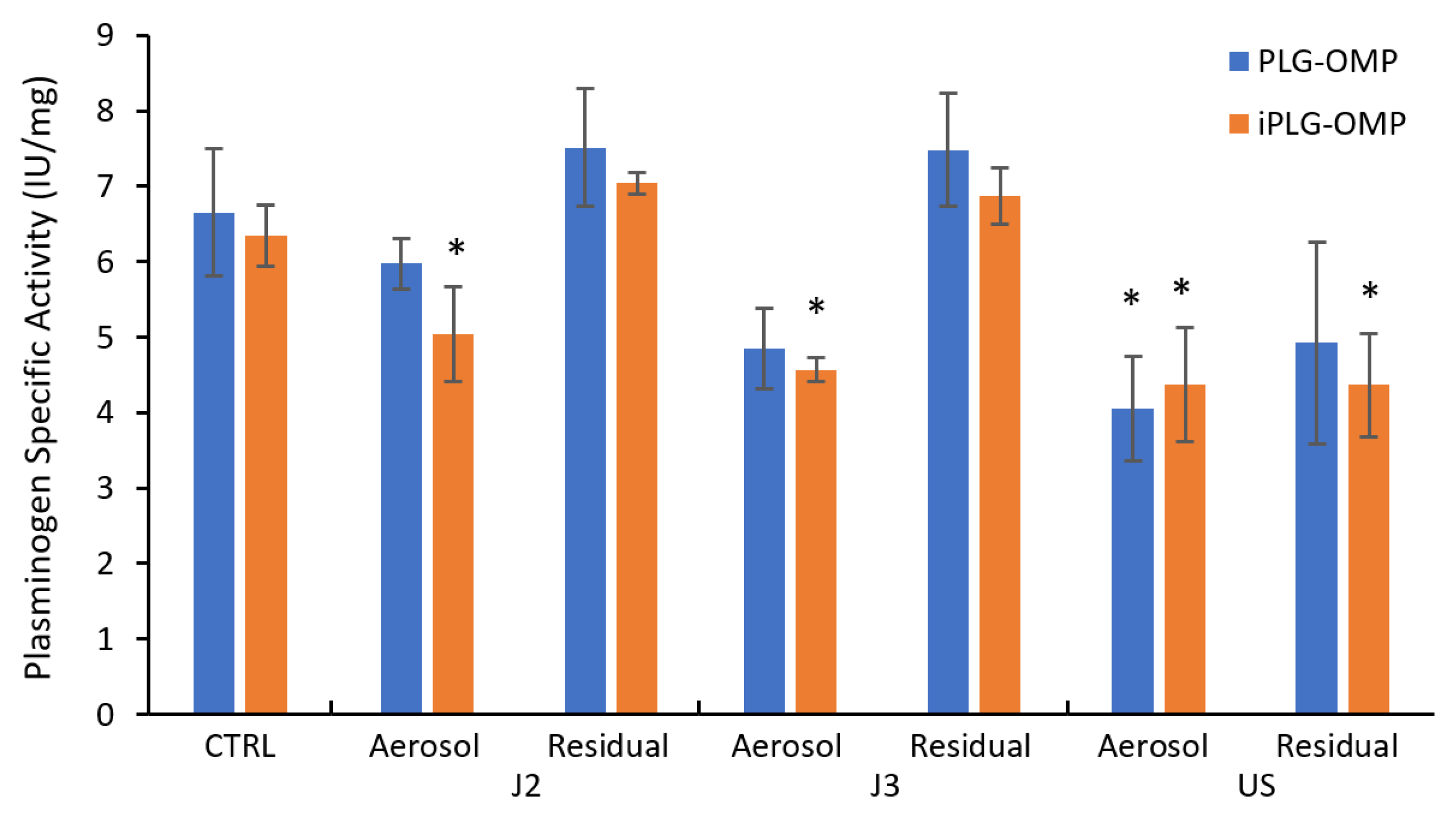
2.2. Nebulization and Biochemical Characterization
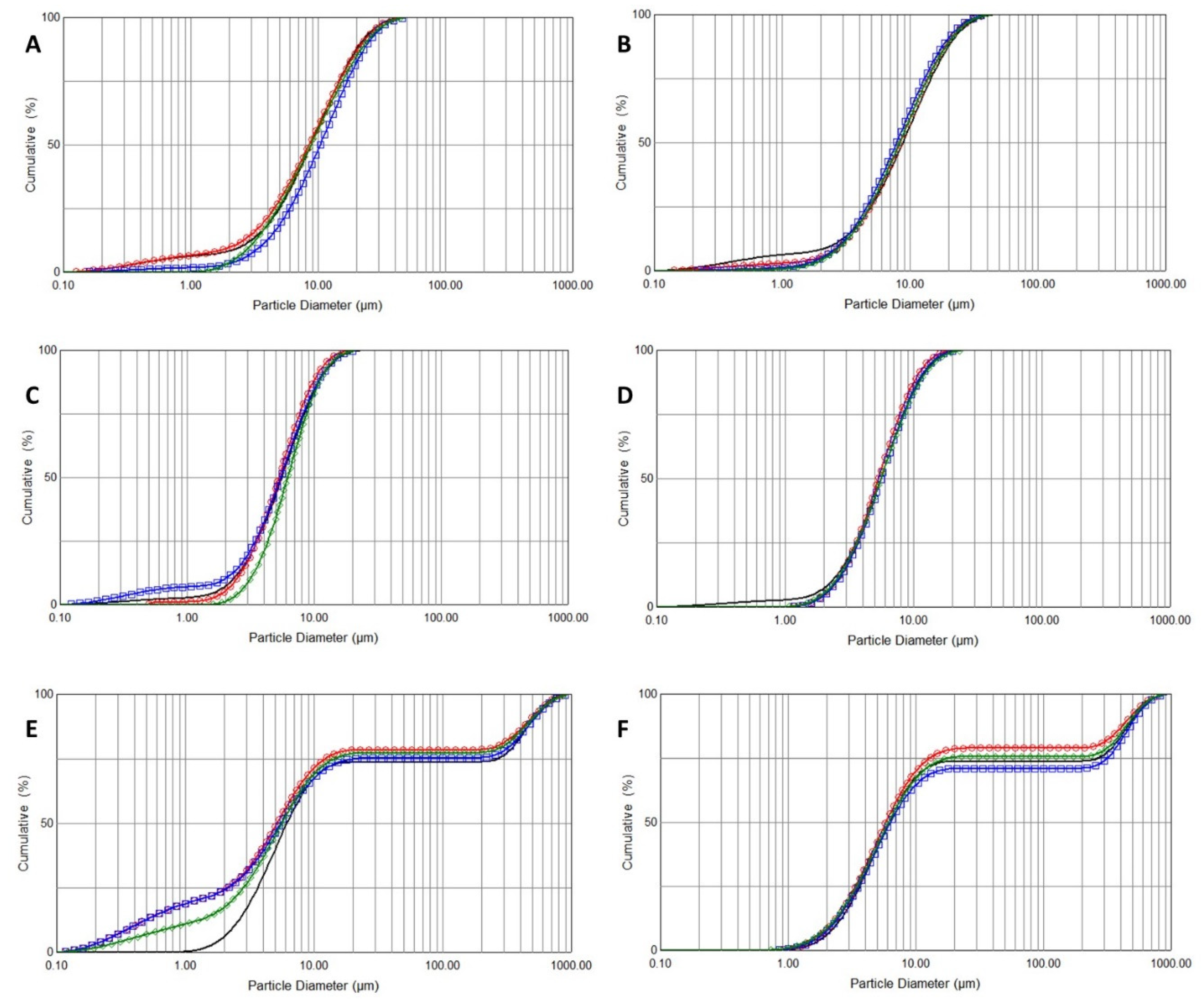
2.3. Droplet Size Distribution
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of Isotonic PLG (iPLG)
4.2.2. Nebulization
4.2.3. Physical Characterization
4.2.4. Biochemical Analytical Methods
- Protein quantification
- Plasminogen specific activity
- Plasmin activity (PL:Act)
- Protein characterization
4.2.5. Droplet Size Distribution
4.2.6. Statistical
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barrett, C.D.; Moore, H.B.; Moore, E.E.; McIntyre, R.C.; Moore, P.K.; Burke, J.; Hua, F.; Apgar, J.; Talmor, D.S.; Sauaia, A.; et al. Fibrinolytic therapy for refractory COVID-19 acute respiratory distress syndrome: Scientific rationale and review. Res. Pr. Thromb. Haemost. 2020, 4, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Whyte, C.S.; Morrow, G.B.; Mitchell, J.L.; Chowdary, P.; Mutch, N.J. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J. Thromb. Haemost. 2020, 18, 1548–1555. [Google Scholar] [CrossRef]
- Belen-Apak, F.; Sarıalioğlu, F. Pulmonary intravascular coagulation in COVID-19: Possible pathogenesis and recommendations on anticoagulant/thrombolytic therapy. J. Thromb. Thrombolysis 2020, 50, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Fibrinolytic Therapy to Treat ARDS in the Setting of COVID-19 Infection. ClinicalTrials.gov Identifier NCT04357730. Available online: https://clinicaltrials.gov/ct2/show/NCT04357730 (accessed on 11 October 2020).
- Impact of Tissue Plasminogen Activator (tPA) Treatment for an Atypical Acute Respiratory Distress Syndrome (COVID-19) (AtTAC). ClinicalTrials.gov Identifier NCT04453371. Available online: https://clinicaltrials.gov/ct2/show/NCT04453371 (accessed on 11 October 2020).
- Tenecteplase in Patients with COVID-19. ClinicalTrials.gov Identifier NCT04505592. Available online: https://clinicaltrials.gov/ct2/show/NCT04505592 (accessed on 11 October 2020).
- Low-Dose Tenecteplase in Covid-19. ClinicalTrials.gov Identifier NCT04558125. Available online: https://clinicaltrials.gov/ct2/show/NCT04558125 (accessed on 11 October 2020).
- Camprubí-Rimblas, M.; Tantinyà, N.; Bringué, J.; Guillamat-Prats, R.; Artigas, A. Anticoagulant therapy in acute respiratory distress syndrome. Ann. Transl. Med. 2018, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Matsuzaki, Y.; Shimonaka, M. Impact of plasminogen on an in vitro wound healing model based on a perfusion cell culture system. Mol. Cell. Biochem. 2008, 322, 1–13. [Google Scholar] [CrossRef]
- Sulniute, R.; Shen, Y.; Guo, Y.-Z.; Fallah, M.; Ahlskog, N.; Ny, L.; Rakhimova, O.; Broden, J.; Boija, H.; Moghaddam, A.; et al. Plasminogen is a critical regulator of cutaneous wound healing. Thromb. Haemost. 2016, 115, 1001–1009. [Google Scholar] [CrossRef]
- Fallah, M.; Viklund, E.; Bäckman, A.; Brodén, J.; Lundskog, B.; Johansson, M.; Blomquist, M.; Wilczynska, M.; Ny, T. Plasminogen is a master regulator and a potential drug candidate for the healing of radiation wounds. Cell Death Dis. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Heissig, B.; Salama, Y.; Takahashi, S.; Osada, T.; Hattori, K. The multifaceted role of plasminogen in inflammation. Cell. Signal. 2020, 75, 109761. [Google Scholar] [CrossRef]
- Vago, J.P.; Sugimoto, M.A.; Lima, K.M.; Negreiros-Lima, G.L.; Baik, N.; Teixeira, M.M.; Perretti, M.; Parmer, R.J.; Miles, L.A.; Sousa, L.P. Plasminogen and the Plasminogen Receptor, Plg-RKT, Regulate Macrophage Phenotypic, and Functional Changes. Front. Immunol. 2019, 10, 1458. [Google Scholar] [CrossRef]
- Henry, B.M.; Benoit, S.W.; Hoehn, J.; Lippi, G.; Favaloro, E.J.; Benoit, J.L. Circulating Plasminogen Concentration at Admission in Patients with Coronavirus Disease 2019 (COVID-19). Semin. Thromb. Hemost. 2020, 46, 859–862. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T.; Guo, C.; Zhang, D.; Ge, X.; Huang, Z.; Zhou, X.; Li, Y.; Peng, Q.; Li, J. Plasminogen improves lung lesions and hypoxemia in patients with COVID-19. QJM Int. J. Med. 2020, 113, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Videira, M.; Llop, J.; Sousa, C.; Kreutzer, B.; Cossío, U.; Forbes, B.; Vieira, I.; Gil, N.; Silva-Lima, B. Pulmonary Administration: Strengthening the Value of Therapeutic Proximity. Front. Med. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Hertel, S.; Winter, G.; Friess, W. Protein stability in pulmonary drug delivery via nebulization. Adv. Drug Deliv. Rev. 2015, 93, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Moon, S.-H.; Oh, J.Y.; Yoon, Y.-S.; Gu, N.; Lim, C.-Y.; Park, B.J.; Nam, K.C. Comparison of Salbutamol Delivery Efficiency for Jet versus Mesh Nebulizer Using Mice. Pharmaceutics 2019, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Adorni, G.; Seifert, G.; Buttini, F.; Colombo, G.; Stecanella, L.A.; Krämer, I.; Rossi, A. Aerosolization Performance of Jet Nebulizers and Biopharmaceutical Aspects. Pharmaceutics 2019, 11, 406. [Google Scholar] [CrossRef]
- Steckel, H.; Eskandar, F. Factors affecting aerosol performance during nebulization with jet and ultrasonic nebulizers. Eur. J. Pharm. Sci. 2003, 19, 443–455. [Google Scholar] [CrossRef]
- Tsuda, A.; Henry, F.S.; Butler, J.P. Particle Transport and Deposition: Basic Physics of Particle Kinetics. Compr. Physiol. 2013, 3, 1437–1471. [Google Scholar] [CrossRef]
- Zhang, Z.; Kleinstreuer, C.; Kim, C.S. Isotonic and Hypertonic Saline Droplet Deposition in a Human Upper Airway Model. J. Aerosol. Med. 2006, 19, 184–198. [Google Scholar] [CrossRef]
- Laube, B.L.; Jashnani, R.; Dalby, R.N.; Zeitlin, P.L. Targeting Aerosol Deposition in Patients With Cystic Fibrosis. Chest 2000, 118, 1069–1076. [Google Scholar] [CrossRef]
- Khan, S.Y.; O’Driscoll, B.R. Is nebulized saline a placebo in COPD? BMC Pulm. Med. 2004, 4, 9. [Google Scholar] [CrossRef]
- Cheng, Y.S. Mechanisms of Pharmaceutical Aerosol Deposition in the Respiratory Tract. AAPS PharmSciTech 2014, 15, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Kassam, G.; Kwon, M.; Yoon, C.-S.; Graham, K.S.; Young, M.K.; Gluck, S.; Waisman, D.M. Purification and Characterization of A61. J. Biol. Chem. 2000, 276, 8924–8933. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.-Y.; Wu, H.-L. Differential autolysis of human plasmin at various pH levels. Thromb. Res. 1988, 51, 355–364. [Google Scholar] [CrossRef]
- Vonarburg, C.; Loetscher, M.; Spycher, M.O.; Kropf, A.; Illi, M.; Salmon, S.; Roberts, S.; Steinfuehrer, K.; Campbell, I.; Koernig, S.; et al. Topical application of nebulized human IgG, IgA and IgAM in the lungs of rats and non-human primates. Respir. Res. 2019, 20, 1–16. [Google Scholar] [CrossRef]
- Council of Europe. 2.9.44. Preparation for Nebulisation: Characterisation. In European Pharmacopoeia. (Ph. Eur.), 10th ed.; Council of Europe: Strasbourg, France, 2019. [Google Scholar]
- Surasarang, S.H.; Sahakijpijarn, S.; Florova, G.; Komissarov, A.A.; Nelson, C.L.; Perenlei, E.; Fukuda, S.; Wolfson, M.R.; Shaffer, T.H.; Idell, S.; et al. Nebulization of single-chain tissue-type and single-chain urokinase plasminogen activator for treatment of inhalational smoke-induced acute lung injury. J. Drug Deliv. Sci. Technol. 2018, 46, 19–27. [Google Scholar] [CrossRef]
- Deb, P.K.; Abed, S.N.; Maher, H.; Al-Aboudi, A.; Paradkar, A.; Bandopadhyay, S.; Tekade, R.K. Aerosols in pharmaceutical product development. In Drug Delivery Systems; Academic Press: Cambridge, MA, USA, 2020; pp. 521–577. [Google Scholar]
- Respaud, R.; Marchand, D.; Parent, C.; Pelat, T.; Thullier, P.; Tournamille, J.-F.; Viaud-Massuard, M.-C.; Diot, P.; Si-Tahar, M.; Vecellio, L.; et al. Effect of formulation on the stability and aerosol performance of a nebulized antibody. mAbs 2014, 6, 1347–1355. [Google Scholar] [CrossRef]
- Röhm, M.; Carle, S.; Maigler, F.; Flamm, J.; Kramer, V.; Mavoungou, C.; Schmid, O.; Schindowski, K. A comprehensive screening platform for aerosolizable protein formulations for intranasal and pulmonary drug delivery. Int. J. Pharm. 2017, 532, 537–546. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use (CHMP). Guideline on Immunogenicity Assessment of Therapeutic Proteins; EMEA/CHMP/BMWP/14327/2006 Rev 1; Committee for Medicinal Products for Human Use (CHMP): Amsterdam, The Netherlands, 2017. [Google Scholar]
- Rosenberg, A.S.; Sauna, Z.E. Immunogenicity assessment during the development of protein therapeutics. J. Pharm. Pharmacol. 2018, 70, 584–594. [Google Scholar] [CrossRef]
- Nebulized Heparin in Severe Acute Respiratory Syndrome COVID-19 (NEBUHEPA). ClinicalTrials.gov Identifier: NCT04530578. Available online: https://clinicaltrials.gov/ct2/show/NCT04530578 (accessed on 18 November 2020).
- Chimenti, L.; Camprubí-Rimblas, M.; Guillamat-Prats, R.; Gomez, M.N.; Tijero, J.; Blanch, L.; Artigas, A. Nebulized Heparin Attenuates Pulmonary Coagulopathy and Inflammation through Alveolar Macrophages in a Rat Model of Acute Lung Injury. Thromb. Haemost. 2017, 117, 2125–2134. [Google Scholar] [CrossRef]
- Camprubí-Rimblas, M.; Tantinyà, N.; Guillamat-Prats, R.; Bringué, J.; Puig, F.; Gómez, M.N.; Blanch, L.; Artigas, A. Effects of nebulized antithrombin and heparin on inflammatory and coagulation alterations in an acute lung injury model in rats. J. Thromb. Haemost. 2020, 18, 571–583. [Google Scholar] [CrossRef]
- Bodier-Montagutelli, E.; Respaud, R.; Perret, G.; Baptista, L.; Duquenne, P.; Heuzé-Vourc’H, N.; Vecellio, L. Protein stability during nebulization: Mind the collection step! Eur. J. Pharm. Biopharm. 2020, 152, 23–34. [Google Scholar] [CrossRef] [PubMed]
- ICH. International Council on Harmonisation of Technical. Requirements for Registration of Pharmaceuticals for Human Use (ICH) Guideline; Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology; CPMP/ICH/381/95; ICH: Geneva, Switzerland, 1995. [Google Scholar]
- Hammond, J.B.W.; Kruger, N.J. The Bradford Method for Protein Quantitation. In New Protein Techniques. Methods in Molecular Biology™; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 1988; Volume 3. [Google Scholar]
- ClinicalTrials.gov. Nebulised Rt-PA for ARDS Due to COVID-19 (PACA). Identifier: NCT04356833. Available online: https://clinicaltrials.gov/ct2/show/NCT04356833 (accessed on 10 October 2020).
| Physical Parameter | PLG-OMP | iPLG-OMP | H2O | NaCl 0.9% |
|---|---|---|---|---|
| pH | 6.6 ± 0.3 | 6.4 ± 0.2 | 6.9 ± 0.1 | 6.0 ± 0.2 |
| density g/mL | 0.9710 ± 0.0060 | 0.9834 ± 0.0048 | 0.9972 ± 0.0234 | 1.0051 ± 0.0226 |
| Osmolality mOsm/Kg H2O | 205 ± 10 | 316 ± 12 | - | 300 ± 4 |
| Kinematic Viscosity cSt | 0.969 ± 0.010 | 0.981 ± 0.008 | 0.957 ± 0.008 | 1.010 ± 0.008 |
| Dynamic Viscosity cP | 0.941 ± 0.007 | 0.965 ± 0.007 | 0.954 ±0.002 | 1.015 ± 0.024 |
| Surface Tension * mN/m | 62.7 ± 0.6 | 63.0 ± 0.2 | 71.5 ± 0.6 | 62.8 ± 0.8 |
| CTRL | J2 | J3 | US | |||||
|---|---|---|---|---|---|---|---|---|
| Aerosol | Residual | Aerosol | Residual | Aerosol | Residual | |||
| PLG-OMP | PL:Act (U/mL) | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 |
| %PLG-band | 95.6 ± 2.2 | 92.2 ± 1.6 | 95.0 ± 2.3 | 91.4 ± 0.4 | 94.0 ± 0.3 | 90.8 ± 2.2 | 92.9 ± 2.9 | |
| % GLU I | 45.0 ± 2.6 | 47.8 ± 5.1 | 48.5 ± 3.1 | 46.5 ± 5.5 | 47.5 ± 3.1 | 45.8 ± 4.0 | 46.9 ± 4.6 | |
| % GLU II | 55.0 ± 2.6 | 52.2 ± 5.1 | 51.5 ± 3.1 | 53.5 ± 5.5 | 52.5 ± 3.1 | 55.2 ± 3.0 | 53.1 ± 4.6 | |
| % Lys I & II | - | - | - | - | - | - | - | |
| iPLG-OMP | PL:Act (U/mL) | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 |
| %PLG-band | 96.7 ± 1.6 | 95.0 ± 1.8 | 95.3 ± 2.1 | 93.9 ± 2.4 | 95.8 ± 2.6 | 88.6 ± 2.6 | 94.8 ± 2.2 | |
| % GLU I | 43.5 ± 1.6 | 45.3 ± 0.4 | 45.4 ± 0.4 | 45.4 ± 4.6 | 45.5 ± 0.6 | 44.7 ± 1.3 | 43.3 ± 1.0 | |
| % GLU II | 56.6 ± 1.6 | 54.7 ± 0.4 | 54.3 ± 0.8 | 54.6 ± 4.6 | 54.5 ± 0.6 | 55.3 ± 1.3 | 56.5 ± 1.3 | |
| % Lys I & II | - | - | - | - | - | - | - | |
| J2 | J3 | US | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PLG-OMP | iPLG-OMP | NaCl | PLG-OMP | iPLG-OMP | NaCl | PLG-OMP | iPLG-OMP | NaCl | |
| dv(50) (μm ± sd) | 9.2 ± 1.0 | 8.1 ± 0.2 | 8.79 | 5.6 ± 0.5 | 5.5 ± 0.1 | 5.5 | 4.9 ± 0.3 | 5.1 ± 0.1 | 5.5 |
| SPAN (value ± sd) | 2.2 ± 0.2 | 2.1 ± 0.1 | 2.17 | 1.5 ± 0.2 | 1.6 ± 0.1 | 1.7 | 78.8 ± 5.0 | 76.8 ± 6.1 | 75.7 |
| %V < 5 µm (% ± sd) | 24.6 ± 5.1 | 27.0 ± 1.3 | 25.39 | 41.9 ± 6.5 | 43.9 ± 1.6 | 43.51 | 50.6 ± 2.9 | 48.7 ± 1.1 | 43.91 |
| %V < 10 µm (% ± sd) | 53.8 ± 5.0 | 60.5 ± 1.7 | 56.53 | 85.5 ± 2.7 | 85.1 ± 2.1 | 83.92 | 78.4 ± 0.6 | 77.1 ± 1.1 | 75.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piras, A.M.; Zambito, Y.; Lugli, M.; Ferro, B.; Roncucci, P.; Mori, F.; Salvatore, A.; Ascione, E.; Bellini, M.; Crea, R. Repurposing of Plasminogen: An Orphan Medicinal Product Suitable for SARS-CoV-2 Inhalable Therapeutics. Pharmaceuticals 2020, 13, 425. https://doi.org/10.3390/ph13120425
Piras AM, Zambito Y, Lugli M, Ferro B, Roncucci P, Mori F, Salvatore A, Ascione E, Bellini M, Crea R. Repurposing of Plasminogen: An Orphan Medicinal Product Suitable for SARS-CoV-2 Inhalable Therapeutics. Pharmaceuticals. 2020; 13(12):425. https://doi.org/10.3390/ph13120425
Chicago/Turabian StylePiras, Anna Maria, Ylenia Zambito, Maurizio Lugli, Baldassare Ferro, Paolo Roncucci, Filippo Mori, Alfonso Salvatore, Ester Ascione, Marta Bellini, and Roberto Crea. 2020. "Repurposing of Plasminogen: An Orphan Medicinal Product Suitable for SARS-CoV-2 Inhalable Therapeutics" Pharmaceuticals 13, no. 12: 425. https://doi.org/10.3390/ph13120425
APA StylePiras, A. M., Zambito, Y., Lugli, M., Ferro, B., Roncucci, P., Mori, F., Salvatore, A., Ascione, E., Bellini, M., & Crea, R. (2020). Repurposing of Plasminogen: An Orphan Medicinal Product Suitable for SARS-CoV-2 Inhalable Therapeutics. Pharmaceuticals, 13(12), 425. https://doi.org/10.3390/ph13120425








