S-Methyl-(2-Methoxycarbonylamino-Benzimidazole-5) Thiosulfonate as a Potential Antiparasitic Agent—Its Action on the Development of Ascaris suum Eggs In Vitro
Abstract
1. Introduction
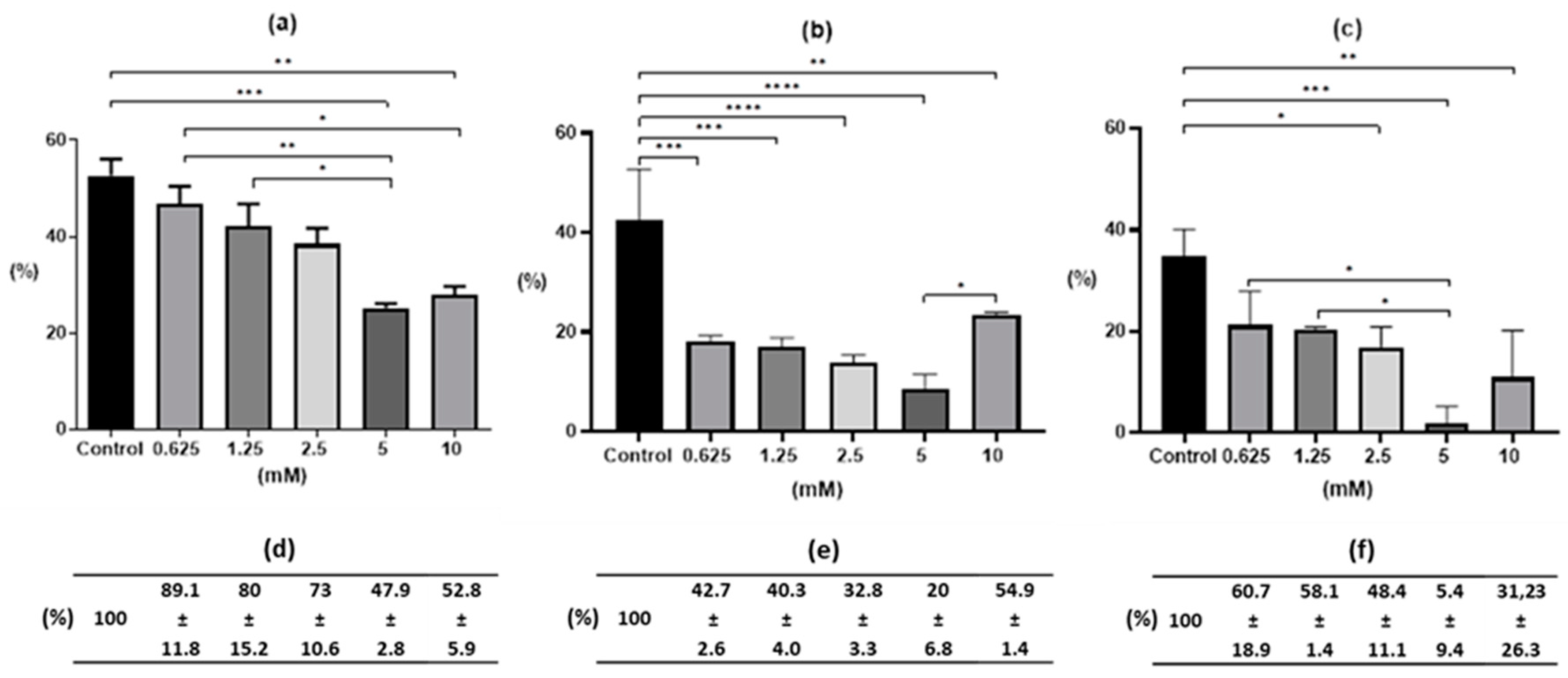
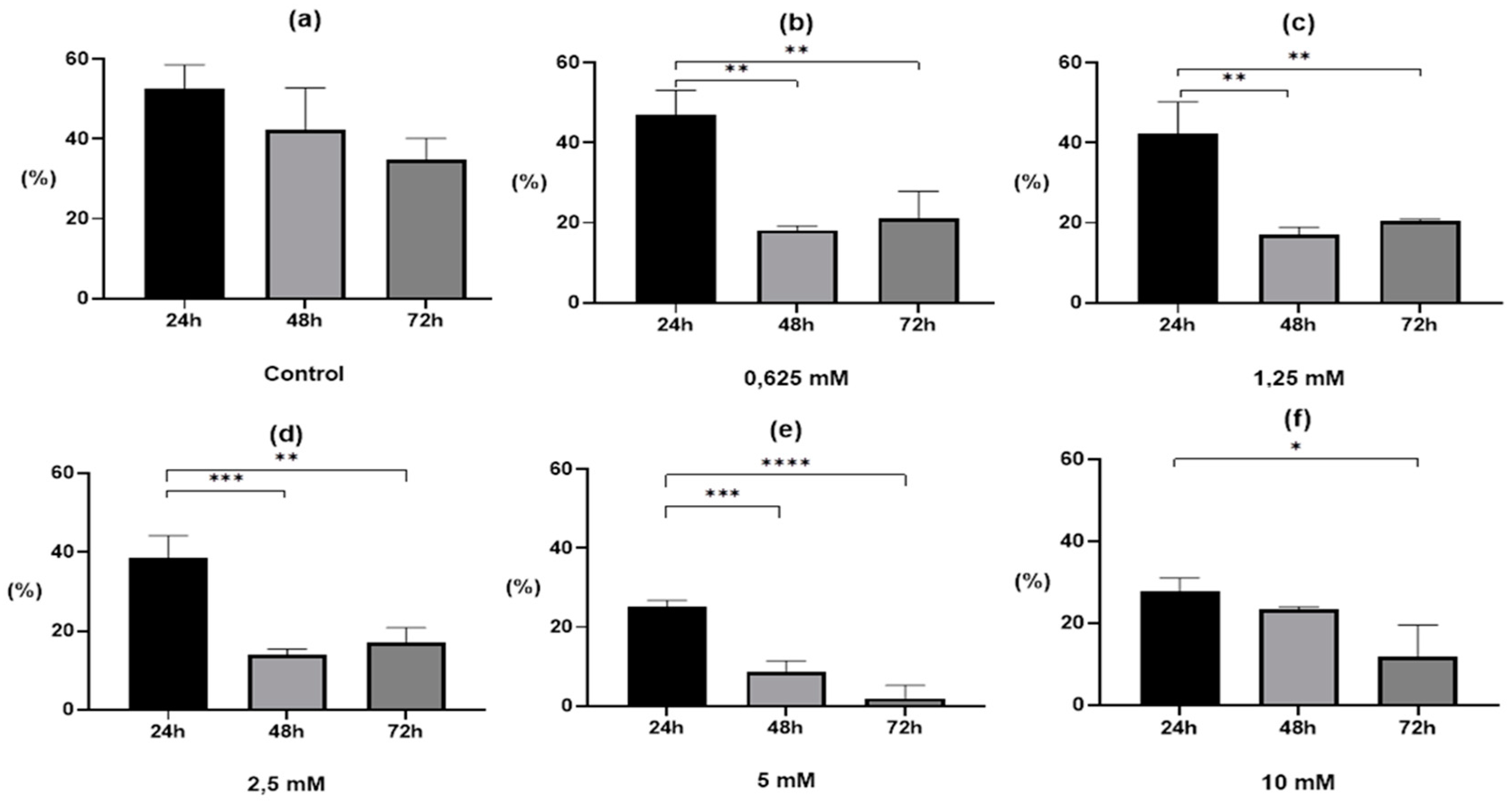
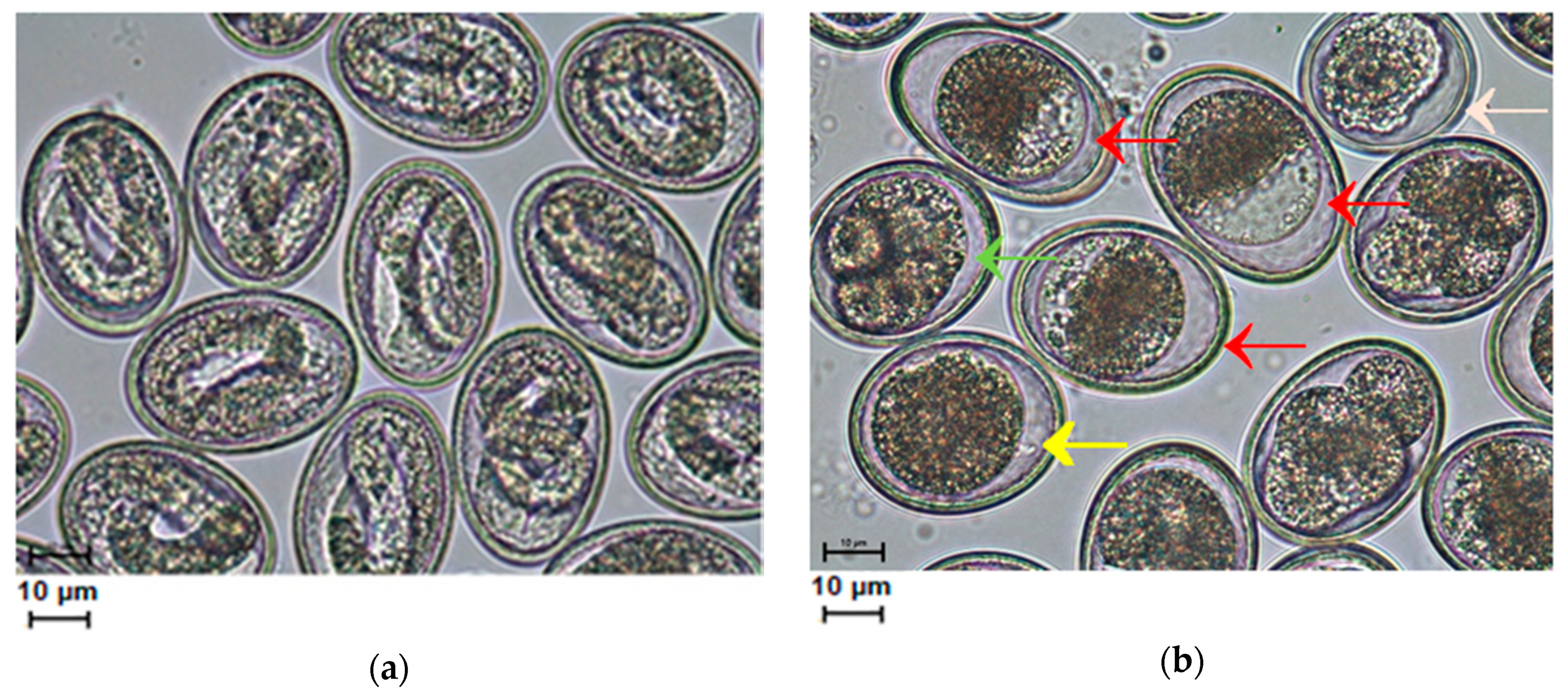
2. Results
2.1. Egg Development after Drug Treatment
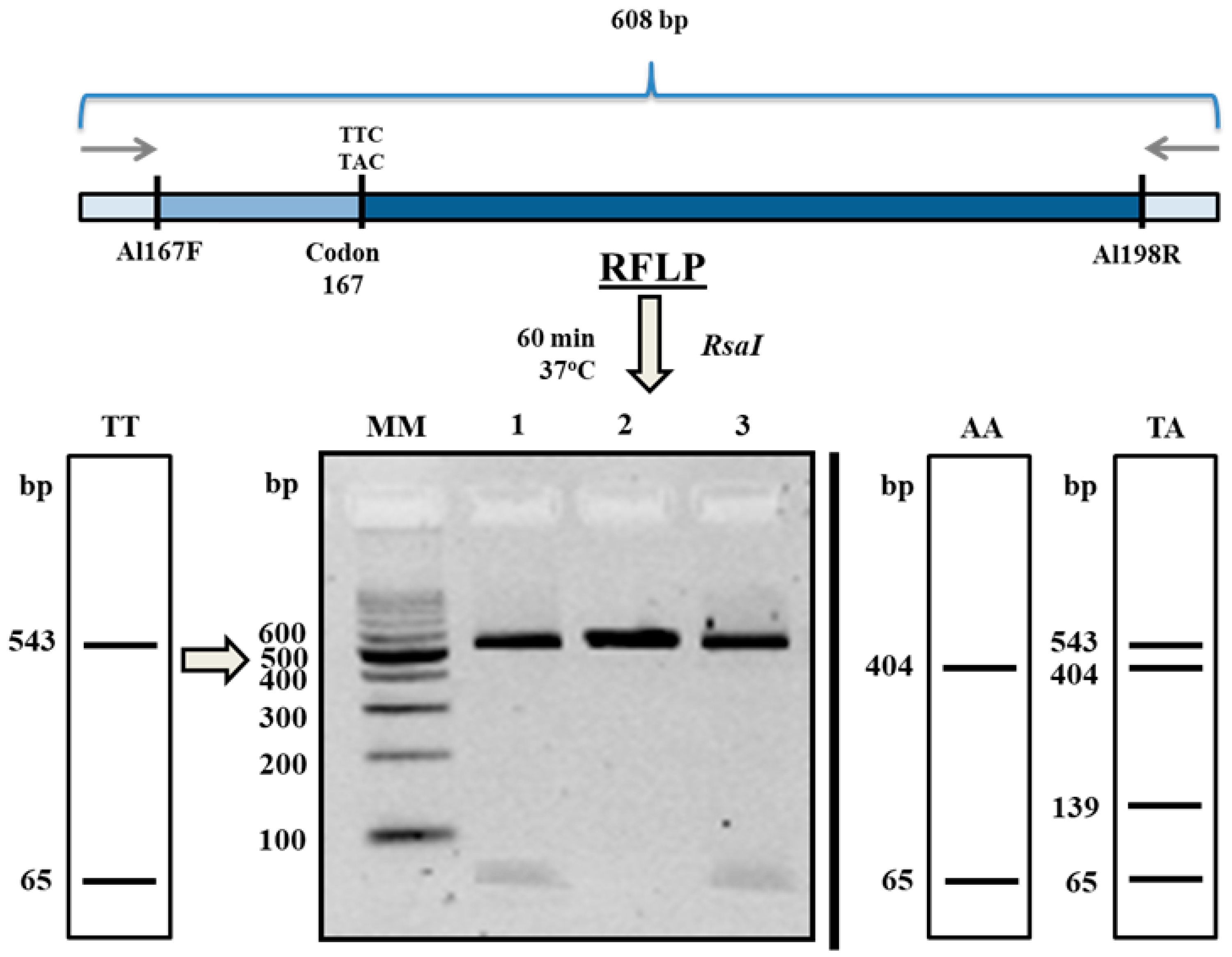
2.2. β-Tubulin SNP Genotyping
3. Discussion
4. Materials and Methods
4.1. Drug Synthesis
4.2. In Vitro Culture and Preparation of Test Samples
4.3. DNA Extraction
4.4. PCR Conditions
4.5. PCR-RFLP Analysis
4.6. DNA Sequencing and Data Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boes, J.; Kanora, A.; Havn, K.T.; Christiansen, S.; Vestergaard-Nielsen, K.; Jacobs, J.; Alban, L. Effect of Ascaris suum infection on performance of fattening pigs. Vet. Parasitol. 2010, 172, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Criscione, C.D. Ascariasis in people and pigs: New inferences from DNA analysis of worm populations. Infect. Genet. Evol. 2012, 12, 227–235. [Google Scholar] [CrossRef]
- Pullan, R.L.; Smith, J.L.; Jasrasaria, R.; Brooker, S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites Vectors 2014, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, P.M.; Lamberton, P.H.L.; Fenwick, A.; Addiss, D.G. Soil-transmitted helminth infections. Lancet 2018, 391, 252–265. [Google Scholar] [CrossRef]
- Diosdado, A.; Simón, F.; Morchón, R.; González-Miguel, J. Pro-fibrinolytic potential of the third larval stage of Ascaris suum as a possible mechanism facilitating its migration through the host tissues. Parasites Vectors 2020, 13, 1–13. [Google Scholar] [CrossRef]
- Zuccherato, L.W.; Furtado, L.F.; Medeiros, C.D.S.; Pinheiro, C.D.S.; Rabelo, É.M. PCR-RFLP screening of polymorphisms associated with benzimidazole resistance in Necator americanus and Ascaris lumbricoides from different geographical regions in Brazil. PLoS Negl. Trop. Dis. 2018, 12, e0006766. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.I.; Mottier, M.L.; Lanusse, C.E. Drug transfer into target helminth parasites. Trends Parasitol. 2007, 23, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.M.; Flemming, A.J.; Urwin, P.E. NHR-176 regulates cyp-35d1 to control hydroxylation-dependent metabolism of thiabendazole in caenorhabditis elegans. Biochem. J. 2015, 466, 37–44. [Google Scholar] [CrossRef]
- Diawara, A.; Drake, L.J.; Suswillo, R.R.; Kihara, J.; Bundy, D.A.P.; Scott, M.E.; Halpenny, C.; Stothard, J.R.; Prichard, R.K. Assays to detect β-tubulin codon 200 polymorphism in Trichuris trichiura and Ascaris lumbricoides. PLoS Negl. Trop. Dis. 2009, 3, e397. [Google Scholar] [CrossRef]
- Rashwan, N.; Scott, M.; Prichard, R. Rapid Genotyping of β-tubulin Polymorphisms in Trichuris trichiura and Ascaris lumbricoides. PLoS Negl. Trop. Dis. 2017, 11, e0005205. [Google Scholar] [CrossRef]
- Boes, J.; Eriksen, L.; Nansen, P. Embryonation and infectivity of Ascaris suum eggs isolated from worms expelled by pigs treated with albendazole, pyrantel pamoate, ivermectin or piperazine dihydrochloride. Vet. Parasitol. 1998, 75, 181–190. [Google Scholar] [CrossRef]
- Rogers, W.P.; Petronoijevic, T. The invasive stage and the development of Nematodes. In Biology and Control of Endoparasites; Symons, L.E.A., Donald, A.D., Dineen, J.K., Eds.; Academy Press: Sydney, Australia; Academy Press: New York, NY, USA; Academy Press: London, UK, 1982; pp. 3–28. [Google Scholar]
- Conn, D.B. Atlas of Invertebrate Reproduction and Development, 1st ed.; Wiley-Liss: New York, NY, USA, 1991; pp. 70–89. [Google Scholar]
- Murrel, K.D.; Eriksen, L.; Nansen, P.; Slotved, H.C.; Rasmusen, T. Ascaris suum: A revision of its early migratory path and implications for human ascariasistle. J. Parasitol. 1997, 83, 255–266. [Google Scholar] [CrossRef]
- Geenen, P.L.; Bresciani, J.; Boes, J.P.; Pedersen, A.; Eriksen, L.; Fagerholm, H.P.; Nansen, P. The morphogenesis of Ascaris suum to the infective third-stage larvae within the egg. J. Parasitol. 2016, 85, 616–622. [Google Scholar] [CrossRef]
- Urban, J.; Kokoska, L.; Langrova, I.; Matejkova, J. In vitro anthelmintic effects of medicinal plants used in Czech Republic. Pharm. Biol. 2008, 46, 808–813. [Google Scholar] [CrossRef]
- Zenebe, S.; Feyera, T.; Assefa, S. In vitro anthelmintic activity of crude extracts of aerial parts of Cissus quadrangularis l. and leaves of Schinus molle l. against Haemonchus contortus. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Karonen, M.; Ahern, J.R.; Legroux, L.; Suvanto, J.; Engström, M.T.; Sinkkonen, J.; Salminen, J.-P.; Hoste, H. Ellagitannins inhibit the exsheathment of Haemonchus contortus and Trichostrongylus colubriformis larvae: The efficiency increases together with the molecular size. J. Agric. Food Chem. 2020, 68, 4176–4186. [Google Scholar] [CrossRef]
- Salehi, B.; Zucca, P.; Orhan, I.E.; Azzini, E.; Adetunji, C.O.; Mohammed, S.A.; Banerjee, S.K.; Sharopov, F.; Rigano, D.; Sharifi-Rad, J.; et al. Allicin and health: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 502–516. [Google Scholar] [CrossRef]
- Lubenets, V.; Vasylyuk, S.; Monka, N.; Bolibrukh, K.; Komarovska-Porokhnyavets, O.; Baranovych, D.; Musyanovych, R.; Zaczynska, E.; Czarny, A.; Nawrot, U.; et al. Synthesis and antimicrobial properties of 4-acylaminobenzenethiosulfoacid S-esters. Saudi Pharm. J. 2017, 25, 266–274. [Google Scholar] [CrossRef]
- Lubenets, V.; Stadnytska, N.; Baranovych, D.; Vasylyuk, S.; Karpenko, O.; Havryliak, V.; Novikov, V. Thiosulfonates: The Prospective Substances against Fungal Infections; de Loreto, É.S., Moraes Tondolo, J.S., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Oriabinska, L.B.; Starovoitova, S.O.; Vasylyuk, S.V.; Novikov, V.P.; Lubenets, V.I. Ethylthiosulfanilate effect on Candida tropicalis. Ukr. Biochem. J. 2017, 89, 70–76. [Google Scholar] [CrossRef][Green Version]
- Pylypets, A.Z.; Iskra, R.Y.; Havryliak, V.V.; Nakonechna, A.V.; Novikov, V.P.; Lubenets, V.I. Effects of thiosulfonates on the lipid composition of rat tissues. Ukr. Biochem. J. 2017, 89, 56–62. [Google Scholar] [CrossRef]
- Lubenets, V.I.; Havryliak, V.V.; Pylypets, A.Z.; Nakonechna, A.V. Changes in the spectrum of proteins and phospholipids in tissues of rats exposed to thiosulfonates. Regul. Mech. Biosyst. 2018, 9, 495–500. [Google Scholar] [CrossRef]
- Lubenets, V.; Parashchyn, Z.; Vasylyuk, S.; Novikov, V. The S-Methyl-(2-Methoxycarbonylamino- Benzimidazole-5) Thiosulfonate as potential anticancer agents. Glob. J. Pharm. Pharm. Sci. 2017, 3, 3–5. [Google Scholar] [CrossRef]
- Weeks, J.C.; Roberts, W.M.; Robinson, K.J.; Keaney, M.; Vermeire, J.J.; Urban, J.F.; Lockery, S.R.; Hawdon, J.M. Microfluidic platform for electrophysiological recordings from host-stage hookworm and Ascaris suum larvae: A new tool for anthelmintic research. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 314–328. [Google Scholar] [CrossRef]
- Massara, C.L.; Ferreira, R.S.; Guerra, H.L.; Dos Santos Carvalho, O. In vitro study on thiabendazole action on viability of Ascaris lumbricoides (Lineu, 1758) eggs. Rev. Soc. Bras. Med. Trop. 2001, 34, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ellis, B.L.; Yiu, Y.Y.; Miller, M.M.; Urban, J.F.; Shi, L.Z.; Aroian, R.V. An extensive comparison of the effect of anthelmintic classes on diverse nematodes. PLoS ONE 2013, 8, e70702. [Google Scholar] [CrossRef]
- Polyakova-Krusteva, O. Brunanska, M.; Dubinsky, P. The influence of fertilization on the ultrastructure of Ascaris suum eggs. Helminthologia 1985, 22, 233–244. [Google Scholar]
- Diawara, A.; Halpenny, C.M.; Churcher, T.S.; Mwandawiro, C.; Kihara, J.; Kaplan, R.M.; Streit, T.G.; Idaghdour, Y.; Scott, M.E.; Basáñez, M.G.; et al. Association between response to albendazole treatment and β-tubulin genotype frequencies in soil-transmitted helminths. PLoS Negl. Trop. Dis. 2013, 7, e2247. [Google Scholar] [CrossRef]
- Parashchyn, Z.; Lubenets, V.; Novikow, V. Organic, Thiosulphonates-derivatives of benzimidazoleitle. Russ. J. Org. Chem. 1998, 34, 280–284. [Google Scholar]
- Ho, N.F.H.; Geary, T.G.; Barsuhn, C.L.; Sims, S.; Thompson, D.P. Mechanistic studies in the transcuticular delivery of antiparasitic drugs II: Ex vivo/in vitro correlation of solute transport by Ascaris suum. Mol. Biochem. Parasitol. 1992, 52, 1–13. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NTe. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitryjuk, M.; Szczotko, M.; Kubiak, K.; Trojanowicz, R.; Parashchyn, Z.; Khomitska, H.; Lubenets, V. S-Methyl-(2-Methoxycarbonylamino-Benzimidazole-5) Thiosulfonate as a Potential Antiparasitic Agent—Its Action on the Development of Ascaris suum Eggs In Vitro. Pharmaceuticals 2020, 13, 332. https://doi.org/10.3390/ph13110332
Dmitryjuk M, Szczotko M, Kubiak K, Trojanowicz R, Parashchyn Z, Khomitska H, Lubenets V. S-Methyl-(2-Methoxycarbonylamino-Benzimidazole-5) Thiosulfonate as a Potential Antiparasitic Agent—Its Action on the Development of Ascaris suum Eggs In Vitro. Pharmaceuticals. 2020; 13(11):332. https://doi.org/10.3390/ph13110332
Chicago/Turabian StyleDmitryjuk, Małgorzata, Magdalena Szczotko, Katarzyna Kubiak, Radosław Trojanowicz, Zhanna Parashchyn, Halyna Khomitska, and Vira Lubenets. 2020. "S-Methyl-(2-Methoxycarbonylamino-Benzimidazole-5) Thiosulfonate as a Potential Antiparasitic Agent—Its Action on the Development of Ascaris suum Eggs In Vitro" Pharmaceuticals 13, no. 11: 332. https://doi.org/10.3390/ph13110332
APA StyleDmitryjuk, M., Szczotko, M., Kubiak, K., Trojanowicz, R., Parashchyn, Z., Khomitska, H., & Lubenets, V. (2020). S-Methyl-(2-Methoxycarbonylamino-Benzimidazole-5) Thiosulfonate as a Potential Antiparasitic Agent—Its Action on the Development of Ascaris suum Eggs In Vitro. Pharmaceuticals, 13(11), 332. https://doi.org/10.3390/ph13110332





