Liraglutide Activates Glucagon-Like Peptide 1 Receptor to Attenuate Hyperglycemia through Endogenous Beta-Endorphin in Diabetic Rats
Abstract
1. Introduction
2. Results
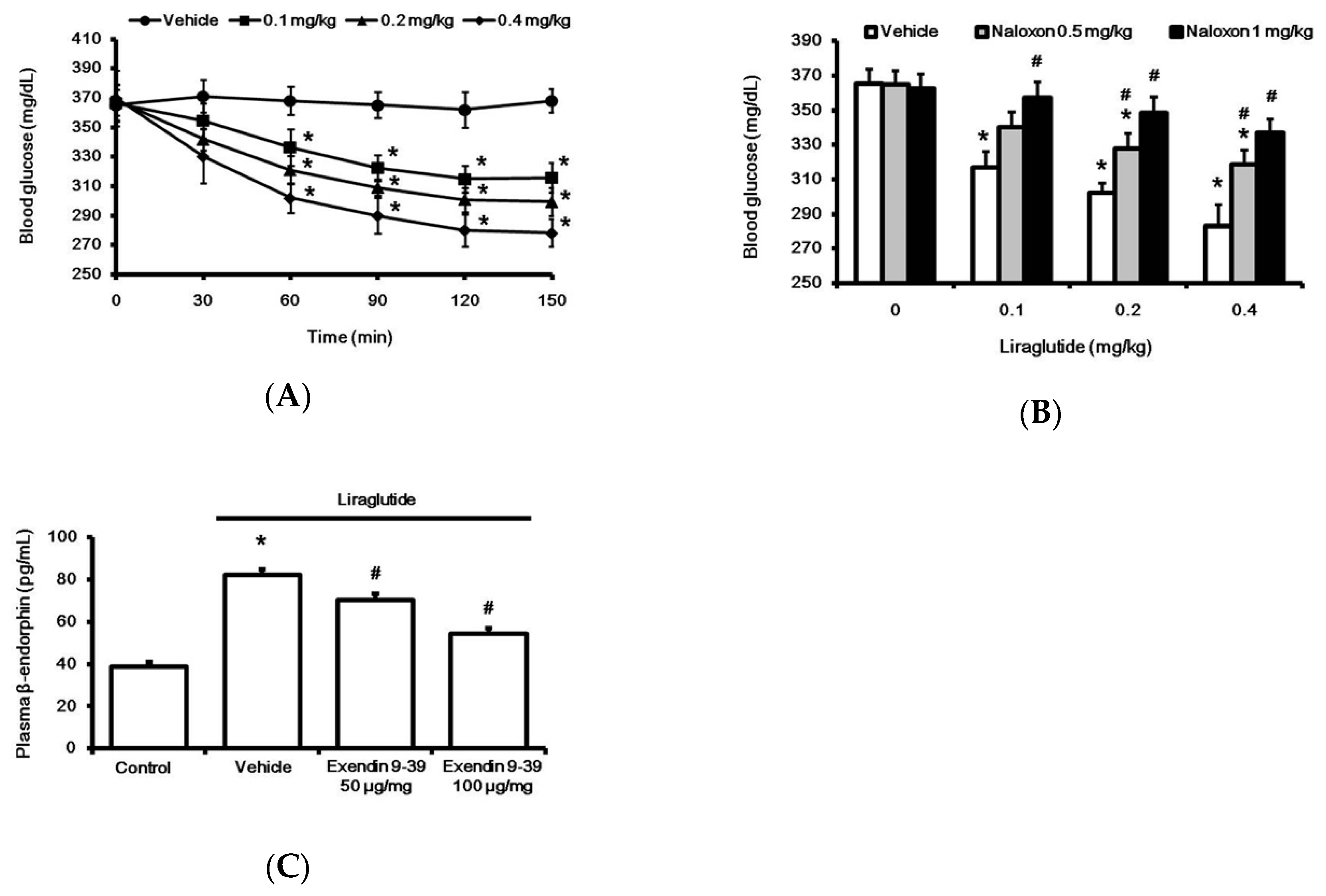
2.1. Liraglutide Attenuates Hyperglycemia with Increased Plasma BER Levels in Rats with Type-1 Diabetes
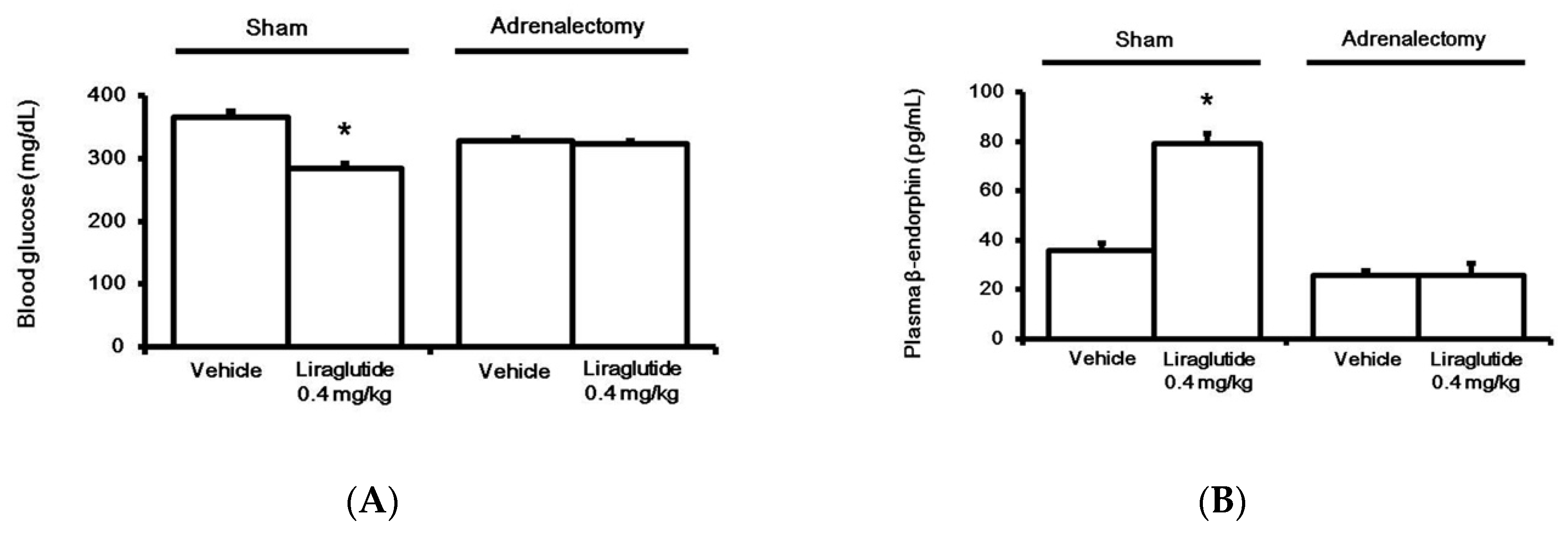
2.2. Liraglutide Induced BER Secretion from Adrenal Gland in Rats with Type-1 Diabetes
2.3. Direct Effect of Liraglutide on The Adrenal Medulla to Induce BER Secretion In Vitro
2.4. Liraglutide Modified Glucose Homeostasis through BER in Rats with Type-1 Diabetes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Model
4.3. Laboratory Determinations
4.4. Plasma BER Level in Rats with STZ-induced Diabetes
4.5. Adrenalectomy of Rats with STZ-induced Diabetes
4.6. Isolation of Adrenal Medulla
4.7. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
4.8. Western Blotting Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Knudsen, L.B.; Nielsen, P.F.; Huusfeldt, P.O.; Johansen, N.L.; Madsen, K.; Pedersen, F.Z.; Thogersen, H.; Wilken, M.; Agerso, H. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J. Med. Chem. 2000, 43, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Montanya, E.; Sesti, G. A review of efficacy and safety data regarding the use of liraglutide, a once-daily human glucagon-like peptide 1 analogue, in the treatment of type 2 diabetes mellitus. Clin. Ther. 2009, 31, 2472–2488. [Google Scholar] [CrossRef] [PubMed]
- Rutti, S.; Sauter, N.S.; Bouzakri, K.; Prazak, R.; Halban, P.A.; Donath, M.Y. In vitro proliferation of adult human beta-cells. PLoS ONE 2012, 7, e35801. [Google Scholar] [CrossRef]
- McClean, P.L.; Holscher, C. Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology 2014, 76 Pt A, 57–67. [Google Scholar] [CrossRef]
- Raun, K.; von Voss, P.; Gotfredsen, C.F.; Golozoubova, V.; Rolin, B.; Knudsen, L.B. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes 2007, 56, 8–15. [Google Scholar] [CrossRef]
- Nikolaidis, L.A.; Mankad, S.; Sokos, G.G.; Miske, G.; Shah, A.; Elahi, D.; Shannon, R.P. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 2004, 109, 962–965. [Google Scholar] [CrossRef]
- Hattori, Y.; Jojima, T.; Tomizawa, A.; Satoh, H.; Hattori, S.; Kasai, K.; Hayashi, T. A glucagon-like peptide-1 (GLP-1) analogue, liraglutide, upregulates nitric oxide production and exerts anti-inflammatory action in endothelial cells. Diabetologia 2010, 53, 2256–2263. [Google Scholar] [CrossRef]
- Buse, J.B.; Sesti, G.; Schmidt, W.E.; Montanya, E.; Chang, C.T.; Xu, Y.; Blonde, L.; Rosenstock, J. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care 2010, 33, 1300–1303. [Google Scholar] [CrossRef]
- Plutzky, J.; Garber, A.J.; Falahati, A.; Toft, A.D.; Poulter, N.R. The once-daily human GLP-1 analogue, liraglutide, significantly reduces markers of cardiovascular risk in type 2 diabetes: A meta-analysis of six clinical trials. Eur. Heart J. 2009, 30, 917. [Google Scholar]
- Rowlands, J.; Heng, J.; Newsholme, P.; Carlessi, R. Pleiotropic Effects of GLP-1 and Analogs on Cell Signaling, Metabolism, and Function. Front. Endocrinol. 2018, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- Seufert, J.; Gallwitz, B. The extra-pancreatic effects of GLP-1 receptor agonists: A focus on the cardiovascular, gastrointestinal and central nervous systems. Diabetes Obes. Metab. 2014, 16, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. JAMA 2016, 315, 1624–1645. [Google Scholar] [CrossRef] [PubMed]
- Radosevich, P.M.; Williams, P.E.; McRae, J.R.; Lacy, W.W.; Orth, D.N.; Abumrad, N.N. Beta-endorphin inhibits glucose production in the conscious dog. J. Clin. Investig. 1984, 73, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Shieh, J.P.; Cheng, K.C.; Chung, H.H.; Kerh, Y.F.; Yeh, C.H.; Cheng, J.T. Plasma glucose lowering mechanisms of catalpol, an active principle from roots of Rehmannia glutinosa, in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2011, 59, 3747–3753. [Google Scholar] [CrossRef]
- Liu, I.M.; Liou, S.S.; Cheng, J.T. Mediation of beta-endorphin by myricetin to lower plasma glucose in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2006, 104, 199–206. [Google Scholar] [CrossRef]
- Liu, I.M.; Cheng, J.T. Mediation of Endogenous beta-Endorphin in the Plasma Glucose-Lowering Action of Herbal Products Observed in Type 1-Like Diabetic Rats. Evid.-Based Complement. Altern. Med. eCAM 2011, 2011, 987876. [Google Scholar] [CrossRef]
- Jia, Y.; Gong, N.; Li, T.F.; Zhu, B.; Wang, Y.X. Peptidic exenatide and herbal catalpol mediate neuroprotection via the hippocampal GLP-1 receptor/beta-endorphin pathway. Pharmacol. Res. 2015, 102, 276–285. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Yi, X.; Liu, C.; Kong, D.; Zhang, J.; Gong, M. Myricetin: A potent approach for the treatment of type 2 diabetes as a natural class B GPCR agonist. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 2603–2611. [Google Scholar] [CrossRef]
- Krieger, J.P.; Langhans, W.; Lee, S.J. Novel role of GLP-1 receptor signaling in energy expenditure during chronic high fat diet feeding in rats. Physiol. Behav. 2018, 192, 194–199. [Google Scholar] [CrossRef]
- Pinyo, J.; Hira, T.; Hara, H. Enhanced postprandial glucagon-like peptide-1 secretion during obesity development has a protective role against glucose intolerance induction in rats. Br. J. Nutr. 2019, 122, 411–422. [Google Scholar] [CrossRef]
- Hsu, C.C.; Lin, M.H.; Cheng, J.T.; Wu, M.C. Antihyperglycaemic action of diosmin, a citrus flavonoid, is induced through endogenous beta-endorphin in type I-like diabetic rats. Clin. Exp. Pharmacol. Physiol. 2017, 44, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]
- Graaf, C.; Donnelly, D.; Wootten, D.; Lau, J.; Sexton, P.M.; Miller, L.J.; Ahn, J.M.; Liao, J.; Fletcher, M.M.; Yang, D.; et al. Glucagon-Like Peptide-1 and Its Class B G Protein-Coupled Receptors: A Long March to Therapeutic Successes. Pharmacol. Rev. 2016, 68, 954–1013. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.M.; Steen, O. Semaglutide: Review and Place in Therapy for Adults with Type 2 Diabetes. Can. J. Diabetes 2019, 43, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A. Binding selectivity profiles for ligands of multiple receptor types: Focus on opioid receptors. Trends Pharmacol. Sci. 1987, 8, 456–459. [Google Scholar] [CrossRef]
- Hsu, C.T.; Liu, I.M.; Cheng, J.T. Increase of beta-endorphin biosynthesis in the adrenal gland of streptozotocin-induced diabetic rats. Neurosci. Lett. 2002, 318, 57–60. [Google Scholar] [CrossRef]
- Cheng, J.T.; Liu, I.M.; Chi, T.C.; Tzeng, T.F.; Lu, F.H.; Chang, C.J. Plasma glucose-lowering effect of tramadol in streptozotocin-induced diabetic rats. Diabetes 2001, 50, 2815–2821. [Google Scholar] [CrossRef]
- Johansen, O.; Vaaler, S.; Jorde, R.; Reikeras, O. Increased plasma glucose levels after Hypnorm anaesthesia, but not after Pentobarbital anaesthesia in rats. Lab. Anim. 1994, 28, 244–248. [Google Scholar] [CrossRef]
- Hwang, S.L.; Liu, I.M.; Tzeng, T.F.; Cheng, J.T. Activation of imidazoline receptors in adrenal gland to lower plasma glucose in streptozotocin-induced diabetic rats. Diabetologia 2005, 48, 767–775. [Google Scholar] [CrossRef]
- Kuo, S.C.; Li, Y.; Cheng, Y.Z.; Lee, W.J.; Cheng, J.T.; Cheng, K.C. Molecular mechanisms regarding potassium bromateinduced cardiac hypertrophy without apoptosis in H9c2 cells. Mol. Med. Rep. 2018, 18, 4700–4708. [Google Scholar] [CrossRef]
- Niu, H.S.; Chao, P.C.; Ku, P.M.; Niu, C.S.; Lee, K.S.; Cheng, J.T. Amarogentin ameliorates diabetic disorders in animal models. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 1215–1223. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, K.-C.; Li, Y.-X.; Shieh, P.-C.; Cheng, J.-T.; Hsu, C.-C. Liraglutide Activates Glucagon-Like Peptide 1 Receptor to Attenuate Hyperglycemia through Endogenous Beta-Endorphin in Diabetic Rats. Pharmaceuticals 2020, 13, 407. https://doi.org/10.3390/ph13110407
Cheng K-C, Li Y-X, Shieh P-C, Cheng J-T, Hsu C-C. Liraglutide Activates Glucagon-Like Peptide 1 Receptor to Attenuate Hyperglycemia through Endogenous Beta-Endorphin in Diabetic Rats. Pharmaceuticals. 2020; 13(11):407. https://doi.org/10.3390/ph13110407
Chicago/Turabian StyleCheng, Kai-Chun, Ying-Xiao Li, Po-Chuen Shieh, Juei-Tang Cheng, and Chia-Chen Hsu. 2020. "Liraglutide Activates Glucagon-Like Peptide 1 Receptor to Attenuate Hyperglycemia through Endogenous Beta-Endorphin in Diabetic Rats" Pharmaceuticals 13, no. 11: 407. https://doi.org/10.3390/ph13110407
APA StyleCheng, K.-C., Li, Y.-X., Shieh, P.-C., Cheng, J.-T., & Hsu, C.-C. (2020). Liraglutide Activates Glucagon-Like Peptide 1 Receptor to Attenuate Hyperglycemia through Endogenous Beta-Endorphin in Diabetic Rats. Pharmaceuticals, 13(11), 407. https://doi.org/10.3390/ph13110407




