Central Serous Chorioretinopathy: Treatment with Laser
Abstract
1. Introduction
2. Results
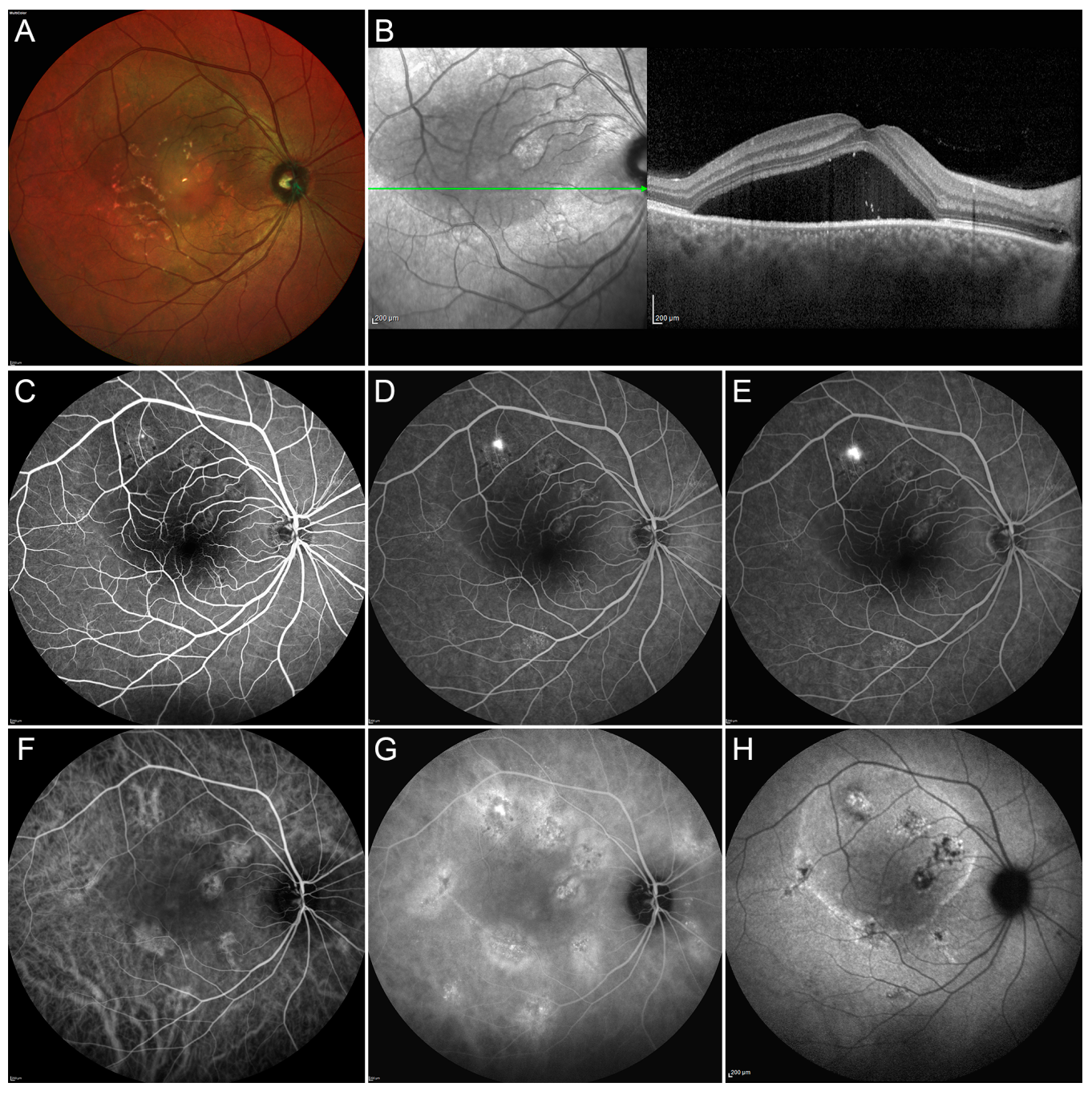
2.1. Conventional Laser Treatment
2.2. Subthreshold Laser Treatment (STLT)
2.2.1. Mechanism of Action
2.2.2. Outcomes and Safety of STLT
2.3. Subthreshold Laser Treatment versus PDT
2.4. STLT versus Conventional Laser Treatment
2.5. STLT versus Eplerenone
2.6. STLT versus Antivascular Endothelial Growth Factor (VEGF)
2.7. Subvisible Damaging Laser
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iacono, P.; Battaglia Parodi, M.; Falcomatà, B.; Bandello, F. Central Serous Chorioretinopathy Treatments: A Mini Review. Ophthalmic Res. 2015, 55, 76–83. [Google Scholar] [CrossRef]
- Inomata, H. Wound healing after xenon arc photocoagulation in the rabbit retina. Identification of the proliferating cells in the lesion by light and electron microscopic autoradiography using 3H-thymidine. Ophthalmologica 1975, 170, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.N.; McNaught, E.I.; Foulds, W.S. Effect of photocoagulation on the barrier function of the pigment epithelium. II. A study by electron microscopy. Trans. Ophthalmol. Soc. UK 1977, 97, 640–651. [Google Scholar] [PubMed]
- Daruich, A.; Matet, A.; Dirani, A.; Bousquet, E.; Zhao, M.; Farman, N.; Jaisser, F.; Behar-Cohen, F. Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog. Retin. Eye Res. 2015, 48, 82–118. [Google Scholar] [CrossRef]
- Lim, J.W.; Kang, S.W.; Kim, Y.-T.; Chung, S.E.; Lee, S.W. Comparative study of patients with central serous chorioretinopathy undergoing focal laser photocoagulation or photodynamic therapy. Br. J. Ophthalmol. 2011, 95, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Leaver, P.; Williams, C. Argon laser photocoagulation in the treatment of central serous retinopathy. Br. J. Ophthalmol. 1979, 63, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Brancato, R.; Pratesi, R.; Leoni, G.; Trabucchi, G.; Vanni, U. Histopathology of diode and argon laser lesions in rabbit retina: A comparative study. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1504–1510. [Google Scholar]
- Loo, R.H.; Scott, I.U.; Flynn, H.W., Jr.; Gass, J.D.; Murray, T.G.; Lewis, M.L.; Rosenfeld, P.J.; Smiddy, W.E. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina 2002, 22, 19–24. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Dorin, G. Subthreshold Diode Micropulse Laser Photocoagulation (SDM) as Invisible Retinal Phototherapy for Diabetic Macular Edema: A Review. Curr. Diabetes Rev. 2012, 8, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Verma, L.; Sinha, R.; Venkatesh, P.; Tewari, H.K. Comparative evaluation of diode laser versus argon laser photocoagulation in patients with central serous retinopathy: A pilot, randomized controlled trial [ISRCTN84128484]. BMC Ophthalmol. 2004, 4, 15. [Google Scholar] [CrossRef]
- Lanzetta, P.; Furlan, F.; Morgante, L.; Veritti, D.; Bandello, F. Nonvisible subthreshold micropulse diode laser (810 nm) treatment of central serous chorioretinopathy: A pilot study. Eur. J. Ophthalmol. 2008, 18, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Hwang, J.F.; Tseng, L.F.; Lin, C.J. Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmology 2008, 115, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Scholz, P.; Ersoy, L.; Boon, C.J.; Fauser, S. Subthreshold micropulse laser (577 nm) treatment in chronic central serous chorioretinopathy. Ophthalmologica 2015, 234, 189–194. [Google Scholar] [CrossRef]
- Yadav, N.K.; Jayadev, C.; Mohan, A.; Vijayan, P.; Battu, R.; Dabir, S.; Shetty, B.; Shetty, R. Subthreshold micropulse yellow laser (577 nm) in chronic central serous chorioretinopathy: Safety profile and treatment outcome. Eye 2015, 29, 258–264. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, H.S.; Kim, S.Y. Short-term efficacy of subthreshold micropulse yellow laser (577 nm) photocoagulation for chronic central serous chorioretinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 2129–2135. [Google Scholar] [CrossRef]
- Luttrull, J.K. Low-intensity/high-density subthreshold diode micropulse laser for central serous chorioretinopathy. Retina 2016, 36, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Arsan, A.; Kanar, H.S.; Sonmez, A. Visual outcomes and anatomic changes after sub-threshold micropulse yellow laser (577 nm) treatment for chronic central serous chorioretinopathy: Long-term follow-up. Eye 2018, 32, 726–733. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, S.Y.; Ha, S.; Moon, D.; Seong, S.; Kwon, O.W.; Park, H.S. Short-duration multiple-session subthreshold micropulse yellow laser (577 nm) for chronic central serous chorioretinopathy: Results at 3 years. Eye 2019, 33, 819–825. [Google Scholar] [CrossRef]
- Işık, M.U.; Değirmenci, M.F.K.; Sağlık, A. Efficacy of the subthreshold micropulse yellow wavelength laser photostimulation in the treatment of chronic central serous chorioretinopathy. Int. J. Ophthalmol. 2020, 13, 1404–1410. [Google Scholar] [CrossRef]
- Van Dijk, E.H.; Fauser, S.; Breukink, M.B.; Blanco-Garavito, R.; Groenewoud, J.M.; Keunen, J.E.; Peters, P.J.; Dijkman, G.; Souied, E.H.; MacLaren, R.E.; et al. Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy: The PLACE trial. Ophthalmology 2018, 125, 1547–1555. [Google Scholar] [CrossRef]
- Scholz, P.; Altay, L.; Fauser, S. Comparison of subthreshold micropulse laser (577 nm) treatment and half-dose photodynamic therapy in patients with chronic central serous chorioretinopathy. Eye 2016, 30, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Özmert, E.; Demirel, S.; Yanık, Ö.; Batıoğlu, F. Low-Fluence Photodynamic Therapy versus Subthreshold Micropulse Yellow Wavelength Laser in the Treatment of Chronic Central Serous Chorioretinopathy. J. Ophthalmol. 2016, 2016, 3513794. [Google Scholar] [CrossRef]
- Roca, J.A.; Wu, L.; Fromow-Guerra, J.; Rodríguez, F.J.; Berrocal, M.H.; Rojas, S.; Lima, L.H.; Gallego-Pinazo, R.; Chhablani, J.; Arevalo, J.F.; et al. Yellow (577 nm) micropulse laser versus half-dose verteporfin photodynamic therapy in eyes with chronic central serous chorioretinopathy: Results of the Pan-American Collaborative Retina Study (PACORES) Group. Br. J. Ophthalmol. 2018, 102, 1696–1700. [Google Scholar] [CrossRef]
- Ntomoka, C.G.; Rajesh, B.; Muriithi, G.M.; Goud, A.; Chhablani, J. Comparison of photodynamic therapy and navigated microsecond laser for chronic central serous chorioretinopathy. Eye 2018, 32, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Lai, F.H.P.; Ng, D.S.C.; Iu, L.P.L.; Chen, L.J.; Mak, A.C.Y.; Yip, Y.; Cheung, C.; Young, A.L.; Brelen, M. Analysis of choriocapillaris perfusion and choroidal layer changes in patients with chronic central serous chorioretinopathy randomised to micropulse laser or photodynamic therapy. Br. J. Ophthalmol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kretz, F.T.; Beger, I.; Koch, F.; Nowomiejska, K.; Auffarth, G.U.; Koss, M.J. Randomized Clinical Trial to Compare Micropulse Photocoagulation Versus Half-dose Verteporfin Photodynamic Therapy in the Treatment of Central Serous Chorioretinopathy. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 837–843. [Google Scholar] [CrossRef]
- Battaglia Parodi, M.; Iacono, P. Re: Van Dijk et al.: Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy: The PLACE trial (Ophthalmology 2018, 125, 1547–1555). Ophthalmology 2019, 126, e29–e30. [Google Scholar] [CrossRef]
- Luttrull, J.K. Comment on: Focal and Diffuse Chronic Central Serous Chorioretinopathy Treated with Half-Dose Photodynamic Therapy or Subthreshold Micropulse Laser: PLACE Trial Report No. 3. Am. J. Ophthalmol. 2020, 212, 186–187. [Google Scholar] [CrossRef]
- Keunen, J.E.E.; Battaglia Parodi, M.; Vujosevic, S.; Luttrull, J.K. International Retinal Laser Society Guidelines for Subthreshold Laser Treatment. Transl. Vis. Sci. Technol. 2020, 9, 15. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, Y.; Nie, C.; Wang, Z.; Pei, J.; Lin, B.; Zhou, R.; Zhang, J.; Chong, V.; Liu, X. Efficacy and safety of subthreshold micropulse laser compared with threshold conventional laser in central serous chorioretinopathy. Eye 2020, 34, 1592–1599. [Google Scholar] [CrossRef]
- Maruko, I.; Koizumi, H.; Hasegawa, T.; Arakawa, H.; Iida, T. Subthreshold 577 nm micropulse laser treatment for central serous chorioretinopathy. PLoS ONE 2017, 12, e0184112. [Google Scholar] [CrossRef]
- Vignesh, T.P.; Maitray, A.; Sen, S.; Chakrabarti, A.; Kannan, N.B.; Ramasamy, K. Subthreshold Micro-Pulse Yellow Laser and Eplerenone Drug Therapy in Chronic Central Serous Chorio-Retinopathy Patients: A Comparative Study. Semin. Ophthalmol. 2020, 35, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Koss, M.J.; Beger, I.; Koch, F.H. Subthreshold diode laser micropulse photocoagulation versus intravitreal injections of bevacizumab in the treatment of central serous chorioretinopathy. Eye 2012, 26, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Park, Y.G.; Kim, J.R.; Seifert, E.; Theisen-Kunde, D.; Brinkmann, R.; Roh, Y.J. Selective Retina Therapy in Patients with Chronic Central Serous Chorioretinopathy: A Pilot Study. Medicine (Baltim) 2016, 95, e2524. [Google Scholar] [CrossRef] [PubMed]
- Framme, C.; Walter, A.; Berger, L.; Prahs, P.; Alt, C.; Theisen-Kunde, D.; Kowal, J.; Brinkmann, R. Selective Retina Therapy in Acute and Chronic-Recurrent Central Serous Chorioretinopathy. Ophthalmologica 2015, 234, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Yasui, A.; Yamamoto, M.; Hirayama, K.; Shiraki, K.; Theisen-Kunde, D.; Brinkmann, R.; Miura, Y.; Kohno, T. Retinal sensitivity after selective retina therapy (SRT) on patients with central serous chorioretinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 243–254. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, Y.G.; Lee, D.W.; Kim, J.H. Selective Retina Therapy with Real-Time Feedback-Controlled Dosimetry for Treating Acute Idiopathic Central Serous Chorioretinopathy in Korean Patients. J. Ophthalmol. 2018, 2018, 6027871. [Google Scholar] [CrossRef]
- Park, Y.G.; Kang, S.; Kim, M.; Yoo, N.; Roh, Y.J. Selective retina therapy with automatic real-time feedback-controlled dosimetry for chronic central serous chorioretinopathy in Korean patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1375–1383. [Google Scholar] [CrossRef]
- Klatt, C.; Saeger, M.; Oppermann, T.; Pörksen, E.; Treumer, F.; Hillenkamp, J.; Fritzer, E.; Brinkmann, R.; Birngruber, R.; Roider, J. Selective retina therapy for acute central serous chorioretinopathy. Br. J. Ophthalmol. 2011, 95, 83–88. [Google Scholar] [CrossRef]
- Elsner, H.; Pörksen, E.; Klatt, C.; Bunse, A.; Theisen-Kunde, D.; Brinkmann, R.; Birngruber, R.; Laqua, H.; Roider, J. Selective retina therapy in patients with central serous chorioretinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 1638–1645. [Google Scholar] [CrossRef]




| Authors (P/R) | F-UP | N° Patients | BCVA | RS (dB) | NSD Resolution | NSD Recurrences | N° Treatments | Side Effects |
|---|---|---|---|---|---|---|---|---|
| Kim [18] (R) | 3.7 years | 27 | +0.18 LogMar | - | 81.5% | 6 (22%) | 2.5 | None |
| Isik [19] (R) | 11.4 months | 58 | +0.22 LogMar | - | 67.2% | 6 (10.3%) | 1–2 | None |
| Arsan [17] (P) | 17 months | 39 | +0.40 LogMar | - | 92.3% | 9 (23%) | 1.4 (1–4) | None |
| Lutrull [16] (R) | 14 months | 11 | Improved in all pts | - | 100% | 1 | 1 | None |
| Lanzetta [11] (P) | 14 months | 24 | +0.10 LogMar | - | 75% | - | 1 | None |
| Authors (P/R) | Protocol | F-UP | N° pts | BCVA | NSD Resolution | NSD Recurrences | N° Treatment | Side Effects |
|---|---|---|---|---|---|---|---|---|
| PLACE trial [20] (P) | HD-PDT | 7–8 months | 67 | +6.7 letters | 67.2 % | 4 (5%) | 1–2 | 0 |
| Scholz [21] (R) | HD-PDT | 6 weeks | 58 | +0.04 Logmar | 21% | - | 1–2 | 1 CNV |
| STLT-577 nm | 6 weeks | 42 | +0.08 Logmar | 36% | - | 1–2 | 0 | |
| Ozmert [22] (R) | HF-PDT | 1 year | 18 | +4 letters | 72% | 1 | 1–2 | 0 |
| STLT-Microsecond | 1 year | 15 | +4 letters | 80% | 2 | 1 | 1 Hypofluorescent spot | |
| Roca [23] (R) | HD-PDT | 17.4 months | 67 | +0.03 Logmar | 95.5% | - | 1 2 (5 eyes) 3 (1 eye) | 1 CNV |
| STLT-577 nm | 15.8 months | 92 | +0.21 Logmar | 92.4 % | - | 1 2 (12 eyes) 3 (2 eyes) 4 (2 eyes) | 0 | |
| Ntomoka [24] (R) | HF-PDT | 6 months | 22 | +0.1 Logmar | 21.7% | - | 1 | 0 |
| STLT-577 nm | 6 months | 23 | +0.2 Logmar | 59% | - | 1 | 0 | |
| Ho [25] (P) | HD-PDT | 6 months | 15 | +0.14 Logmar | - | - | 1 | - |
| STLT-577 nm | 6 months | 18 | +0.20 Logmar | - | - | 1 | - | |
| STLT-810nm | 7–8 months | 66 | +4.4 letters | 28.8% | 1 (1.3) | 1–2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battaglia Parodi, M.; Arrigo, A.; Iacono, P.; Falcomatà, B.; Bandello, F. Central Serous Chorioretinopathy: Treatment with Laser. Pharmaceuticals 2020, 13, 359. https://doi.org/10.3390/ph13110359
Battaglia Parodi M, Arrigo A, Iacono P, Falcomatà B, Bandello F. Central Serous Chorioretinopathy: Treatment with Laser. Pharmaceuticals. 2020; 13(11):359. https://doi.org/10.3390/ph13110359
Chicago/Turabian StyleBattaglia Parodi, Maurizio, Alessandro Arrigo, Pierluigi Iacono, Bruno Falcomatà, and Francesco Bandello. 2020. "Central Serous Chorioretinopathy: Treatment with Laser" Pharmaceuticals 13, no. 11: 359. https://doi.org/10.3390/ph13110359
APA StyleBattaglia Parodi, M., Arrigo, A., Iacono, P., Falcomatà, B., & Bandello, F. (2020). Central Serous Chorioretinopathy: Treatment with Laser. Pharmaceuticals, 13(11), 359. https://doi.org/10.3390/ph13110359






