Examining the Characteristics and Applications of Mesenchymal, Induced Pluripotent, and Embryonic Stem Cells for Tissue Engineering Approaches across the Germ Layers
Abstract
1. Introduction
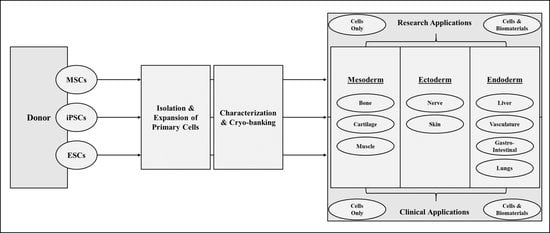
2. Stem Cell Basics
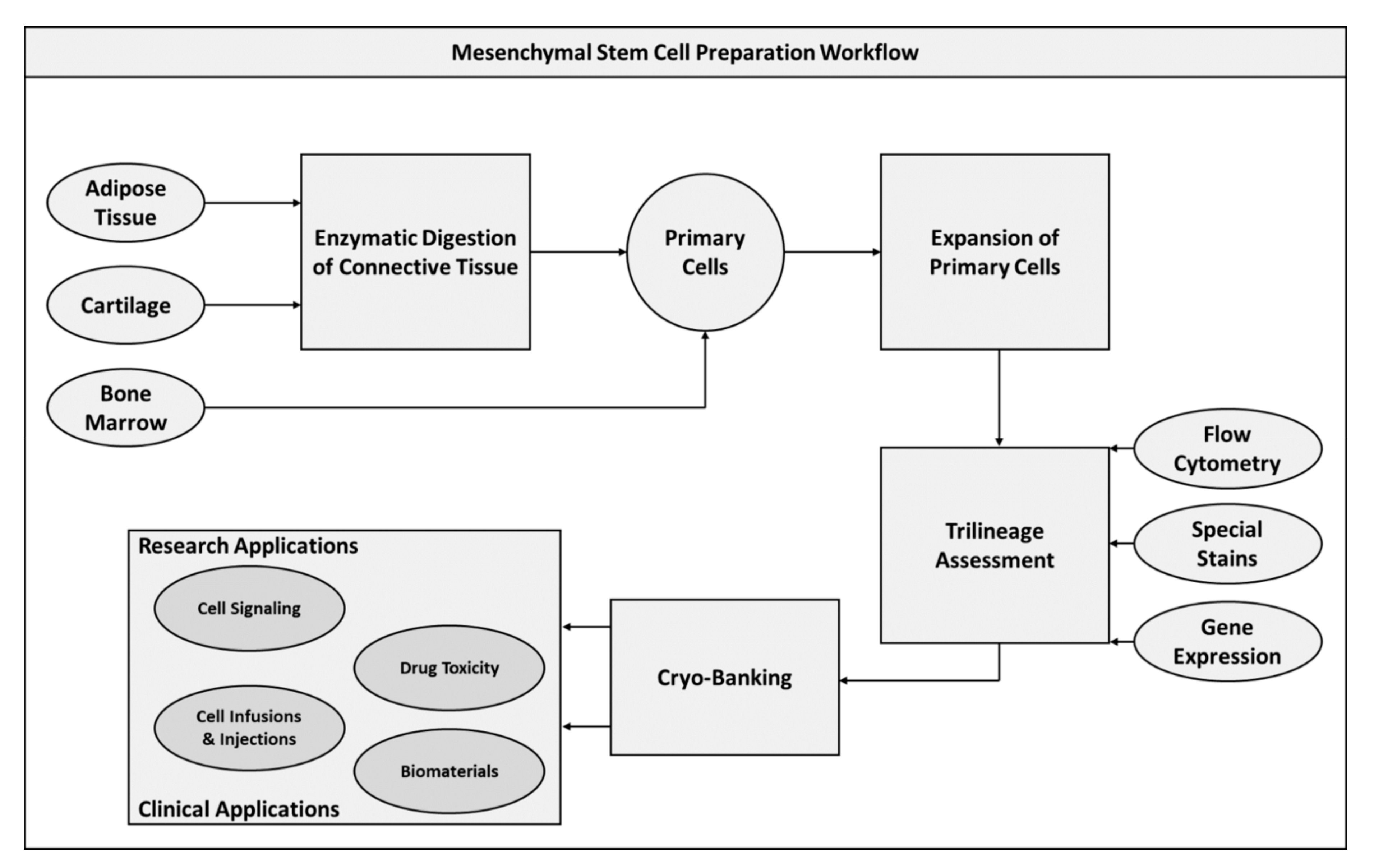
2.1. Mesenchymal Stem Cells
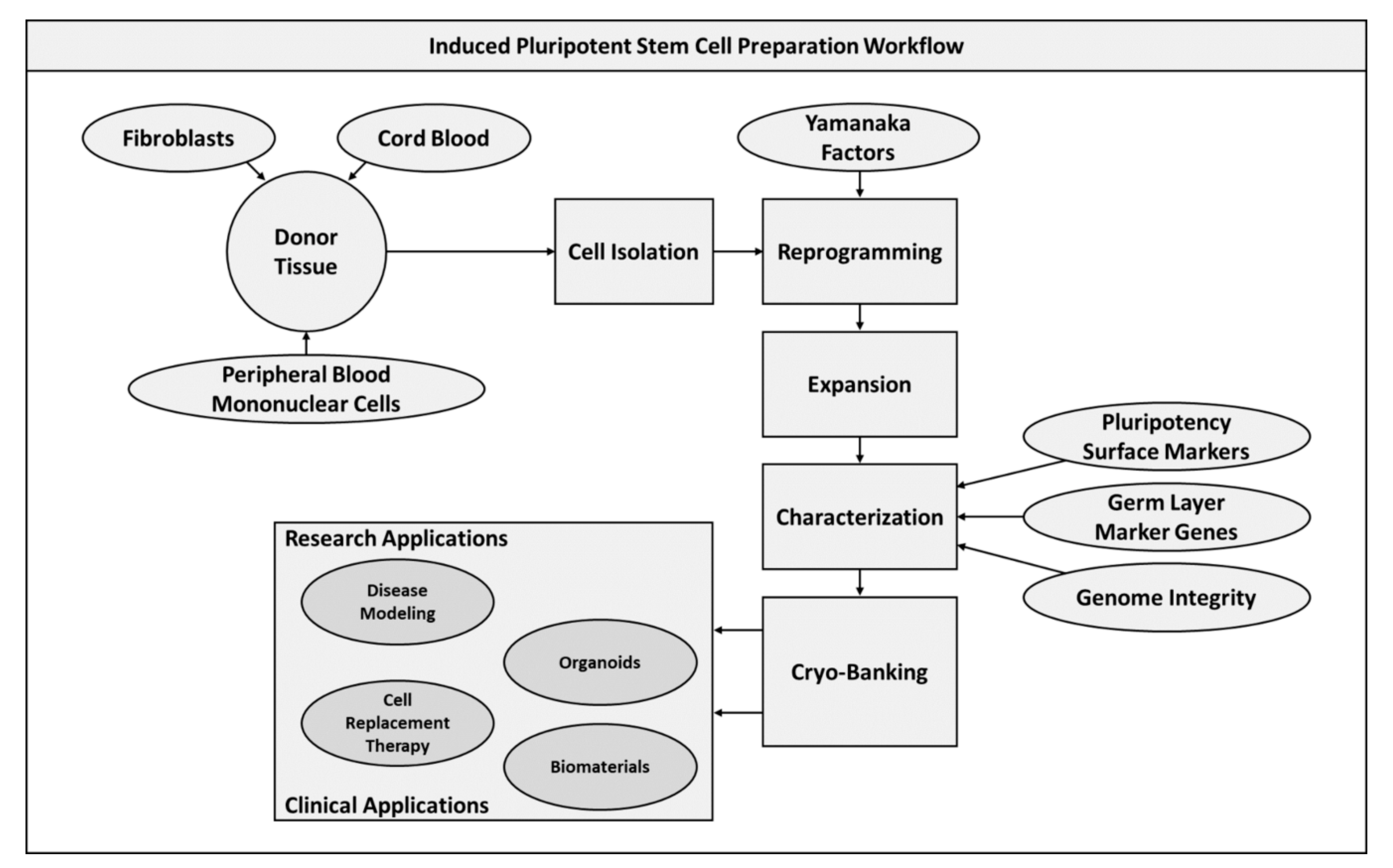
2.2. Induced Pluripotent Stem Cells
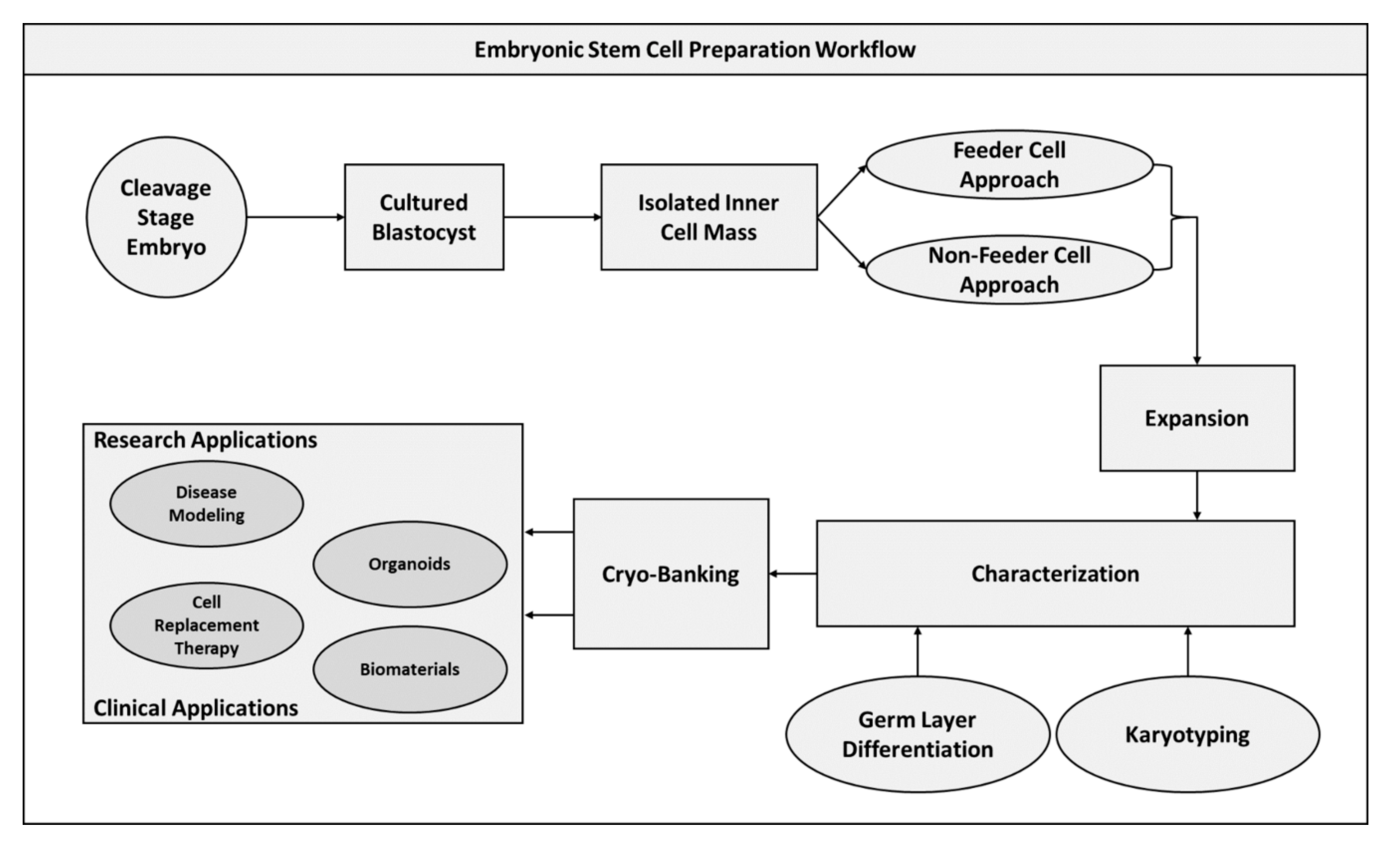
2.3. Embryonic Stem Cells
3. Applications of Stem Cells in Regenerative Medicine
3.1. Mesodermal Applications
3.1.1. Bone
3.1.2. Cartilage
3.1.3. Muscle
3.2. Ectodermal Applications
3.2.1. Nerve
3.2.2. Skin
3.3. Endodermal Applications
3.3.1. Liver
3.3.2. Vasculature
3.3.3. Gastro-Intestinal
3.3.4. Lungs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sobhani, A.; Khanlarkhani, N.; Baazm, M.; Mohammadzadeh, F.; Najafi, A.; Mehdinejadiani, S.; Aval, F.S. Multipotent stem cell and current application. Acta Med. Iran. 2017, 55, 6–23. [Google Scholar] [PubMed]
- Kalra, K.; Tomar, P. Stem cell: Basics, classification and applications. Am. J. Phytomedicine Clin. Ther. 2014, 2, 919–930. [Google Scholar]
- Bongso, A.; Lee, E.H. Stem cells: Their definition, classification and sources. Stem Cells Bench Bedside 2005, 1, 1–13. [Google Scholar]
- Arrighi, N. Definition and Classification of Stem Cells. Stem Cells 2018, 2018, 1–45. [Google Scholar]
- Ratajczak, M.Z.; Zuba-Surma, E.K.; Wojakowski, W.; Ratajczak, J.; Kucia, M. Bone marrow–home of versatile stem cells. Transfus. Med. Hemotherapy 2008, 35, 248–259. [Google Scholar] [CrossRef]
- Rodriguez, A.-M.; Elabd, C.; Amri, E.-Z.; Ailhaud, G.; Dani, C. The human adipose tissue is a source of multipotent stem cells. Biochimie 2005, 87, 125–128. [Google Scholar] [CrossRef]
- Dulak, J.; Szade, K.; Szade, A.; Nowak, W.; Józkowicz, A. Adult stem cells: Hopes and hypes of regenerative medicine. Acta Biochim. Pol. 2015, 62, 329–337. [Google Scholar] [CrossRef]
- Glicksman, M.A. Induced pluripotent stem cells: The most versatile source for stem cell therapy. Clin. Ther. 2018, 40, 1060–1065. [Google Scholar] [CrossRef]
- Mascetti, V.L.; Pedersen, R.A. Contributions of Mammalian Chimeras to Pluripotent Stem Cell Research. Cell Stem Cell 2016, 19, 163–175. [Google Scholar] [CrossRef]
- Zunder, E.R.; Lujan, E.; Goltsev, Y.; Wernig, M.; Nolan, G.P. A continuous molecular roadmap to iPSC reprogramming through progression analysis of single-cell mass cytometry. Cell Stem Cell 2015, 16, 323–337. [Google Scholar] [CrossRef]
- Rao, M.S.; Malik, N. Assessing iPSC reprogramming methods for their suitability in translational medicine. J. Cell. Biochem. 2012, 113, 3061–3068. [Google Scholar] [CrossRef]
- Martins, J.G.; Camargo, S.E.; Bishop, T.T.; Popat, K.C.; Kipper, M.J.; Martins, A.F. Pectin-chitosan membrane scaffold imparts controlled stem cell adhesion and proliferation. Carbohydr. Polym. 2018, 197, 47–56. [Google Scholar] [CrossRef]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 942–965. [Google Scholar] [CrossRef] [PubMed]
- Damaraju, S.M.; Shen, Y.; Elele, E.; Khusid, B.; Eshghinejad, A.; Li, J.; Jaffe, M.; Arinzeh, T.L. Three-dimensional piezoelectric fibrous scaffolds selectively promote mesenchymal stem cell differentiation. Biomaterials 2017, 149, 51–62. [Google Scholar] [CrossRef]
- Bow, A.; Anderson, D.E.; Dhar, M. Commercially available bone graft substitutes: The impact of origin and processing on graft functionality. Drug Metab. Rev. 2019, 51, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, J.A.; Yang, Z.; Wu, Y.N.; Lee, E.H. Towards standardized stem cell therapy in type 2 diabetes mellitus: A systematic review. Curr. Stem Cell Res. Ther. 2018, 13, 476–488. [Google Scholar] [CrossRef]
- Smith, J.R.; Pfeifer, K.; Petry, F.; Powell, N.; Delzeit, J.; Weiss, M.L. Standardizing umbilical cord mesenchymal stromal cells for translation to clinical use: Selection of GMP-compliant medium and a simplified isolation method. Stem Cells Int. 2016, 2016, 6810980. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, X.; Yang, X.; Ei-Hashash, A. Stem cells in lung repair and regeneration: Current applications and future promise. J. Cell. Physiol. 2018, 233, 6414–6424. [Google Scholar] [CrossRef]
- Wang, L.; Tran, I.; Seshareddy, K.; Weiss, M.L.; Detamore, M.S. A Comparison of Human Bone Marrow–Derived Mesenchymal Stem Cells and Human Umbilical Cord–Derived Mesenchymal Stromal Cells for Cartilage Tissue Engineering. Tissue Eng. Part A 2009, 15, 2259–2266. [Google Scholar] [CrossRef]
- Mortada, I.; Mortada, R. Epigenetic changes in mesenchymal stem cells differentiation. Eur. J. Med. Genet. 2018, 61, 114–118. [Google Scholar] [CrossRef]
- Ranera, B.; Lyahyai, J.; Romero, A.; Vázquez, F.J.; Remacha, A.R.; Bernal, M.L.; Zaragoza, P.; Rodellar, C.; Martín-Burriel, I. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet. Immunol. Immunopathol. 2011, 144, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.-J.; Tuan, R.S.; Cheung, K.M.C.; Leung, V.Y.L. Concise Review: The Surface Markers and Identity of Human Mesenchymal Stem Cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2018, 19, 360. [Google Scholar] [CrossRef] [PubMed]
- Elkhenany, H.; Amelse, L.; Caldwell, M.; Abdelwahed, R.; Dhar, M. Impact of the source and serial passaging of goat mesenchymal stem cells on osteogenic differentiation potential: Implications for bone tissue engineering. J. Anim. Sci. Biotechnol. 2016, 7, 16. [Google Scholar] [CrossRef]
- Elkhenany, H.; Bourdo, S.; Biris, A.; Anderson, D.; Dhar, M. Important Considerations in the Therapeutic Application of Stem Cells in Bone Healing and Regeneration. Stem Cells Toxicol. Med. 2016, 458–480. [Google Scholar] [CrossRef]
- Guo, X.; Bai, Y.; Zhang, L.; Zhang, B.; Zagidullin, N.; Carvalho, K.; Du, Z.; Cai, B. Cardiomyocyte differentiation of mesenchymal stem cells from bone marrow: New regulators and its implications. Stem Cell Res. Ther. 2018, 9, 44. [Google Scholar] [CrossRef]
- De La Garza-Rodea, A.S.; Van Der Velde-Van Dijke, I.; Boersma, H.; Gonçalves, M.A.F.V.; Van Bekkum, D.W.; De Vries, A.A.F.; Knaän-Shanzer, S. Myogenic Properties of Human Mesenchymal Stem Cells Derived from Three Different Sources. Cell Transp. 2012, 21, 153–173. [Google Scholar] [CrossRef]
- Witt, R.; Weigand, A.; Boos, A.; Cai, A.; Dippold, D.; Boccaccini, A.; Schubert, D.; Hardt, M.; Lange, C.; Arkudas, A. Mesenchymal stem cells and myoblast differentiation under HGF and IGF-1 stimulation for 3D skeletal muscle tissue engineering. BMC Cell Biol. 2017, 18, 15. [Google Scholar] [CrossRef]
- Park, Y.G.; Baek, A.M.; Do, B.R.; Choi, J.H.; Do Kim, S. Myogenic Differentiation of Human Adipose-Derived Stem Cells. J. Korean Acad. Rehabil. Med. 2011, 35, 8–13. [Google Scholar]
- Stöckl, S.; Bauer, R.J.; Bosserhoff, A.K.; Göttl, C.; Grifka, J.; Grässel, S. Sox9 modulates cell survival and adipogenic differentiation of multipotent adult rat mesenchymal stem cells. J. Cell Sci. 2013, 126, 2890. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Schiller, P.C.; Ricordi, C.; Roos, B.A.; Howard, G.A. Age-Related Osteogenic Potential of Mesenchymal Stromal Stem Cells from Human Vertebral Bone Marrow. J. Bone Miner. Res. 1999, 14, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.K.; Zhu, M.; Wulur, I.; Alfonso, Z. Adipose-Derived Stem Cells. In Mesenchymal Stem Cells: Methods and Protocols; Prockop, D.J., Bunnell, B.A., Phinney, D.G., Eds.; Humana Press: Totowa, NJ, USA, 2008; pp. 59–67. [Google Scholar]
- Kolaparthy, L.K.; Sanivarapu, S.; Moogla, S.; Kutcham, R.S. Adipose Tissue—Adequate, Accessible Regenerative Material. Int. J. Stem Cells 2015, 8, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H. Adipose-derived stem cells for tissue repair and regeneration: Ten years of research and a literature review. J. Nippon Med. Sch. 2009, 76, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Stensløkken, K.O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef]
- Xu, G.-P.; Zhang, X.-F.; Sun, L.; Chen, E.-M. Current and future uses of skeletal stem cells for bone regeneration. World J. Stem Cells 2020, 12, 339–350. [Google Scholar] [CrossRef]
- Ambrosi, T.H.; Longaker, M.T.; Chan, C.K.F. A Revised Perspective of Skeletal Stem Cell Biology. Front. Cell Dev. Biol. 2019, 7, 189. [Google Scholar] [CrossRef]
- Halim, T.Y.; Rana, B.M.; Walker, J.A.; Kerscher, B.; Knolle, M.D.; Jolin, H.E.; Serrao, E.M.; Haim-Vilmovsky, L.; Teichmann, S.A.; Rodewald, H.-R. Tissue-restricted adaptive type 2 immunity is orchestrated by expression of the costimulatory molecule OX40L on group 2 innate lymphoid cells. Immunity 2018, 48, 1195–1207. [Google Scholar] [CrossRef]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Gong, L.; Yu, D.; An, C.; Bunpetch, V.; Dai, J.; Huang, H.; Zou, X.; Ouyang, H.; et al. The Plasticity of Mesenchymal Stem Cells in Regulating Surface HLA-I. iScience 2019, 15, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Ogilvie, C. Concise review: Human embryonic stem cells-what have we done. What are we ng? Stem Cells 2017, 35, 17–25. [Google Scholar] [CrossRef]
- Zheng, Y.L. Some ethical concerns about human induced pluripotent stem cells. Sci. Eng. Ethics 2016, 22, 1277–1284. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Su, Y.; Huang, C.; Yin, Y.; Chu, A.; Knupp, A.; Tang, Y. NANOG and LIN28 dramatically improve human cell reprogramming by modulating LIN41 and canonical WNT activities. Biol. Open 2019, 8, bio047225. [Google Scholar] [CrossRef]
- Lin, T.; Ambasudhan, R.; Yuan, X.; Li, W.; Hilcove, S.; Abujarour, R.; Lin, X.; Hahm, H.S.; Hao, E.; Hayek, A.; et al. A chemical platform for improved induction of human iPSCs. Nat. Methods 2009, 6, 805–808. [Google Scholar] [CrossRef]
- Yilmazer, A.; de Lázaro, I.; Bussy, C.; Kostarelos, K. In vivo Reprogramming of Adult Somatic Cells to Pluripotency by Overexpression of Yamanaka Factors. JoVE 2013, e50837. [Google Scholar] [CrossRef] [PubMed]
- Narsinh, K.H.; Plews, J.; Wu, J.C. Comparison of human induced pluripotent and embryonic stem cells: Fraternal or identical twins? Mol. Ther. 2011, 19, 635–638. [Google Scholar] [CrossRef]
- Gutierrez-Aranda, I.; Ramos-Mejia, V.; Bueno, C.; Munoz-Lopez, M.; Real, P.J.; Mácia, A.; Sanchez, L.; Ligero, G.; Garcia-Parez, J.L.; Menendez, P. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells (Dayt. Ohio) 2010, 28, 1568–1570. [Google Scholar] [CrossRef] [PubMed]
- Ohnuki, M.; Takahashi, K.; Yamanaka, S. Generation and Characterization of Human Induced Pluripotent Stem Cells. Curr. Protoc. Stem Cell Biol. 2009, 9, 4A.2.1–4A.2.25. [Google Scholar] [CrossRef]
- Paull, D.; Sevilla, A.; Zhou, H.; Hahn, A.K.; Kim, H.; Napolitano, C.; Tsankov, A.; Shang, L.; Krumholz, K.; Jagadeesan, P.; et al. Automated, high-throughput derivation, characterization and differentiation of induced pluripotent stem cells. Nat. Methods 2015, 12, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Yamanaka, S. Recent Stem Cell Advances: Induced Pluripotent Stem Cells for Disease Modeling and Stem Cell–Based Regeneration. Circulation 2010, 122, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Chaudhari, P.; Jang, Y.-Y. Applications of patient-specific induced pluripotent stem cells; focused on disease modeling, drug screening and therapeutic potentials for liver disease. Int. J. Biol. Sci. 2010, 6, 796–805. [Google Scholar] [CrossRef]
- Marchetto, M.C.; Brennand, K.J.; Boyer, L.F.; Gage, F.H. Induced pluripotent stem cells (iPSCs) and neurological disease modeling: Progress and promises. Hum. Mol. Genet. 2011, 20, R109–R115. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Yang, Y.; Shi, Y.; Chen, J.; Gao, R.; Fan, Y.; Yao, H.; Liao, W.; Sun, X.-F.; Gao, S. Modeling Dravet syndrome using induced pluripotent stem cells (iPSCs) and directly converted neurons. Hum. Mol. Genet. 2013, 22, 4241–4252. [Google Scholar] [CrossRef]
- Sayed, N.; Liu, C.; Wu, J.C. Translation of Human-Induced Pluripotent Stem Cells. J. Am. Coll. Cardiol. 2016, 67, 2161. [Google Scholar] [CrossRef]
- Pera, M.F.; Reubinoff, B.; Trounson, A. Human embryonic stem cells. J. Cell Sci. 2000, 113, 5–10. [Google Scholar]
- Biehl, J.K.; Russell, B. Introduction to stem cell therapy. J. Cardiovasc. Nurs. 2009, 24, 98–103. [Google Scholar] [CrossRef]
- Chen, T.; Wang, F.; Wu, M.; Wang, Z.Z. Development of hematopoietic stem and progenitor cells from human pluripotent stem cells. J. Cell. Biochem. 2015, 116, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145. [Google Scholar] [CrossRef]
- Klimanskaya, I.; Chung, Y.; Becker, S.; Lu, S.-J.; Lanza, R. Derivation of human embryonic stem cells from single blastomeres. Nat. Protoc. 2007, 2, 1963. [Google Scholar] [CrossRef] [PubMed]
- Niakan, K.K.; Han, J.; Pedersen, R.A.; Simon, C.; Pera, R.A.R. Human pre-implantation embryo development. Development 2012, 139, 829–841. [Google Scholar] [CrossRef]
- Glasser, S.R.; Julian, J.; Munir, M.I.; Soares, M.J. Biological markers during early pregnancy: Trophoblastic signals of the peri-implantation period. Environ. Health Perspect. 1987, 74, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Sagi, I.; Chia, G.; Golan-Lev, T.; Peretz, M.; Weissbein, U.; Sui, L.; Sauer, M.V.; Yanuka, O.; Egli, D.; Benvenisty, N. Derivation and differentiation of haploid human embryonic stem cells. Nature 2016, 532, 107–111. [Google Scholar] [CrossRef]
- Eiselleova, L.; Peterkova, I.; Neradil, J.; Slaninova, I.; Hampl, A.; Dvorak, P. Comparative study of mouse and human feeder cells for human embryonic stem cells. Int. J. Dev. Biol. 2004, 52, 353–363. [Google Scholar] [CrossRef]
- Klimanskaya, I.; Chung, Y.; Meisner, L.; Johnson, J.; West, M.D.; Lanza, R. Human embryonic stem cells derived without feeder cells. Lancet 2005, 365, 1636–1641. [Google Scholar] [CrossRef]
- Suemori, H.; Yasuchika, K.; Hasegawa, K.; Fujioka, T.; Tsuneyoshi, N.; Nakatsuji, N. Efficient establishment of human embryonic stem cell lines and long-term maintenance with stable karyotype by enzymatic bulk passage. Biochem. Biophys. Res. Commun. 2006, 345, 926–932. [Google Scholar] [CrossRef]
- Lefort, N.; Perrier, A.L.; Laâbi, Y.; Varela, C.; Peschanski, M. Human embryonic stem cells and genomic instability. Regen. Med. 2009, 4, 899–909. [Google Scholar] [CrossRef]
- Heins, N.; Englund, M.C.O.; Sjöblom, C.; Dahl, U.; Tonning, A.; Bergh, C.; Lindahl, A.; Hanson, C.; Semb, H. Derivation, Characterization, and Differentiation of Human Embryonic Stem Cells. Stem Cells 2004, 22, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.M.; Carpenter, M.K. Characterization and culture of human embryonic stem cells. Nat. Biotechnol. 2005, 23, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Colman, A.; Dreesen, O. Pluripotent Stem Cells and Disease Modeling. Cell Stem Cell 2009, 5, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Stevens, K.R.; Pabon, L.; Muskheli, V.; Murry, C.E. Scaffold-Free Human Cardiac Tissue Patch Created from Embryonic Stem Cells. Tissue Eng. Part A 2008, 15, 1211–1222. [Google Scholar] [CrossRef]
- Lenzini, S.; Devine, D.; Shin, J.-W. Leveraging Biomaterial Mechanics to Improve Pluripotent Stem Cell Applications for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 260. [Google Scholar] [CrossRef]
- Vacanti, J. Tissue engineering and regenerative medicine: From first principles to state of the art. J. Pediatr. Surg. 2010, 45, 291–294. [Google Scholar] [CrossRef]
- Richardson, S.M.; Kalamegam, G.; Pushparaj, P.N.; Matta, C.; Memic, A.; Khademhosseini, A.; Mobasheri, R.; Poletti, F.L.; Hoyland, J.A.; Mobasheri, A. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods 2016, 99, 69–80. [Google Scholar] [CrossRef]
- Ha, S.-W.; Weiss, D.; Weitzmann, M.N.; Beck, G.R., Jr. Applications of silica-based nanomaterials in dental and skeletal biology. In Nanobiomaterials in Clinical Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 77–112. [Google Scholar]
- Zareidoost, A.; Yousefpour, M.; Ghaseme, B.; Amanzadeh, A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J. Mater. Sci. Mater. Med. 2012, 23, 1479–1488. [Google Scholar] [CrossRef]
- Le, X.; Poinern, G.E.J.; Ali, N.; Berry, C.M.; Fawcett, D. Engineering a biocompatible scaffold with either micrometre or nanometre scale surface topography for promoting protein adsorption and cellular response. Int. J. Biomater. 2013, 2013, 782549. [Google Scholar] [CrossRef]
- Abdel Meguid, E.; Ke, Y.; Ji, J.; El-Hashash, A.H.K. Stem cells applications in bone and tooth repair and regeneration: New insights, tools, and hopes. J. Cell. Physiol. 2018, 233, 1825–1835. [Google Scholar] [CrossRef]
- Chen, W.; Liu, X.; Chen, Q.; Bao, C.; Zhao, L.; Zhu, Z.; Xu, H.H.K. Angiogenic and osteogenic regeneration in rats via calcium phosphate scaffold and endothelial cell co-culture with human bone marrow mesenchymal stem cells (MSCs), human umbilical cord MSCs, human induced pluripotent stem cell-derived MSCs and human embryonic stem cell-derived MSCs. J. Tissue Eng. Regen. Med. 2018, 12, 191–203. [Google Scholar] [PubMed]
- Trubiani, O.; Marconi, G.D.; Pierdomenico, S.D.; Piattelli, A.; Diomede, F.; Pizzicannella, J. Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair. Int. J. Mol. Sci. 2019, 20, 4987. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, A.; Yin, X.; Dong, S.; Luo, F.; Dou, C.; Lan, X.; Xie, Z.; Hou, T.; Xu, J.; et al. Mesenchymal stem cells promote endothelial progenitor cell migration, vascularization, and bone repair in tissue-engineered constructs via activating CXCR2-Src-PKL/Vav2-Rac1. FASEB J. 2018, 32, 2197–2211. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef]
- Wofford, A.; Bow, A.; Newby, S.; Brooks, S.; Rodriguez, R.; Masi, T.; Stephenson, S.; Gotcher, J.; Anderson, D.E.; Campbell, J. Human Fat-Derived Mesenchymal Stem Cells Xenogenically Implanted in a Rat Model Show Enhanced New Bone Formation in Maxillary Alveolar Tooth Defects. Stem Cells Int. 2020, 2020, 8142938. [Google Scholar] [CrossRef]
- Chen, M.; Xu, Y.; Zhang, T.; Ma, Y.; Liu, J.; Yuan, B.; Chen, X.; Zhou, P.; Zhao, X.; Pang, F.; et al. Mesenchymal stem cell sheets: A new cell-based strategy for bone repair and regeneration. Biotechnol. Lett. 2019, 41, 305–318. [Google Scholar] [CrossRef]
- Ono, N.; Kronenberg, H.M. Bone repair and stem cells. Curr. Opin. Genet. Dev. 2016, 40, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Kumar, S.; Webster, T.J.; Ramalingam, M. Impact of Induced Pluripotent Stem Cells in Bone Repair and Regeneration. Curr. Osteoporos. Rep. 2019, 17, 226–234. [Google Scholar] [CrossRef]
- Hayashi, K.; Ochiai-Shino, H.; Shiga, T.; Onodera, S.; Saito, A.; Shibahara, T.; Azuma, T. Transplantation of human-induced pluripotent stem cells carried by self-assembling peptide nanofiber hydrogel improves bone regeneration in rat calvarial bone defects. BDJ Open 2016, 2, 15007. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Li, C.; Weir, M.D.; Wang, P.; Reynolds, M.A.; Zhao, L.; Xu, H.H.K. Injectable calcium phosphate with hydrogel fibers encapsulating induced pluripotent, dental pulp and bone marrow stem cells for bone repair. Mater. Sci. Eng. C 2016, 69, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, W.; Zhang, C.; Thein-Han, W.; Hu, K.; Reynolds, M.A.; Bao, C.; Wang, P.; Zhao, L.; Xu, H.H.K. Co-Seeding Human Endothelial Cells with Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells on Calcium Phosphate Scaffold Enhances Osteogenesis and Vascularization in Rats. Tissue Eng. Part A 2017, 23, 546–555. [Google Scholar] [CrossRef]
- Zou, L.; Chen, Q.; Quanbeck, Z.; Bechtold, J.E.; Kaufman, D.S. Angiogenic activity mediates bone repair from human pluripotent stem cell-derived osteogenic cells. Sci. Rep. 2016, 6, 22868. [Google Scholar] [CrossRef] [PubMed]
- Fellows, C.R.; Matta, C.; Zakany, R.; Khan, I.M.; Mobasheri, A. Adipose, Bone Marrow and Synovial Joint-Derived Mesenchymal Stem Cells for Cartilage Repair. Front. Genet. 2016, 7, 213. [Google Scholar] [CrossRef]
- De Bari, C.; Roelofs, A.J. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr. Opin. Pharmacol. 2018, 40, 74–80. [Google Scholar] [CrossRef]
- Lo Monaco, M.; Merckx, G.; Ratajczak, J.; Gervois, P.; Hilkens, P.; Clegg, P.; Bronckaers, A.; Vandeweerd, J.-M.; Lambrichts, I. Stem Cells for Cartilage Repair: Preclinical Studies and Insights in Translational Animal Models and Outcome Measures. Stem Cells Int. 2018, 2018, 9079538. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Ju, J.H. Application of Cord Blood and Cord Blood-Derived Induced Pluripotent Stem Cells for Cartilage Regeneration. Cell Transplant. 2018, 28, 529–537. [Google Scholar] [CrossRef]
- Nam, Y.; Rim, Y.A.; Lee, J.; Ju, J.H. Current Therapeutic Strategies for Stem Cell-Based Cartilage Regeneration. Stem Cells Int. 2018, 2018, 8490489. [Google Scholar] [CrossRef]
- Dubey, N.K.; Mishra, V.K.; Dubey, R.; Syed-Abdul, S.; Wang, J.R.; Wang, P.D.; Deng, W.-P. Combating Osteoarthritis through Stem Cell Therapies by Rejuvenating Cartilage: A Review. Stem Cells Int. 2018, 2018, 5421019. [Google Scholar] [CrossRef]
- Lietman, S.A. Induced pluripotent stem cells in cartilage repair. World J. Orthop. 2016, 7, 149–155. [Google Scholar] [CrossRef]
- Lee, W.Y.-W.; Wang, B. Cartilage repair by mesenchymal stem cells: Clinical trial update and perspectives. J. Orthop. Transl. 2017, 9, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, S.; Gao, C.; Liu, X.; Zhou, Y.; Liu, P.; Cai, J. Chemically defined serum-free conditions for cartilage regeneration from human embryonic stem cells. Life Sci. 2016, 164, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.; Talovic, M.; Patel, K.; Patel, A.; Marcinczyk, M.; Garg, K. Biomaterial and stem cell-based strategies for skeletal muscle regeneration. J. Orthop. Res. 2019, 37, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Uchiyama, Y.; Hirata, M.; Hashimoto, H.; Nakajima, N.; Saito, K.; Terachi, T.; Mochida, J. Therapeutic isolation and expansion of human skeletal muscle-derived stem cells for the use of muscle-nerve-blood vessel reconstitution. Front. Physiol. 2015, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Čamernik, K.; Mihelič, A.; Mihalič, R.; Marolt Presen, D.; Janež, A.; Trebše, R.; Marc, J.; Zupan, J. Skeletal-muscle-derived mesenchymal stem/stromal cells from patients with osteoarthritis show superior biological properties compared to bone-derived cells. Stem Cell Res. 2019, 38, 101465. [Google Scholar] [CrossRef]
- Beier, J.; Bitto, F.F.; Lange, C.; Klumpp, D.; Arkudas, A.; Bleiziffer, O.; Boos, A.M.; Horch, R.E.; Kneser, U. Myogenic differentiation of mesenchymal stem cells co-cultured with primary myoblasts. Cell Biol. Int. 2011, 35, 397–406. [Google Scholar] [CrossRef]
- Park, S.; Choi, Y.; Jung, N.; Yu, Y.; Ryu, K.-H.; Kim, H.S.; Jo, I.; Choi, B.-O.; Jung, S.-C. Myogenic differentiation potential of human tonsil-derived mesenchymal stem cells and their potential for use to promote skeletal muscle regeneration. Int. J. Mol. Med. 2016, 37, 1209–1220. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, N.; Zhang, J.; Liu, Y.; Zhu, D.; Kong, Y. Mesenchymal stem cells rejuvenate cardiac muscle through regulating macrophage polarization. Aging 2019, 11, 3900–3908. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, S.; Zhang, H.; Zhu, B.; Su, Y.; Zheng, C.; Tian, R.; Wang, M.; Kuang, H.; Zhao, X.; et al. Mesenchymal stem cells and extracellular matrix scaffold promote muscle regeneration by synergistically regulating macrophage polarization toward the M2 phenotype. Stem Cell Res. Ther. 2018, 9, 88. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Chang, T.-H.; Chang, S.-S.; Chang, G.-J.; Chen, A.C.-Y.; Cheng, C.-Y.; Chen, S.-C.; Fu, J.-F.; Wen, C.-J.; Chan, Y.-S. Application of Bone Marrow–Derived Mesenchymal Stem Cells for Muscle Healing After Contusion Injury in Mice. Am. J. Sports Med. 2020, 48, 1226–1235. [Google Scholar] [CrossRef]
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol. Ther. 2018, 26, 1610–1623. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Wang, H.-J.; Li, Z.-H.; Wang, Y.-L.; Wu, X.-P.; Tan, Y.-Z. Mesenchymal stem cell-loaded cardiac patch promotes epicardial activation and repair of the infarcted myocardium. J. Cell. Mol. Med. 2017, 21, 1751–1766. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Yuan, Y.; Sun, X.; Zhou, M.; Guo, G.; Zhang, Q.; Hescheler, J.; Xi, J. Skeletal Extracellular Matrix Supports Cardiac Differentiation of Embryonic Stem Cells: A Potential Scaffold for Engineered Cardiac Tissue. Cell. Physiol. Biochem. 2018, 45, 319–331. [Google Scholar] [CrossRef]
- Van der Wal, E.; Herrero-Hernandez, P.; Wan, R.; Broeders, M.; In’t Groen, S.L.M.; van Gestel, T.J.M.; van Ijcken, W.F.J.; Cheung, T.H.; van der Ploeg, A.T.; Schaaf, G.J.; et al. Large-Scale Expansion of Human iPSC-Derived Skeletal Muscle Cells for Disease Modeling and Cell-Based Therapeutic Strategies. Stem Cell Rep. 2018, 10, 1975–1990. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, S.; Miki, K.; Takaki, T.; Okubo, C.; Hatani, T.; Chonabayashi, K.; Nishikawa, M.; Takei, I.; Oishi, A.; Narita, M.; et al. Enhanced engraftment, proliferation and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci. Rep. 2016, 6, 19111. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.C.; Levi, K.; Schwan, J.; Luo, J.; Bartulos, O.; Wu, H.; Qiu, C.; Yi, T.; Ren, Y.; Campbell, S.; et al. Tissue-Engineered Vascular Rings from Human iPSC-Derived Smooth Muscle Cells. Stem Cell Rep. 2016, 7, 19–28. [Google Scholar] [CrossRef]
- Danisovic, L.; Culenova, M.; Csobonyeiova, M. Induced pluripotent stem cells for Duchenne muscular dystrophy modeling and therapy. Cells 2018, 7, 253. [Google Scholar] [CrossRef]
- Del Carmen Ortuño-Costela, M.; García-López, M.; Cerrada, V.; Gallardo, M.E. iPSCs: A powerful tool for skeletal muscle tissue engineering. J. Cell. Mol. Med. 2019, 23, 3784–3794. [Google Scholar] [CrossRef]
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int. J. Mol. Sci. 2019, 20, 2698. [Google Scholar] [CrossRef]
- Tasnim, N.; Thakur, V.; Chattopadhyay, M.; Joddar, B. The Efficacy of Graphene Foams for Culturing Mesenchymal Stem Cells and Their Differentiation into Dopaminergic Neurons. Stem Cells Int. 2018, 2018, 3410168. [Google Scholar] [CrossRef]
- Duncan, T.; Valenzuela, M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res. Ther. 2017, 8, 111. [Google Scholar] [CrossRef]
- Kikuchi, T.; Morizane, A.; Doi, D.; Onoe, H.; Hayashi, T.; Kawasaki, T.; Saiki, H.; Miyamoto, S.; Takahashi, J. Survival of Human Induced Pluripotent Stem Cell–Derived Midbrain Dopaminergic Neurons in the Brain of a Primate Model of Parkinson’s Disease. J. Parkinsons Dis. 2011, 1, 395–412. [Google Scholar] [CrossRef]
- Doi, D.; Magotani, H.; Kikuchi, T.; Ikeda, M.; Hiramatsu, S.; Yoshida, K.; Amano, N.; Nomura, M.; Umekage, M.; Morizane, A.; et al. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson’s disease. Nat. Commun. 2020, 11, 3369. [Google Scholar] [CrossRef]
- Barker, R.A.; Parmar, M.; Studer, L.; Takahashi, J. Human Trials of Stem Cell-Derived Dopamine Neurons for Parkinson’s Disease: Dawn of a New Era. Cell Stem Cell 2017, 21, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.M.; Rodrigues, G.M.C.; Kulkarni, R.U.; Rao, A.T.; Chernavsky, N.E.; Miller, E.W.; Schaffer, D.V. Efficient generation of hPSC-derived midbrain dopaminergic neurons in a fully defined, scalable, 3D biomaterial platform. Sci. Rep. 2017, 7, 40573. [Google Scholar] [CrossRef]
- Adil, M.M.; Rao, A.T.; Ramadoss, G.N.; Chernavsky, N.E.; Kulkarni, R.U.; Miller, E.W.; Kumar, S.; Schaffer, D.V. Dopaminergic Neurons Transplanted Using Cell-Instructive Biomaterials Alleviate Parkinsonism in Rodents. Adv. Funct. Mater. 2018, 28, 1804144. [Google Scholar] [CrossRef]
- Hedlund, E.; Pruszak, J.; Lardaro, T.; Ludwig, W.; Viñuela, A.; Kim, K.-S.; Isacson, O. Embryonic Stem Cell-Derived Pitx3-Enhanced Green Fluorescent Protein Midbrain Dopamine Neurons Survive Enrichment by Fluorescence-Activated Cell Sorting and Function in an Animal Model of Parkinson’s Disease. Stem Cells 2008, 26, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Midha, S.; Giri, S.; Mohanty, S. Functional Skin Grafts: Where Biomaterials Meet Stem Cells. Stem Cells Int. 2019, 2019, 1286054. [Google Scholar] [CrossRef] [PubMed]
- Tartarini, D.; Mele, E. Adult Stem Cell Therapies for Wound Healing: Biomaterials and Computational Models. Front. Bioeng. Biotechnol. 2016, 3, 206. [Google Scholar] [CrossRef]
- Shin, T.-H.; Kim, H.-S.; Choi, S.W.; Kang, K.-S. Mesenchymal Stem Cell Therapy for Inflammatory Skin Diseases: Clinical Potential and Mode of Action. Int. J. Mol. Sci. 2017, 18, 244. [Google Scholar] [CrossRef]
- Chen, L.; Xing, Q.; Zhai, Q.; Tahtinen, M.; Zhou, F.; Chen, L.; Xu, Y.; Qi, S.; Zhao, F. Pre-vascularization Enhances Therapeutic Effects of Human Mesenchymal Stem Cell Sheets in Full Thickness Skin Wound Repair. Theranostics 2017, 7, 117–131. [Google Scholar] [CrossRef]
- Abaci, H.E.; Guo, Z.; Coffman, A.; Gillette, B.; Lee, W.-h.; Sia, S.K.; Christiano, A.M. Human Skin Constructs with Spatially Controlled Vasculature Using Primary and iPSC-Derived Endothelial Cells. Adv. Healthc. Mater. 2016, 5, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, L.; Zhang, C.; Hu, J.; Chen, J.; Du, W.; Liu, F.; Ren, W.; Wang, J.; Quan, R. Feasibility of repairing full-thickness skin defects by iPSC-derived epithelial stem cells seeded on a human acellular amniotic membrane. Stem Cell Res. Ther. 2019, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Chen, M.; Hu, C.; Qin, S.; Chu, C.; Xiang, L.; Man, Y.; Qu, Y. Endowing iPSC-Derived MSCs with Angiogenic and Keratinogenic Differentiation Potential: A Promising Cell Source for Skin Tissue Engineering. BioMed Res. Int. 2018, 2018, 8459503. [Google Scholar] [CrossRef]
- Liu, L.-P.; Li, Y.-M.; Guo, N.-N.; Li, S.; Ma, X.; Zhang, Y.-X.; Gao, Y.; Huang, J.-L.; Zheng, D.-X.; Wang, L.-Y.; et al. Therapeutic Potential of Patient iPSC-Derived iMelanocytes in Autologous Transplantation. Cell Rep. 2019, 27, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Movahednia, M.M.; Kidwai, F.K.; Jokhun, D.S.; Squier, C.A.; Toh, W.S.; Cao, T. Potential applications of keratinocytes derived from human embryonic stem cells. Biotechnol. J. 2016, 11, 58–70. [Google Scholar] [CrossRef]
- Yoon, D.; Yoon, D.; Sim, H.; Hwang, I.; Lee, J.-S.; Chun, W. Accelerated Wound Healing by Fibroblasts Differentiated from Human Embryonic Stem Cell-Derived Mesenchymal Stem Cells in a Pressure Ulcer Animal Model. Stem Cells Int. 2018, 2018, 4789568. [Google Scholar] [CrossRef]
- Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef]
- Kuo, T.K.; Hung, S.P.; Chuang, C.H.; Chen, C.T.; Shih, Y.R.V.; Fang, S.C.Y.; Yang, V.W.; Lee, O.K. Stem Cell Therapy for Liver Disease: Parameters Governing the Success of Using Bone Marrow Mesenchymal Stem Cells. Gastroenterology 2008, 134, 2111–2121. [Google Scholar] [CrossRef]
- Zhao, W.; Li, J.-J.; Cao, D.-Y.; Li, X.; Zhang, L.-Y.; He, Y.; Yue, S.-Q.; Wang, D.-S.; Dou, K.-F. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J. Gastroenterol. 2012, 18, 1048–1058. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.-R.; Ueno, Y.; Zheng, Y.-W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Kimura, M.; Yoshizawa, E.; Ayano, S.; Koido, M.; Funayama, S.; Nakanishi, N.; Hisai, T.; Kobayashi, T.; et al. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Rep. 2017, 21, 2661–2670. [Google Scholar] [CrossRef]
- Nagamoto, Y.; Takayama, K.; Ohashi, K.; Okamoto, R.; Sakurai, F.; Tachibana, M.; Kawabata, K.; Mizuguchi, H. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J. Hepatol. 2016, 64, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-R.; Koido, M.; Tadokoro, T.; Ouchi, R.; Matsuno, T.; Ueno, Y.; Sekine, K.; Takebe, T.; Taniguchi, H. Human iPSC-Derived Posterior Gut Progenitors Are Expandable and Capable of Forming Gut and Liver Organoids. Stem Cell Rep. 2018, 10, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Tan, Z.; Su, Y.; Liu, J.; Chang, M.; Yan, F.; Chen, J.; Chen, T.; Li, C.; et al. Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Res. 2019, 29, 1009–1026. [Google Scholar] [CrossRef] [PubMed]
- Luong, E.; Gerecht, S. Stem cells and scaffolds for vascularizing engineered tissue constructs. Adv. Biochem. Eng. Biotechnol. 2009, 114, 129–172. [Google Scholar] [PubMed]
- Bhardwaj, N.; Chouhan, D.; Mandal, B.B. Tissue Engineered Skin and Wound Healing: Current Strategies and Future Directions. Curr. Pharm. Des. 2017, 23, 3455–3482. [Google Scholar] [CrossRef]
- Aguirre, A.; Planell, J.A.; Engel, E. Dynamics of bone marrow-derived endothelial progenitor cell/mesenchymal stem cell interaction in co-culture and its implications in angiogenesis. Biochem. Biophys. Res. Commun. 2010, 400, 284–291. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, W.; Ma, D.; Yang, Y. Endothelial cells promote neural stem cell proliferation and differentiation associated with VEGF activated Notch and Pten signaling. Dev. Dyn. 2010, 239, 2345–2353. [Google Scholar] [CrossRef]
- Nguyen, B.-N.B.; Moriarty, R.A.; Kamalitdinov, T.; Etheridge, J.M.; Fisher, J.P. Collagen hydrogel scaffold promotes mesenchymal stem cell and endothelial cell coculture for bone tissue engineering. J. Biomed. Mater. Res. Part A 2017, 105, 1123–1131. [Google Scholar] [CrossRef]
- Unger, R.E.; Dohle, E.; Kirkpatrick, C.J. Improving vascularization of engineered bone through the generation of pro-angiogenic effects in co-culture systems. Adv. Drug Deliv. Rev. 2015, 94, 116–125. [Google Scholar] [CrossRef]
- Choi, J.W.; Park, J.K.; Chang, J.W.; Kim, D.Y.; Kim, M.S.; Shin, Y.S.; Kim, C.-H. Small intestine submucosa and mesenchymal stem cells composite gel for scarless vocal fold regeneration. Biomaterials 2014, 35, 4911–4918. [Google Scholar] [CrossRef] [PubMed]
- Voswinkel, J.; Francois, S.; Simon, J.-M.; Benderitter, M.; Gorin, N.-C.; Mohty, M.; Fouillard, L.; Chapel, A. Use of Mesenchymal Stem Cells (MSC) in Chronic Inflammatory Fistulizing and Fibrotic Diseases: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2013, 45, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Soontararak, S.; Chow, L.; Johnson, V.; Coy, J.; Wheat, W.; Regan, D.; Dow, S. Mesenchymal Stem Cells (MSC) Derived from Induced Pluripotent Stem Cells (iPSC) Equivalent to Adipose-Derived MSC in Promoting Intestinal Healing and Microbiome Normalization in Mouse Inflammatory Bowel Disease Model. Stem Cells Transl. Med. 2018, 7, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Kitano, K.; Schwartz, D.M.; Zhou, H.; Gilpin, S.E.; Wojtkiewicz, G.R.; Ren, X.; Sommer, C.A.; Capilla, A.V.; Mathisen, D.J.; Goldstein, A.M.; et al. Bioengineering of functional human induced pluripotent stem cell-derived intestinal grafts. Nat. Commun. 2017, 8, 765. [Google Scholar] [CrossRef]
- Finkbeiner, S.R.; Freeman, J.J.; Wieck, M.M.; El-Nachef, W.; Altheim, C.H.; Tsai, Y.-H.; Huang, S.; Dyal, R.; White, E.S.; Grikscheit, T.C.; et al. Generation of tissue-engineered small intestine using embryonic stem cell-derived human intestinal organoids. Biol. Open 2015, 4, 1462. [Google Scholar] [CrossRef]
- Reza Mokhber Dezfouli, M.; Sadeghian Chaleshtori, S.; Mehdi Dehghan, M.; Tavanaeimanesh, H.; Baharvand, H.; Tahamtani, Y. The therapeutic potential of differentiated lung cells from embryonic stem cells in lung diseases. Curr. Stem Cell Res. Ther. 2017, 12, 80–84. [Google Scholar] [CrossRef]
- Covid-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available online: https://coronavirus.jhu.edu/map.html (accessed on 21 October 2020).
- Weiss, D.J.; Ortiz, L.A. Cell Therapy Trials for Lung Diseases: Progress and Cautions. Am. J. Respir. Crit. Care Med. 2013, 188, 123–125. [Google Scholar] [CrossRef]
- Silva, L.H.A.; Antunes, M.A.; Dos Santos, C.C.; Weiss, D.J.; Cruz, F.F.; Rocco, P.R.M. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res. Ther. 2018, 9, 45. [Google Scholar] [CrossRef]
- Khoury, M.; Cuenca, J.; Cruz, F.F.; Figueroa, F.E.; Rocco, P.R.M.; Weiss, D.J. Current status of cell-based therapies for respiratory virus infections: Applicability to COVID-19. Eur. Respir. J. 2020, 55, 2000858. [Google Scholar] [CrossRef]
- Alghazali, K.M.; Nima, Z.A.; Hamzah, R.N.; Dhar, M.S.; Anderson, D.E.; Biris, A.S. Bone-tissue engineering: Complex tunable structural and biological responses to injury, drug delivery, and cell-based therapies. Drug Metab. Rev. 2015, 47, 431–454. [Google Scholar] [CrossRef]
- Illien-Jünger, S.; Lu, Y.; Purmessur, D.; Mayer, J.E.; Walter, B.A.; Roughley, P.J.; Qureshi, S.A.; Hecht, A.C.; Iatridis, J.C. Detrimental effects of discectomy on intervertebral disc biology can be decelerated by growth factor treatment during surgery: A large animal organ culture model. Spine J. 2014, 14, 2724–2732. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.; Wang, M.; Hu, Z.; Stradner, M.; Zhu, S.; Kong, H.; Yi, H.; Goldrath, A.; Yang, Y.-G.; Xu, Y.; et al. An Effective Approach to Prevent Immune Rejection of Human ESC-Derived Allografts. Cell Stem Cell 2014, 14, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Underhill, G.H.; Zaret, K.S.; Fox, I.J. Cell and tissue engineering for liver disease. Sci. Transl. Med. 2014, 6, 245sr2. [Google Scholar] [CrossRef] [PubMed]
- Song, H.G.; Rumma, R.T.; Ozaki, C.K.; Edelman, E.R.; Chen, C.S. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell 2018, 22, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Hendow, E.K.; Guhmann, P.; Wright, B.; Sofokleous, P.; Parmar, N.; Day, R.M. Biomaterials for hollow organ tissue engineering. Fibrogenesis Tissue Repair 2016, 9, 3. [Google Scholar] [CrossRef]
- Nakamura, T.; Sato, T. Advancing Intestinal Organoid Technology Toward Regenerative Medicine. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 51–60. [Google Scholar] [CrossRef]
- Edgar, L.; Pu, T.; Porter, B.; Aziz, J.M.; La Pointe, C.; Asthana, A.; Orlando, G. Regenerative medicine, organ bioengineering and transplantation. Br. J. Surg. 2020, 107, 793–800. [Google Scholar] [CrossRef]
- Stabler, C.T.; Morrisey, E.E. Developmental pathways in lung regeneration. Cell Tissue Res. 2017, 367, 677–685. [Google Scholar] [CrossRef]
| Mesodermal Stem Cell Applications | |||||
|---|---|---|---|---|---|
| Target Tissue | Utilized Stem Cell Populations | Biomaterial(s) Utilized | Reference(s) | ||
| MSCs | iPSCs | ESCs | |||
| Bone | X | X | X | Yes | [83,86] |
| X | Yes | [84,87,88,89,90] | |||
| X | No | [85] | |||
| X | Yes | [91,93] | |||
| X | X | Yes | [92,95] | ||
| X | X | No | [94] | ||
| Cartilage | X | No | [96,98] | ||
| X | X | Yes | [99,103] | ||
| X | X | No | [100] | ||
| X | X | X | No | [101] | |
| X | No | [102] | |||
| X | Yes | [104] | |||
| X | Yes | [105] | |||
| Muscle | X | X | Yes | [107] | |
| X | No | [108,109,110,112,114] | |||
| X | Yes | [111,113,115,116] | |||
| X | Yes | [117] | |||
| X | No | [118,119,121,122] | |||
| X | Yes | [120] | |||
| Ectodermal Stem Cell Applications | |||||
|---|---|---|---|---|---|
| Target Tissue | Utilized Stem Cell Populations | Biomaterial(s) Utilized | Reference(s) | ||
| MSCs | iPSCs | ESCs | |||
| Nerve | X | Yes | [123,128] | ||
| X | X | X | No | [124] | |
| X | No | [125,126] | |||
| X | X | No | [127] | ||
| X | Yes | [129] | |||
| X | Yes | [130] | |||
| X | No | [131] | |||
| Skin | X | X | X | Yes | [132] |
| X | Yes | [133] | |||
| X | No | [134,135] | |||
| X | Yes | [136,137] | |||
| X | X | No | [138] | ||
| X | No | [139] | |||
| X | No | [140] | |||
| X | X | No | [141] | ||
| X | X | X | No | [142] | |
| Endodermal Stem Cell Applications | |||||
|---|---|---|---|---|---|
| Target Tissue | Utilized Stem Cell Populations | Biomaterial(s) Utilized | Reference(s) | ||
| MSCs | iPSCs | ESCs | |||
| Liver | X | No | [144,145] | ||
| X | No | [146,147,148,161] | |||
| X | No | [149] | |||
| Vasculature | X | X | X | Yes | [86,150,152] |
| X | No | [153,154] | |||
| X | Yes | [155,156] | |||
| Gastro-intestinal | X | Yes | [158] | ||
| X | No | [161] | |||
| X | No | [162] | |||
| X | X | No | [163] | ||
| X | Yes | [164] | |||
| X | Yes | [165] | |||
| Lungs | X | X | X | No | [18,170] |
| X | No | [166,167,168] | |||
| X | No | [169] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priester, C.; MacDonald, A.; Dhar, M.; Bow, A. Examining the Characteristics and Applications of Mesenchymal, Induced Pluripotent, and Embryonic Stem Cells for Tissue Engineering Approaches across the Germ Layers. Pharmaceuticals 2020, 13, 344. https://doi.org/10.3390/ph13110344
Priester C, MacDonald A, Dhar M, Bow A. Examining the Characteristics and Applications of Mesenchymal, Induced Pluripotent, and Embryonic Stem Cells for Tissue Engineering Approaches across the Germ Layers. Pharmaceuticals. 2020; 13(11):344. https://doi.org/10.3390/ph13110344
Chicago/Turabian StylePriester, Caitlin, Amber MacDonald, Madhu Dhar, and Austin Bow. 2020. "Examining the Characteristics and Applications of Mesenchymal, Induced Pluripotent, and Embryonic Stem Cells for Tissue Engineering Approaches across the Germ Layers" Pharmaceuticals 13, no. 11: 344. https://doi.org/10.3390/ph13110344
APA StylePriester, C., MacDonald, A., Dhar, M., & Bow, A. (2020). Examining the Characteristics and Applications of Mesenchymal, Induced Pluripotent, and Embryonic Stem Cells for Tissue Engineering Approaches across the Germ Layers. Pharmaceuticals, 13(11), 344. https://doi.org/10.3390/ph13110344