In Vitro and Ex Vivo Evaluation of Penetratin as a Non-invasive Permeation Enhancer in the Penetration of Salmon Calcitonin through TR146 Buccal Cells and Porcine Buccal Tissues
Abstract
1. Introduction
2. Results
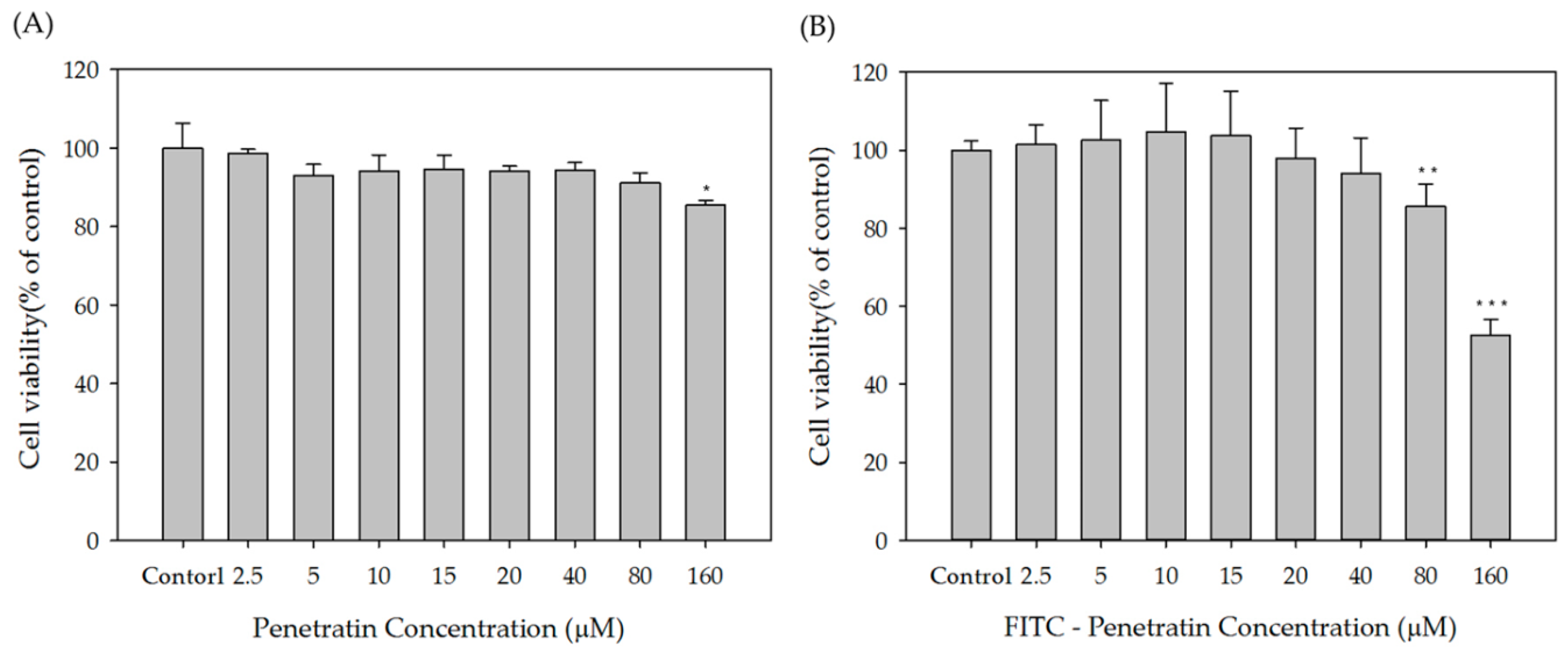
2.1. Cytotoxicity
2.2. FITC-Penetratin and Alexa 647-sCT Internalization into TR146 Cell
2.3. In Vitro Cell Permeation Study
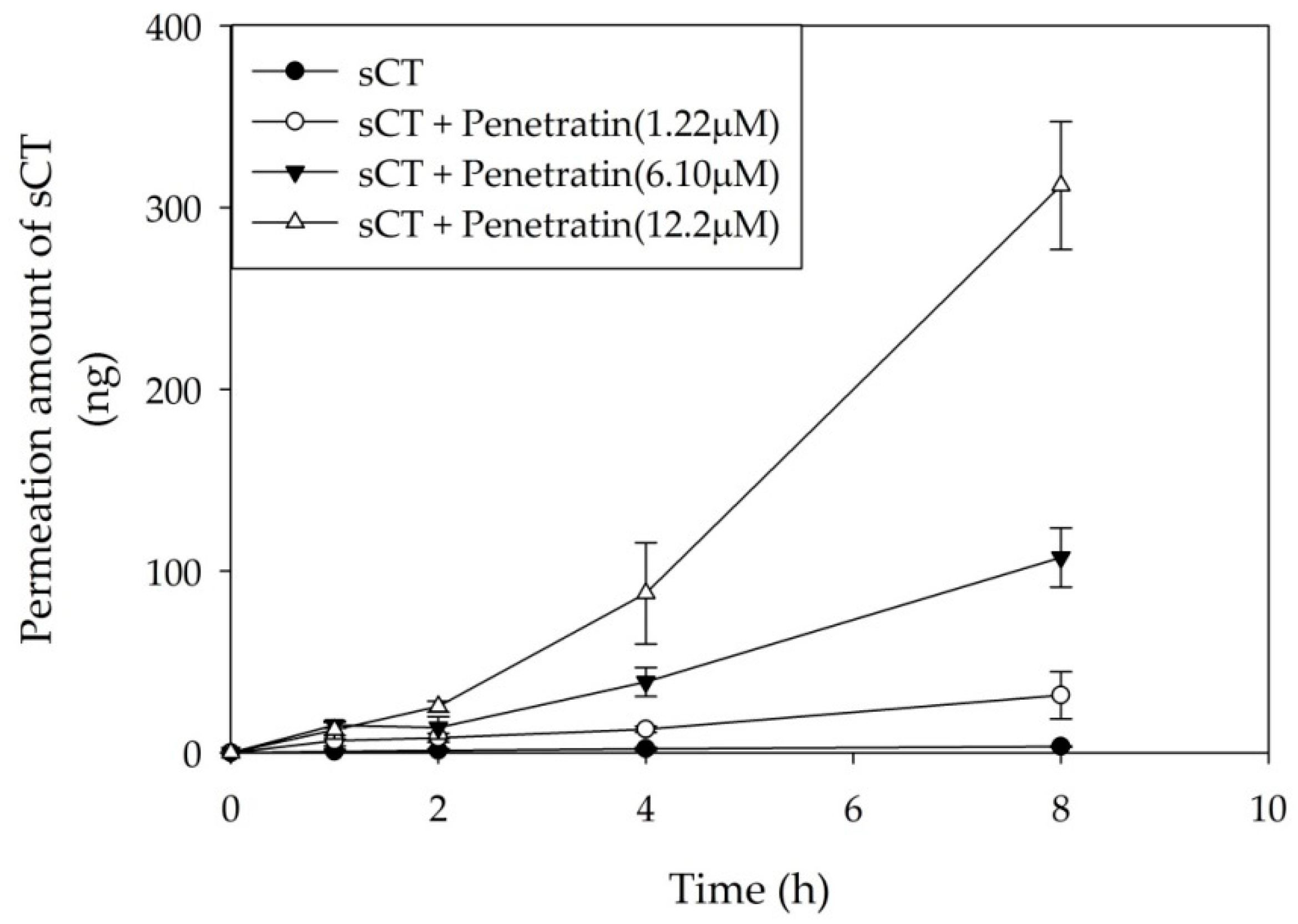
2.4. Ex Vivo Buccal Tissues Permeation
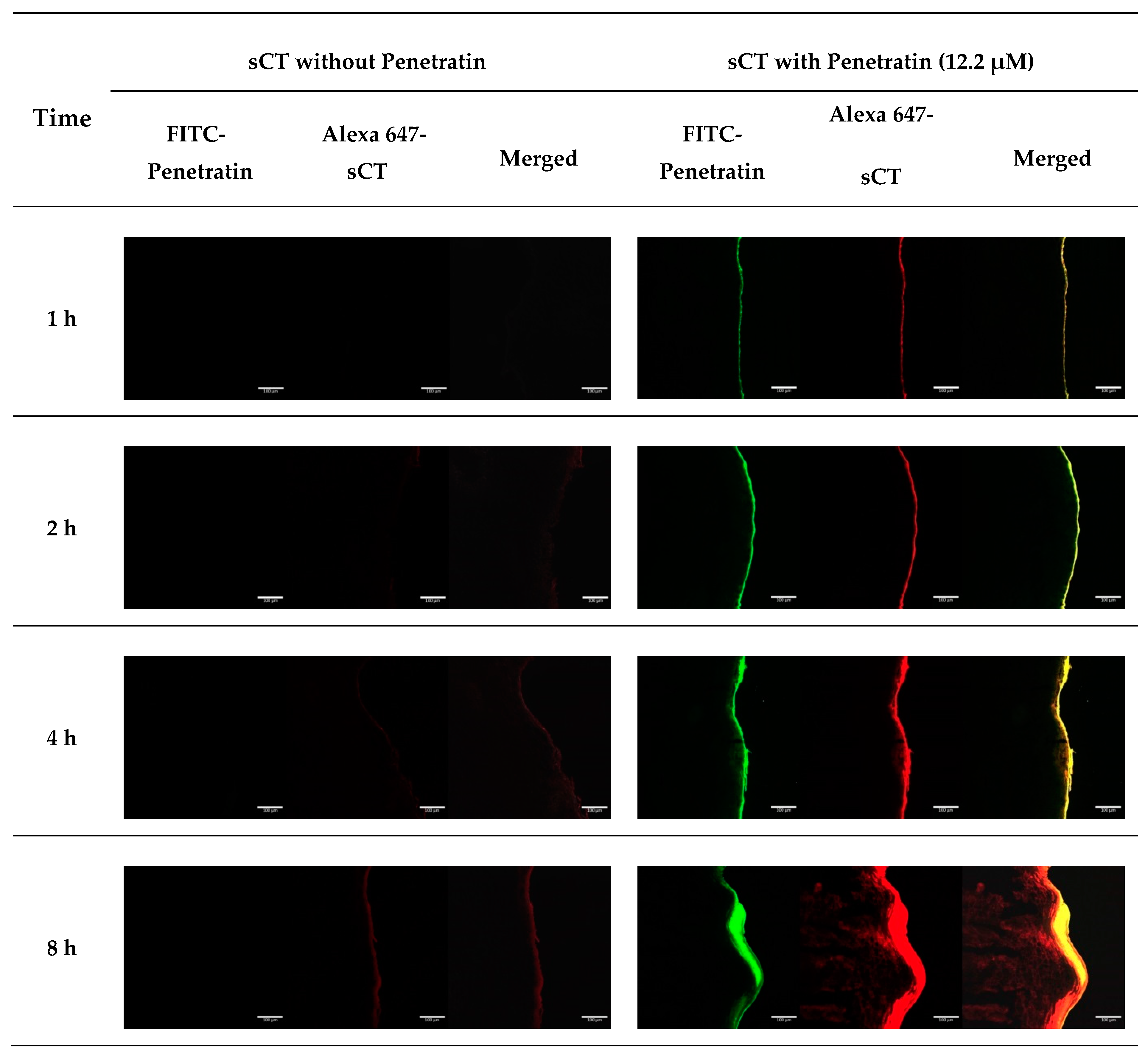
2.5. CLSM Study Using Alexa 647-sCT and FITC-Penetratin in Buccal Tissues
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. TR146 Cell Culture
4.2.2. Cytotoxicity Assay
4.2.3. FITC-Penetratin and Alexa 647-sCT Internalization in TR146 Cell
Flow Cytometry
Confocal Laser Scanning Microscopy
4.2.4. In Vitro Cell Permeation Study
4.2.5. Pretreatment of Porcine Buccal Tissues
4.2.6. Ex Vivo Buccal Tissues Permeation
4.2.7. Permeation Parameters
4.2.8. CLSM Study Using Alexa 647-sCT and FITC-Penetratin in Buccal Tissues
4.2.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghosh, D.; Peng, X.; Leal, J.; Mohanty, R.P. Peptides as drug delivery vehicles across biological barriers. J. Pharm. Investig. 2018, 48, 89–111. [Google Scholar] [CrossRef]
- Cho Lee, A.-R. Microneedle-mediated delivery of cosmeceutically relevant nucleoside and peptides in human skin: Challenges and strategies for dermal delivery. J. Pharm. Investig. 2019, 49, 587–601. [Google Scholar] [CrossRef]
- Palem, C.R.; Gannu, R.; Doodipala, N.; Yamsani, V.V.; Yamsani, M.R. Transmucosal delivery of domperidone from bilayered buccal patches: In vitro, ex vivo and in vivo characterization. Arch Pharm. Res. 2011, 34, 1701–1710. [Google Scholar] [CrossRef]
- Veuillez, F.; Kalia, Y.; Jacques, Y.; Deshusses, J.; Buri, P. Factors and strategies for improving buccal absorption of peptides. Eur. J. Pharm. Biopharm. 2001, 51, 93–109. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261. [Google Scholar] [CrossRef]
- Mathias, N.R.; Hussain, M.A. Non-invasive systemic drug delivery: Developability considerations for alternate routes of administration. J. Pharm. Sci. 2010, 99, 1–20. [Google Scholar] [CrossRef]
- Merkle, H.P.; Wolany, G. Buccal delivery for peptide drugs. J. Control. Release 1992, 21, 155–164. [Google Scholar] [CrossRef]
- Gao, M.; Shen, X.; Mao, S. Factors influencing drug deposition in the nasal cavity upon delivery via nasal sprays. J. Pharm. Investig. 2020, 50, 251–259. [Google Scholar] [CrossRef]
- Padula, C.; Pescina, S.; Nicoli, S.; Santi, P. New Insights on the Mechanism of Fatty Acids as Buccal Permeation Enhancers. Pharmaceutics 2018, 10, 201. [Google Scholar] [CrossRef]
- Wanasathop, A.; Li, S. Iontophoretic drug delivery in the oral cavity. Pharmaceutics 2018, 10, 121. [Google Scholar] [CrossRef]
- Batista, P.; Castro, P.; Madureira, A.R.; Sarmento, B.; Pintado, M. Development and Characterization of Chitosan Microparticles-in-Films for Buccal Delivery of Bioactive Peptides. Pharmaceuticals 2019, 12, 32. [Google Scholar] [CrossRef]
- Eleftheriadis, G.; Monou, P.K.; Andriotis, E.; Mitsouli, E.; Moutafidou, N.; Markopoulou, C.; Bouropoulos, N.; Fatouros, D. Development and Characterization of Inkjet Printed Edible Films for Buccal Delivery of B-Complex Vitamins. Pharmaceuticals 2020, 13, 203. [Google Scholar] [CrossRef]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-penetrating peptides: From basic research to clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef]
- Lopes, L.B.; Brophy, C.M.; Furnish, E.; Flynn, C.R.; Sparks, O.; Komalavilas, P.; Joshi, L.; Panitch, A.; Bentley, M.V.L. Comparative study of the skin penetration of protein transduction domains and a conjugated peptide. Pharm. Res. 2005, 22, 750–757. [Google Scholar] [CrossRef]
- Al-azzawi, S.; Masheta, D. Designing a drug delivery system for improved tumor treatment and targeting by functionalization of a cell-penetrating peptide. J. Pharm. Investig. 2019, 49, 643–654. [Google Scholar] [CrossRef]
- Bashyal, S.; Noh, G.; Keum, T.; Choi, Y.W.; Lee, S. Cell penetrating peptides as an innovative approach for drug delivery; then, present and the future. J. Pharm. Investig. 2016, 46, 205–220. [Google Scholar] [CrossRef]
- Mäe, M.; Langel, Ü. Cell-penetrating peptides as vectors for peptide, protein and oligonucleotide delivery. Curr. Opin. Pharmacol. 2006, 6, 509–514. [Google Scholar] [CrossRef]
- Tréhin, R.; Krauss, U.; Beck-Sickinger, A.G.; Merkle, H.P.; Nielsen, H.M. Cellular uptake but low permeation of human calcitonin-derived cell penetrating peptides and Tat (47-57) through well-differentiated epithelial models. Pharm. Res. 2004, 21, 1248–1256. [Google Scholar] [CrossRef]
- He, H.; Sheng, J.; David, A.E.; Kwon, Y.M.; Zhang, J.; Huang, Y.; Wang, J.; Yang, V.C. The use of low molecular weight protamine chemical chimera to enhance monomeric insulin intestinal absorption. Biomaterials 2013, 34, 7733–7743. [Google Scholar] [CrossRef]
- Liang, J.F.; Yang, V.C. Insulin-cell penetrating peptide hybrids with improved intestinal absorption efficiency. Biochem. Biophys. Res. Commun. 2005, 335, 734–738. [Google Scholar] [CrossRef]
- Patel, L.N.; Wang, J.; Kim, K.-J.; Borok, Z.; Crandall, E.D.; Shen, W.-C. Conjugation with Cationic Cell-Penetrating Peptide Increases Pulmonary Absorption of Insulin. Mol. Pharm. 2009, 6, 492–503. [Google Scholar] [CrossRef]
- Sheng, J.; He, H.; Han, L.; Qin, J.; Chen, S.; Ru, G.; Li, R.; Yang, P.; Wang, J.; Yang, V.C. Enhancing insulin oral absorption by using mucoadhesive nanoparticles loaded with LMWP-linked insulin conjugates. J. Control. Release 2016, 233, 181–190. [Google Scholar] [CrossRef]
- Kamei, N.; Onuki, Y.; Takayama, K.; Takeda-Morishita, M. Mechanistic Study of the Uptake/Permeation of Cell-Penetrating Peptides Across a Caco-2 Monolayer and Their Stimulatory Effect on Epithelial Insulin Transport. J. Pharm. Sci. 2013, 102, 3998–4008. [Google Scholar] [CrossRef]
- Khafagy, E.-S.; Morishita, M.; Isowa, K.; Imai, J.; Takayama, K. Effect of cell-penetrating peptides on the nasal absorption of insulin. J. Control. Release 2009, 133, 103–108. [Google Scholar] [CrossRef]
- Nielsen, E.J.B.; Yoshida, S.; Kamei, N.; Iwamae, R.; Khafagy, E.-S.; Olsen, J.; Rahbek, U.L.; Pedersen, B.L.; Takayama, K.; Takeda-Morishita, M. In vivo proof of concept of oral insulin delivery based on a co-administration strategy with the cell-penetrating peptide penetratin. J. Control. Release 2014, 189, 19–24. [Google Scholar] [CrossRef]
- Kristensen, M.; Birch, D.; Mørck Nielsen, H. Applications and Challenges for Use of Cell-Penetrating Peptides as Delivery Vectors for Peptide and Protein Cargos. Int. J. Mol. Sci. 2016, 17, 185. [Google Scholar] [CrossRef]
- Nasrollahi, S.A.; Taghibiglou, C.; Azizi, E.; Farboud, E.S. Cell-penetrating peptides as a novel transdermal drug delivery system. Chem Biol Drug Des 2012, 80, 639–646. [Google Scholar] [CrossRef]
- Pescina, S.; Ostacolo, C.; Gomez-Monterrey, I.; Sala, M.; Bertamino, A.; Sonvico, F.; Padula, C.; Santi, P.; Bianchera, A.; Nicoli, S. Cell penetrating peptides in ocular drug delivery: State of the art. J. Control. Release 2018, 284, 84–102. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Shin, M.C.; Min, K.A.; Huang, Y.; Oh, E.; Moon, C. Cell-penetrating peptide-based non-invasive topical delivery systems. J. Pharm. Investig. 2018, 48, 77–87. [Google Scholar] [CrossRef]
- Copolovici, D.M.; Langel, K.; Eriste, E.; Langel, U. Cell-penetrating peptides: Design, synthesis, and applications. ACS Nano 2014, 8, 1972–1994. [Google Scholar] [CrossRef]
- Heitz, F.; Morris, M.C.; Divita, G. Twenty years of cell-penetrating peptides: From molecular mechanisms to therapeutics. Br. J. Pharmacol. 2009, 157, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Munyendo, W.L.; Lv, H.; Benza-Ingoula, H.; Baraza, L.D.; Zhou, J. Cell penetrating peptides in the delivery of biopharmaceuticals. Biomolecules 2012, 2, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Alhakamy, N.A.; Nigatu, A.S.; Berkland, C.J.; Ramsey, J.D. Noncovalently associated cell-penetrating peptides for gene delivery applications. Ther. Deliv. 2013, 4, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Meade, B.R.; Dowdy, S.F. Enhancing the cellular uptake of siRNA duplexes following noncovalent packaging with protein transduction domain peptides. Adv. Drug Deliv. Rev. 2008, 60, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.W.; Chan, M.H.; Hsu, H.R.; Liu, B.R.; Chen, C.P.; Chen, H.H.; Lee, H.J. Transdermal delivery of proteins mediated by non-covalently associated arginine-rich intracellular delivery peptides. Exp. Dermatol. 2007, 16, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Raftery, R.M.; Walsh, D.P.; Blokpoel Ferreras, L.; Mencía Castaño, I.; Chen, G.; LeMoine, M.; Osman, G.; Shakesheff, K.M.; Dixon, J.E.; O’Brien, F.J. Highly versatile cell-penetrating peptide loaded scaffold for efficient and localised gene delivery to multiple cell types: From development to application in tissue engineering. Biomaterials 2019, 216, 119277. [Google Scholar] [CrossRef]
- Gonçalves, E.; Kitas, E.; Seelig, J. Binding of oligoarginine to membrane lipids and heparan sulfate: Structural and thermodynamic characterization of a cell-penetrating peptide. Biochemistry 2005, 44, 2692–2702. [Google Scholar] [CrossRef]
- Dom, G.; Shaw-Jackson, C.; Matis, C.; Bouffioux, O.; Picard, J.J.; Prochiantz, A.; Mingeot-Leclercq, M.-P.; Brasseur, R.; Rezsohazy, R. Cellular uptake of Antennapedia Penetratin peptides is a two-step process in which phase transfer precedes a tryptophan-dependent translocation. Nucleic Acids Res. 2003, 31, 556–561. [Google Scholar] [CrossRef]
- Bashyal, S.; Seo, J.-E.; Keum, T.; Noh, G.; Choi, Y.W.; Lee, S. Facilitated permeation of insulin across TR146 cells by cholic acid derivatives-modified elastic bilosomes. Int. J. Nanomed. 2018, 13, 5173–5186. [Google Scholar] [CrossRef]
- Jacobsen, J.; van Deurs, B.; Pedersen, M.; Rassing, M.R. TR146 cells grown on filters as a model for human buccal epithelium: I. Morphology, growth, barrier properties, and permeability. Int. J. Pharm. 1995, 125, 165–184. [Google Scholar] [CrossRef]
- Tréhin, R.; Nielsen, H.M.; Jahnke, H.G.; Krauss, U.; Beck-Sickinger, A.G.; Merkle, H.P. Metabolic cleavage of cell-penetrating peptides in contact with epithelial models: Human calcitonin (hCT)-derived peptides, Tat(47–57) and penetratin(43–58). Biochem. J. 2004, 382, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-H.; Chun, K.-H.; Jeon, S.-O.; Kang, J.-W.; Lee, S. Enhanced transbuccal salmon calcitonin (sCT) delivery: Effect of chemical enhancers and electrical assistance on in vitro sCT buccal permeation. Eur. J. Pharm. Biopharm. 2011, 79, 357–363. [Google Scholar] [CrossRef] [PubMed]





| sCT (μg) | Concentration of Penetratin (μM) | Js (ng·cm−2·h−1) | Kp (cm·h−2) × 10−3 | ER |
|---|---|---|---|---|
| 40 | 0 | 16.091 ± 0.560 | 0.201 ± 0.007 | 1.0 |
| 1.22 | 22.004 ± 3.247 | 0.275 ± 0.041 | 1.4 | |
| 6.10 | 54.075 ± 5.053 | 0.676 ± 0.063 | 3.4 | |
| 12.2 | 89.190 ± 11.227 | 1.115 ± 0.140 | 5.5 |
| sCT (μg) | Concentration of Penetratin (μM) | Recovery (%) | ||
|---|---|---|---|---|
| Before | After | |||
| 40 | 0 | 62.72 ± 5.20 | 60.48 ± 5.88 | 96.31 ± 1.78 |
| 1.22 | 63.47 ± 3.99 | 59.73 ± 2.94 | 94.21 ± 1.90 | |
| 6.10 | 61.23 ± 6.00 | 60.11 ± 6.00 | 98.17 ± 2.64 | |
| 12.2 | 63.84 ± 6.42 | 61.60 ± 6.62 | 96.52 ± 4.97 | |
| sCT (μg) | Concentration of Penetratin (μM) | Js (ng·cm−2·h−1) | Kp (cm·h−2) × 10−3 | ER |
|---|---|---|---|---|
| 40 | 0 | 0.208 ± 0.018 | 0.005 ± 0.000 | 1.0 |
| 1.22 | 1.973 ± 0.660 | 0.049 ± 0.017 | 9.5 | |
| 6.10 | 6.707 ± 0.832 | 0.168 ± 0.021 | 32.2 | |
| 12.2 | 19.507 ± 1.794 | 0.488 ± 0.045 | 93.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keum, T.; Noh, G.; Seo, J.-E.; Bashyal, S.; Lee, S. In Vitro and Ex Vivo Evaluation of Penetratin as a Non-invasive Permeation Enhancer in the Penetration of Salmon Calcitonin through TR146 Buccal Cells and Porcine Buccal Tissues. Pharmaceuticals 2020, 13, 408. https://doi.org/10.3390/ph13110408
Keum T, Noh G, Seo J-E, Bashyal S, Lee S. In Vitro and Ex Vivo Evaluation of Penetratin as a Non-invasive Permeation Enhancer in the Penetration of Salmon Calcitonin through TR146 Buccal Cells and Porcine Buccal Tissues. Pharmaceuticals. 2020; 13(11):408. https://doi.org/10.3390/ph13110408
Chicago/Turabian StyleKeum, Taekwang, Gyubin Noh, Jo-Eun Seo, Santosh Bashyal, and Sangkil Lee. 2020. "In Vitro and Ex Vivo Evaluation of Penetratin as a Non-invasive Permeation Enhancer in the Penetration of Salmon Calcitonin through TR146 Buccal Cells and Porcine Buccal Tissues" Pharmaceuticals 13, no. 11: 408. https://doi.org/10.3390/ph13110408
APA StyleKeum, T., Noh, G., Seo, J.-E., Bashyal, S., & Lee, S. (2020). In Vitro and Ex Vivo Evaluation of Penetratin as a Non-invasive Permeation Enhancer in the Penetration of Salmon Calcitonin through TR146 Buccal Cells and Porcine Buccal Tissues. Pharmaceuticals, 13(11), 408. https://doi.org/10.3390/ph13110408





