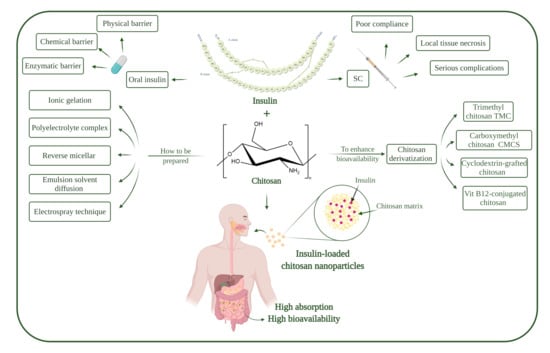
Recent Progress of Chitosan and Chitosan Derivatives-Based Nanoparticles: Pharmaceutical Perspectives of Oral Insulin Delivery
Abstract
1. Introduction
1.1. Diabetes Mellitus
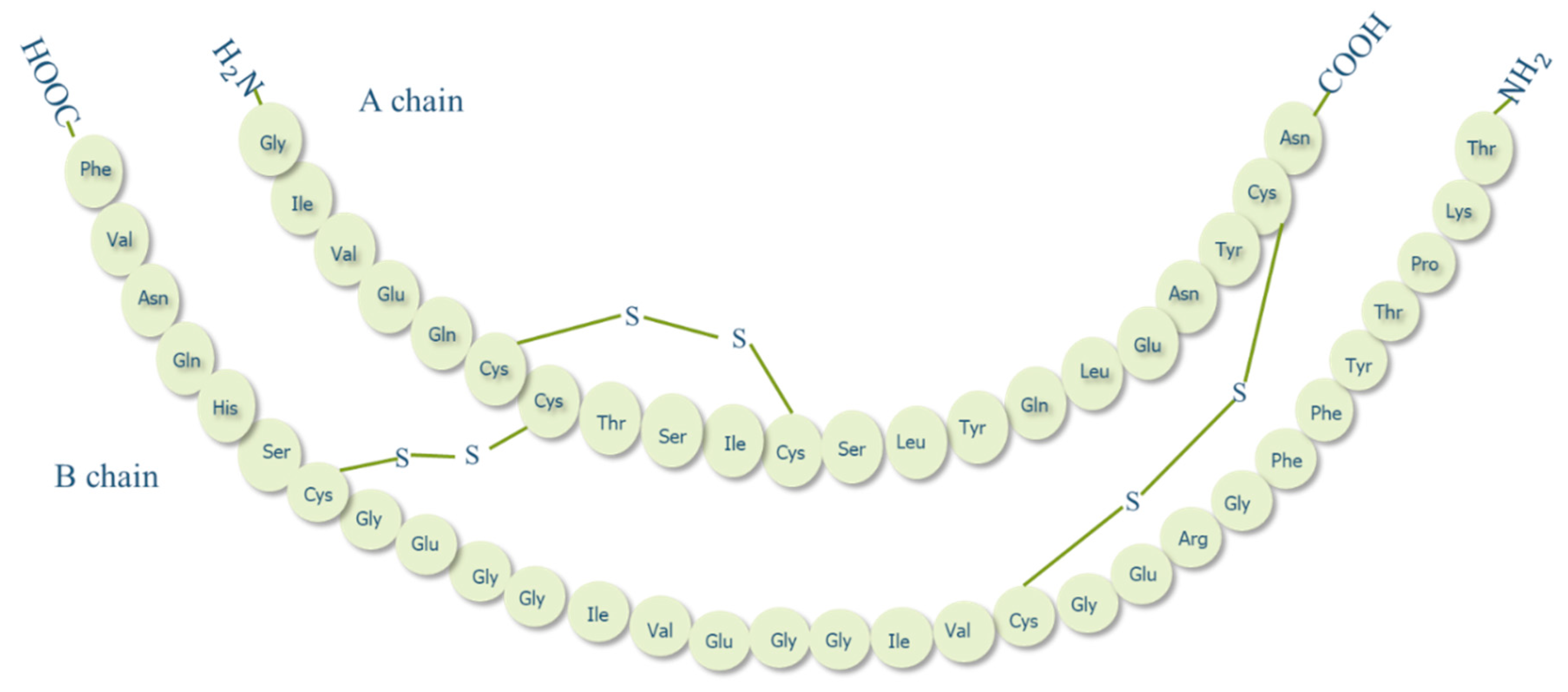
1.2. Insulin
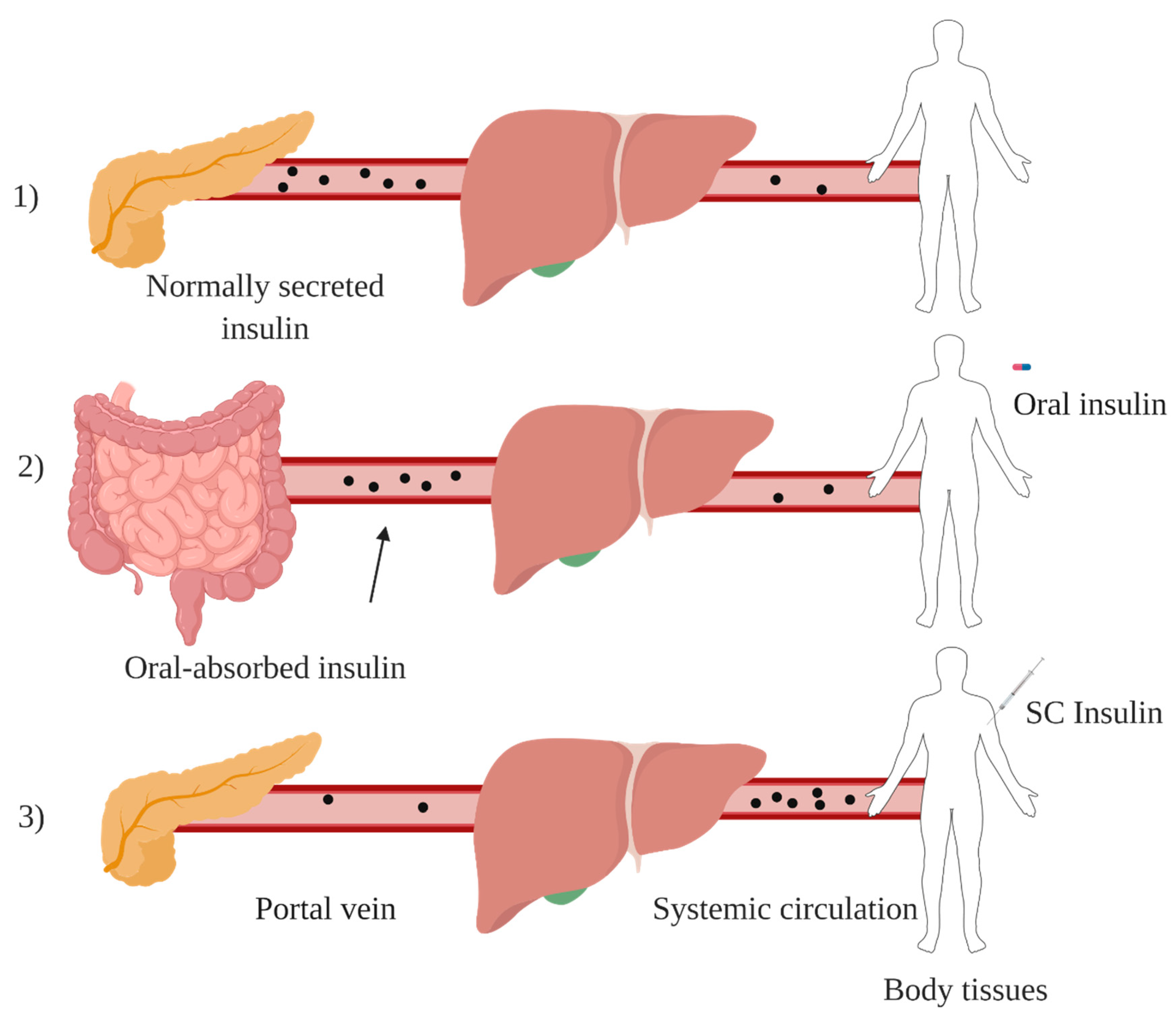
1.3. Oral Insulin Delivery
1.4. Barriers to Oral Insulin Delivery
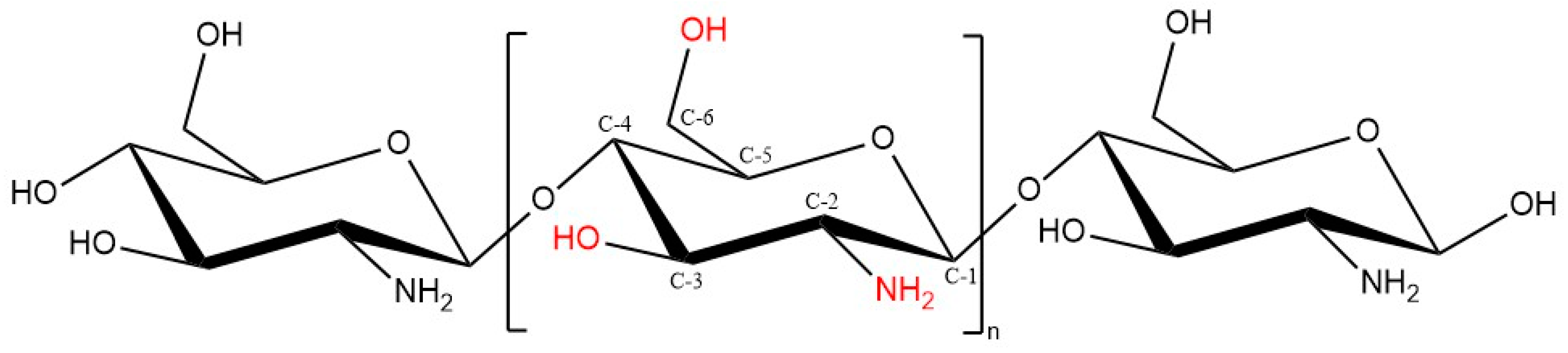
1.5. Chitosan
1.6. Chitosan Nanoparticles
2. Preparation Methods of Chitosan Nanoparticles
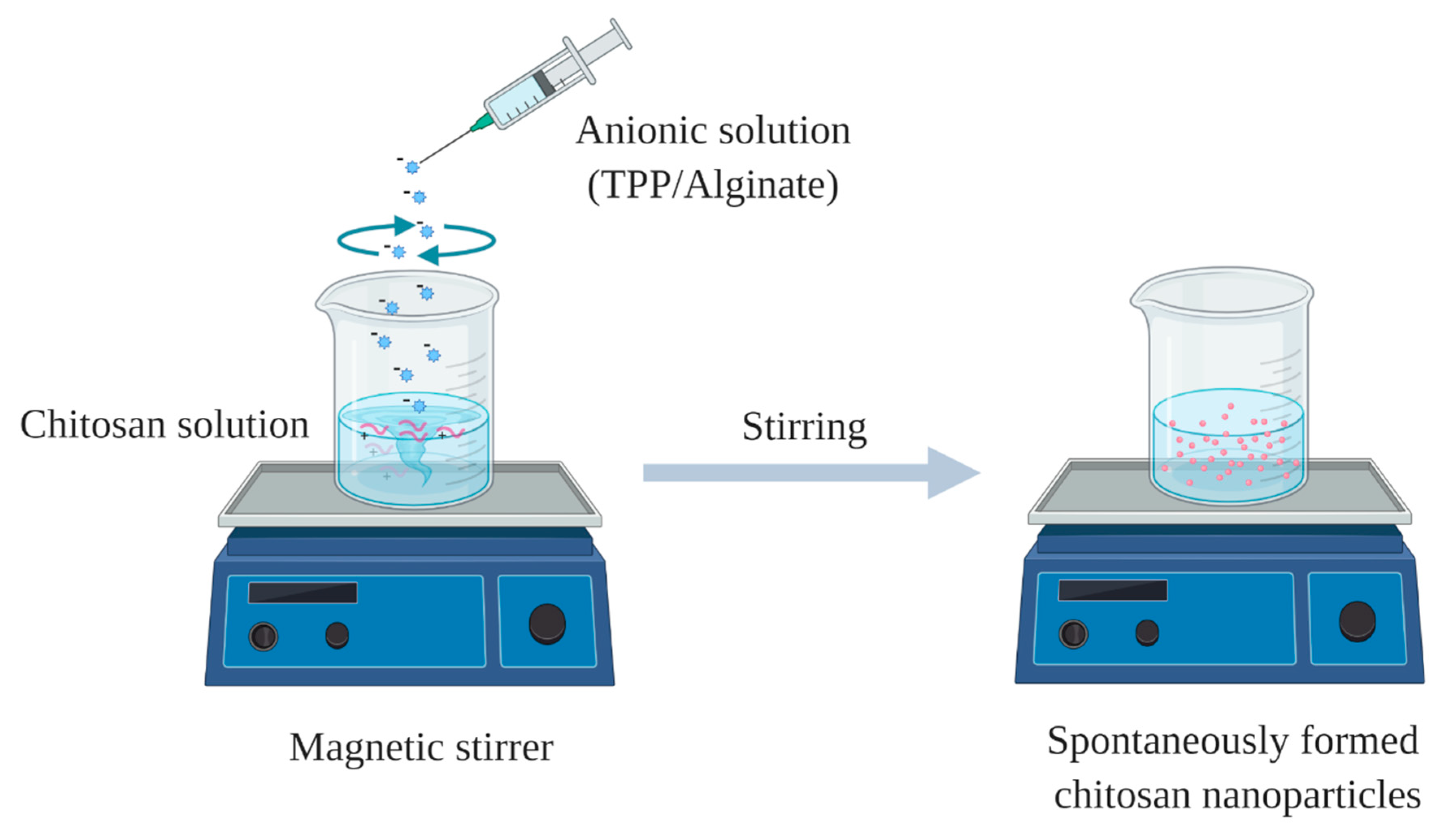
2.1. Ionic Gelation
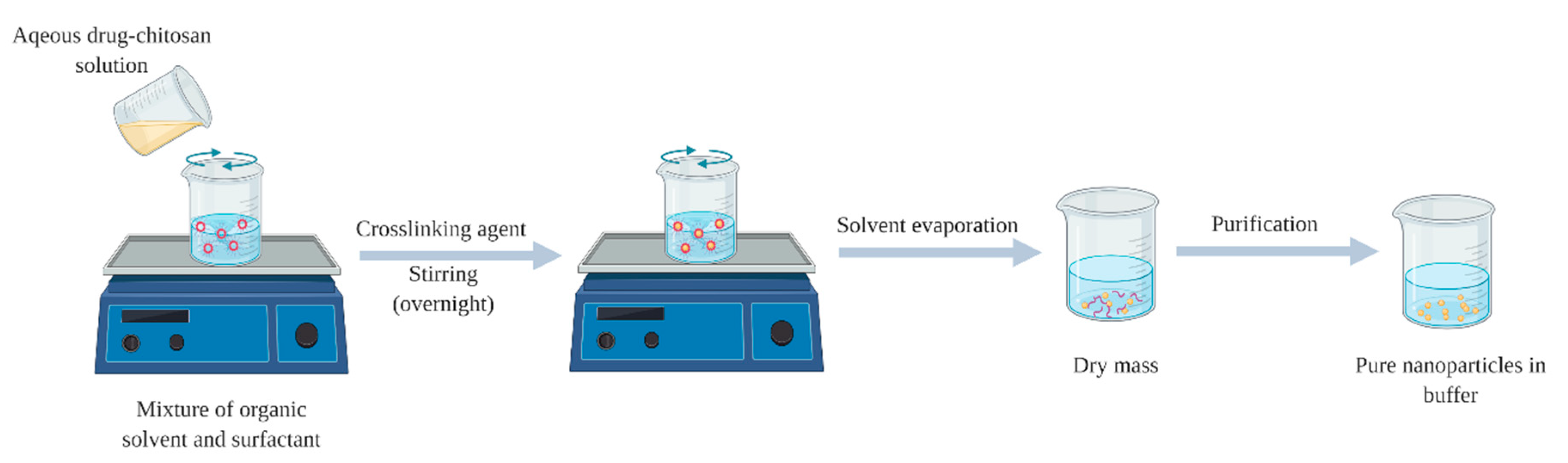
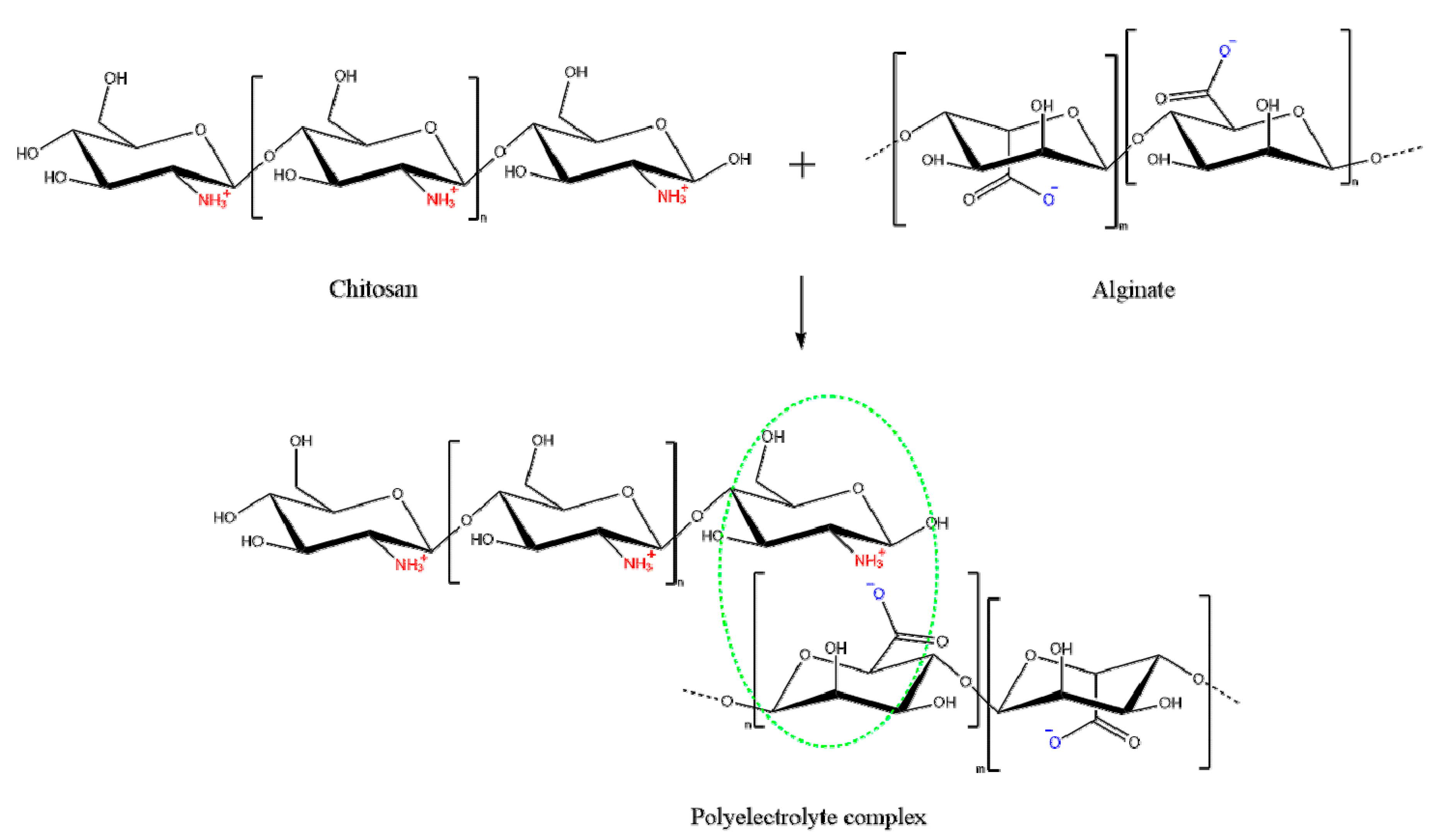
2.2. Polyelectrolyte Complex (PEC)
2.3. Reverse Micellar Method
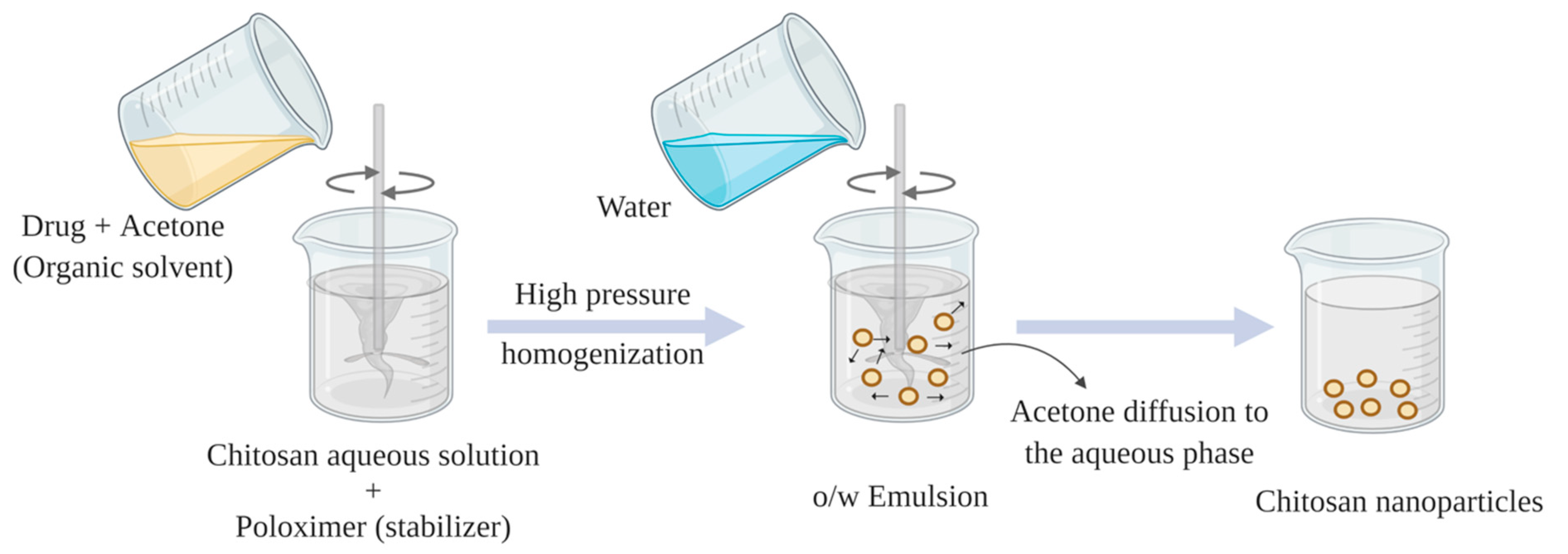
2.4. Emulsion Solvent Diffusion
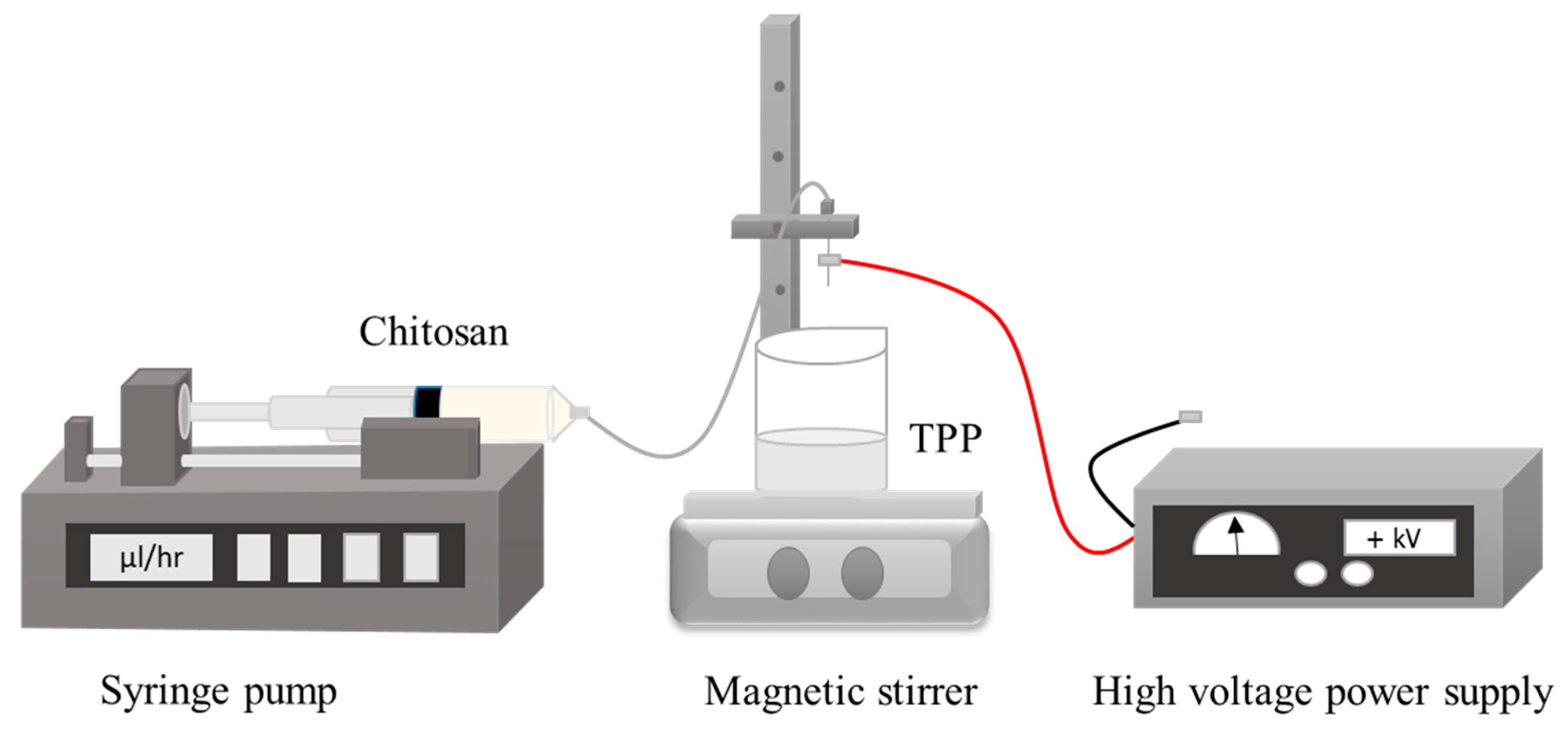
2.5. Electrospraying Technique
3. Insulin-Loaded Chitosan Nanoparticles
4. Chitosan Modification
4.1. Trimethyl Chitosan (TMC)
4.2. Carboxymethyl Chitosan (CMCS)
4.3. Cyclodextrin-Grafted Chitosan
4.4. Vitamin B12-Conjugated Chitosan (VitB12-Chi)
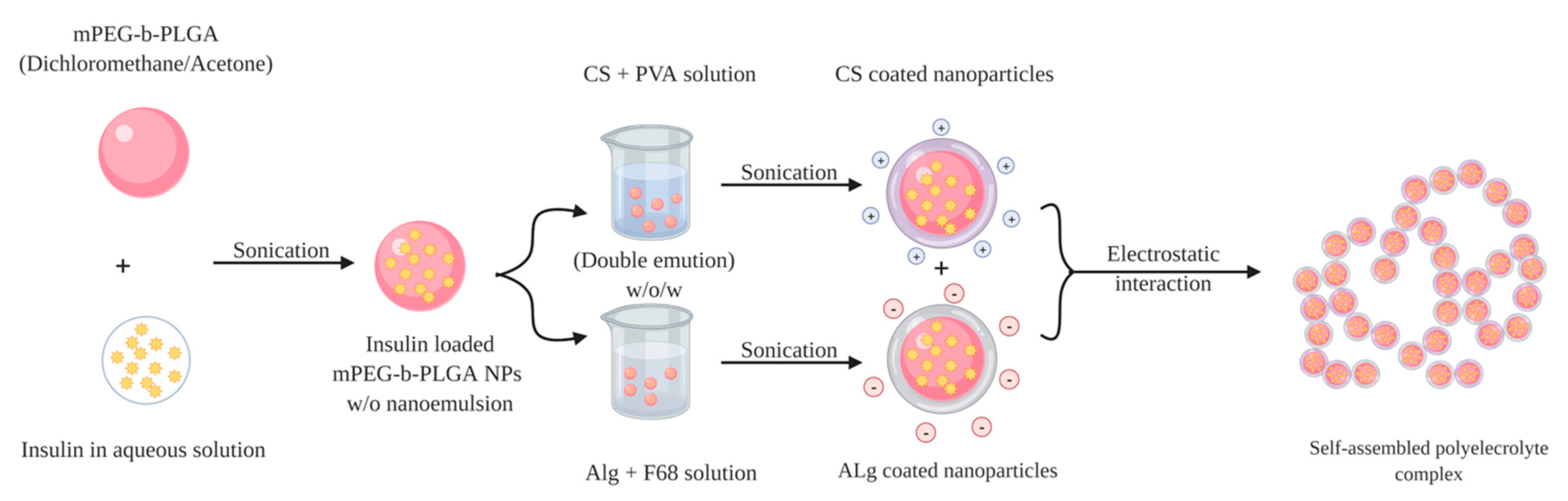
5. Chitosan and Chitosan Derivatives as Coating Material for Insulin Nanoparticles
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Un Kim, J.; Muhammad Shahbaz, H.; Lee, H.; Kim, T.; Yang, K.; Hoon Roh, Y.; Park, J. Optimization of Phytic Acid-Crosslinked Chitosan Microspheres for Oral Insulin Delivery Using Responsive Surface Methodology. Int. J. Pharm. 2020, 588, 119736. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.; Souto, S.B.; Sánchez-López, E.; López Machado, A.; Severino, P.; Jose, S.; Santini, A.; Fortuna, A.; García, M.L.; Silva, A.M. Sugar-Lowering Drugs for Type 2 Diabetes Mellitus and Metabolic Syndrome—Review of Classical and New Compounds: Part-I. Pharmaceuticals 2019, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Chen, S.; Cheong, K.-L.; Teng, B. Carboxymethyl β-Cyclodextrin Grafted Carboxymethyl Chitosan Hydrogel-Based Microparticles for Oral Insulin Delivery. Carbohydr. Polym. 2020, 246, 116617. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.A.; Abrahim, B.A.; Cabral, L.M.; Rodrigues, C.R.; Seiça, R.M.F.; de Baptista Veiga, F.J.; Ribeiro, A.J. Intestinal Absorption of Insulin Nanoparticles: Contribution of M Cells. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1139–1151. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- El-Say, K.M.; El-Sawy, H.S. Polymeric Nanoparticles: Promising Platform for Drug Delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef]
- Santalices, I.; Gonella, A.; Torres, D.; Alonso, M.J. Advances on the Formulation of Proteins Using Nanotechnologies. J. Drug Deliv. Sci. Technol. 2017, 42, 155–180. [Google Scholar] [CrossRef]
- Sadeghi, S.; Lee, W.K.; Kong, S.N.; Shetty, A.; Drum, C.L. Oral Administration of Protein Nanoparticles: An Emerging Route to Disease Treatment. Pharmacol. Res. 2020, 158, 104685. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, J.Z.; Fei, X.; Li, Y.; Song, Y.; Qian, Z.; Peng, Q. Can Nanoparticles and Nano‒protein Interactions Bring a Bright Future for Insulin Delivery? Acta Pharm. Sin. B 2020. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Gardouh, A.R.; Attia, M.A.; Enan, E.T.; Elbahaie, A.M.; Fouad, R.A.; El-Shafey, M.; Youssef, A.M.; Alomar, S.Y.; Ali, Z.A.-E.; Zaitone, S.A.; et al. Synthesis and Antitumor Activity of Doxycycline Polymeric Nanoparticles: Effect on Tumor Apoptosis in Solid Ehrlich Carcinoma. Molecules 2020, 25, 3230. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Wong, S.W.; Yeo, H.L.; Zhao, Y. Nanocarriers for Cancer Treatment: Clinical Impact and Safety. NanoImpact 2020, 20, 100253. [Google Scholar] [CrossRef]
- Wong, K.H.; Lu, A.; Chen, X.; Yang, Z. Natural Ingredient-Based Polymeric Nanoparticles for Cancer Treatment. Molecules 2020, 25, 3620. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, S.; Huang, G. Design and Application of Oral Colon Administration System. J. Enzym. Inhib. Med. Chem. 2019, 34, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Ciro, Y.; Rojas, J.; Alhajj, M.J.; Carabali, G.A.; Salamanca, C.H. Production and Characterization of Chitosan–Polyanion Nanoparticles by Polyelectrolyte Complexation Assisted by High-Intensity Sonication for the Modified Release of Methotrexate. Pharmaceuticals 2020, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.; Wang, J.; Zeng, Y.; Liu, G.; Chen, X. Polysaccharide-based Controlled Release Systems for Therapeutics Delivery and Tissue Engineering: From Bench to Bedside. Adv. Sci. 2018, 5, 1700513. [Google Scholar] [CrossRef] [PubMed]
- Diolosà, M.; Donati, I.; Turco, G.; Cadenaro, M.; Di Lenarda, R.; Breschi, L.; Paoletti, S. Use of Methacrylate-Modified Chitosan to Increase the Durability of Dentine Bonding Systems. Biomacromolecules 2014, 15, 4606–4613. [Google Scholar] [CrossRef] [PubMed]
- Taubner, T.; Marounek, M.; Synytsya, A. Preparation and Characterization of Hydrophobic and Hydrophilic Amidated Derivatives of Carboxymethyl Chitosan and Carboxymethyl β-Glucan. Int. J. Biol. Macromol. 2020, 163, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Alai, M.S.; Lin, W.J.; Pingale, S.S. Application of Polymeric Nanoparticles and Micelles in Insulin Oral Delivery. J. Food Drug Anal. 2015, 23, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Adibah, W.N.; Ahmad, W.; Mahmod, H.; Ali, A.M. A Review of Medicinal Plants and Daily Foods Used in Southeast Asia Possessing Antidiabetic Activity. J. Agrobiotechnol. 2019, 10, 17–35. [Google Scholar]
- Jin, X.; Zhu, D.D.; Chen, B.Z.; Ashfaq, M.; Guo, X.D. Insulin Delivery Systems Combined with Microneedle Technology. Adv. Drug Deliv. Rev. 2018, 127, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Nawi, A.; Mamat, M.; Ahmad, W.M. The Factors That Contribute to Diabetes Mellitus in Malaysia: Alternative Linear Regression Model Approach in the Health Field Involving Diabetes Mellitus Data. Int. J. Public Heal. Clin. Sci. 2018, 5, 2289–7577. [Google Scholar]
- Zhang, Y.; Yu, J.; Kahkoska, A.R.; Wang, J.; Buse, J.B.; Gu, Z. Advances in Transdermal Insulin Delivery. Adv. Drug Deliv. Rev. 2019, 139, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Martinez, J.; Dass, C.R. Oral Delivery of Insulin for Treatment of Diabetes: Status Quo, Challenges and Opportunities. J. Pharm. Pharmacol. 2016, 68, 1093–1108. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.D.; Safavi-Hemami, H. Insulin as a Weapon. Toxicon 2016, 123, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liang, N.; Hiromitsu Yamamoto, Y.K.; Cui, F.; Yan, P. Ph-Sensitive Poly(Lactide-Co-Glycolide) Nanoparticle Composite Microcapsules for Oral Delivery of Insulin. Int. J. Nanomed. 2015, 10, 3489–3498. [Google Scholar] [CrossRef]
- Matteucci, E.; Giampietro, O.; Covolan, V.; Giustarini, D.; Fanti, P.; Rossi, R. Insulin Administration: Present Strategies and Future Directions for a Noninvasive (Possibly More Physiological) Delivery. Drug Des. Devel. Ther. 2015, 9, 3109–3118. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Yu, S.; Zhang, Z.; He, C.; Chen, X. PH- and Amylase-Responsive Carboxymethyl Starch/Poly(2-Isobutyl-Acrylic Acid) Hybrid Microgels as Effective Enteric Carriers for Oral Insulin Delivery. Biomacromolecules 2018, 19, 2123–2136. [Google Scholar] [CrossRef]
- Easa, N.; Alany, R.; Carew, M.; Vangala, A. A Review of Non-Invasive Insulin Delivery Systems for Diabetes Therapy in Clinical Trials over the Past Decade. Drug Discov. Today 2018. [Google Scholar] [CrossRef]
- Gedawy, A.; Martinez, J.; Al-Salami, H.; Dass, C.R. Oral Insulin Delivery: Existing Barriers and Current Counter-Strategies. J. Pharm. Pharmacol. 2018, 70, 197–213. [Google Scholar] [CrossRef]
- Shan, W.; Zhu, X.; Liu, M.; Li, L.; Zhong, J.; Sun, W.; Zhang, Z.; Huang, Y. Overcoming the Diffusion Barrier of Mucus and Absorption Barrier of Epithelium by Self-Assembled Nanoparticles for Oral Delivery of Insulin. ACS Nano 2015, 9, 2345–2356. [Google Scholar] [CrossRef]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Recent Advancements in Oral Administration of Insulin-Loaded Liposomal Drug Delivery Systems for Diabetes Mellitus. Int. J. Pharm. 2018, 549, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The Intestinal Epithelial Barrier: A Therapeutic Target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The Intestinal Barrier: A Fundamental Role in Health and Disease. Expert Rev. Gastroenterol. Hepatol. 2017, 00, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.; Martins, C.; Azevedo, C.; Sarmento, B. Chemical Modification of Drug Molecules as Strategy to Reduce Interactions with Mucus. Adv. Drug Deliv. Rev. 2017, 124, 98–106. [Google Scholar] [CrossRef]
- Muheem, A.; Shakeel, F.; Asadullah, M.; Anwar, M.; Mallick, N.; Kumar, G.; Husain, M.; Jalees, F. A Review on the Strategies for Oral Delivery of Proteins and Peptides and Their Clinical Perspectives. Saudi Pharm. J. 2016, 24, 413–428. [Google Scholar] [CrossRef]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Potential of Insulin Nanoparticle Formulations for Oral Delivery and Diabetes Treatment. J. Control. Release 2017, 264, 247–275. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, Protection, and Delivery of Bioactive Proteins and Peptides Using Nanoparticle and Microparticle Systems: A Review. Adv. Colloid Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef]
- Lundquist, P.; Artursson, P. Oral Absorption of Peptides and Nanoparticles across the Human Intestine: Opportunities, Limitations and Studies in Human Tissues. Adv. Drug Deliv. Rev. 2016, 106, 256–276. [Google Scholar] [CrossRef]
- Chen, M.; Sonaje, K.; Chen, K.; Sung, H. Biomaterials A Review of the Prospects for Polymeric Nanoparticle Platforms in Oral Insulin Delivery. Biomaterials 2011, 32, 9826–9838. [Google Scholar] [CrossRef]
- Sarode, S.; Upadhyay, P.; Khosa, M.A.; Mak, T.; Shakir, A.; Song, S.; Ullah, A. Overview of Wastewater Treatment Methods with Special Focus on Biopolymer Chitin-Chitosan. Int. J. Biol. Macromol. 2019, 121, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of Different Factors Affecting Antimicrobial Properties of Chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Nurhayati, Y.; Manaf, A.A.; Osman, H.; Bakar, A.; Abdullah, C.; Yew, J.; Tang, H. Malaysian Journal of Applied Sciences Effect of Chitosan Oligosaccharides on the Growth of Bifidobacterium Species. Malays. J. Appl. Sci. 2016, 1, 13–23. [Google Scholar]
- Li, L.; Yang, L.; Li, M.; Zhang, L. A Cell-Penetrating Peptide Mediated Chitosan Nanocarriers for Improving Intestinal Insulin Delivery. Carbohydr. Polym. 2017, 174, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Anaya, L.M.; Fernández-Solís, K.G.; Rosselgong, J.; Nano-Rodríguez, J.L.E.; Carvajal, F.; Rinaudo, M. Chitosan-DNA Polyelectrolyte Complex: Influence of Chitosan Characteristics and Mechanism of Complex Formation. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Jangra, A.; Lukhi, M.M.; Sulakhiya, K.; Baruah, C.C.; Lahkar, M. Protective Effect of Mangiferin against Lipopolysaccharide-Induced Depressive and Anxiety-like Behaviour in Mice. Eur. J. Pharmacol. 2014, 740, 337–345. [Google Scholar] [CrossRef]
- Chang, A.K.T.; Frias, R.R.; Alvarez, L.V.; Bigol, U.G.; Guzman, J.P.M.D. Comparative Antibacterial Activity of Commercial Chitosan and Chitosan Extracted from Auricularia Sp. Biocatal. Agric. Biotechnol. 2019, 17, 189–195. [Google Scholar] [CrossRef]
- Gibot, L.; Chabaud, S.; Bouhout, S.; Bolduc, S.; Auger, F.A.; Moulin, V.J. Anticancer Properties of Chitosan on Human Melanoma Are Cell Line Dependent. Int. J. Biol. Macromol. 2015, 72, 370–379. [Google Scholar] [CrossRef]
- Miguel, S.P.; Moreira, A.F.; Correia, I.J. Chitosan Based-Asymmetric Membranes for Wound Healing: A Review. Int. J. Biol. Macromol. 2019, 127, 460–475. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as Biomaterial in Drug Delivery and Tissue Engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current Advancements in Chitosan-Based Film Production for Food Technology; A Review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Baghdan, E.; Pinnapireddy, S.R.; Strehlow, B.; Engelhardt, K.H.; Schäfer, J.; Bakowsky, U. Lipid Coated Chitosan-DNA Nanoparticles for Enhanced Gene Delivery. Int. J. Pharm. 2018, 535, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Shahid-ul-Islam; Butola, B.S. Recent Advances in Chitosan Polysaccharide and Its Derivatives in Antimicrobial Modification of Textile Materials. Int. J. Biol. Macromol. 2019, 121, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Khlibsuwan, R.; Pongjanyakul, T. Chitosan-Clay Matrix Tablets for Sustained-Release Drug Delivery: Effect of Chitosan Molecular Weight and Lubricant. J. Drug Deliv. Sci. Technol. 2016, 35, 303–313. [Google Scholar] [CrossRef]
- He, T.; Wang, W.; Chen, B.; Wang, J.; Liang, Q.; Chen, B. 5-Fluorouracil Monodispersed Chitosan Microspheres: Microfluidic Chip Fabrication with Crosslinking, Characterization, Drug Release and Anticancer Activity. Carbohydr. Polym. 2020, 236, 116094. [Google Scholar] [CrossRef]
- El-Alfy, E.A.; El-Bisi, M.K.; Taha, G.M.; Ibrahim, H.M. Preparation of Biocompatible Chitosan Nanoparticles Loaded by Tetracycline, Gentamycin and Ciprofloxacin as Novel Drug Delivery System for Improvement the Antibacterial Properties of Cellulose Based Fabrics. Int. J. Biol. Macromol. 2020, 161, 1247–1260. [Google Scholar] [CrossRef]
- Stie, M.B.; Gätke, J.R.; Wan, F.; Chronakis, I.S.; Jacobsen, J.; Nielsen, H.M. Swelling of Mucoadhesive Electrospun Chitosan/Polyethylene Oxide Nanofibers Facilitates Adhesion to the Sublingual Mucosa. Carbohydr. Polym. 2020, 242, 116428. [Google Scholar] [CrossRef]
- De Oliveira, R.L.; da Silva, M.F.; da Silva, S.P.; Cavalcanti, J.V.F.L.; Converti, A.; Porto, T.S. Immobilization of a Commercial Aspergillus Aculeatus Enzyme Preparation with Fructosyltransferase Activity in Chitosan Beads: A Kinetic/Thermodynamic Study and Fructo-Oligosaccharides Continuous Production in Enzymatic Reactor. Food Bioprod. Process. 2020, 122, 169–182. [Google Scholar] [CrossRef]
- da Silva, T.N.; Reynaud, F.; de Souza Picciani, P.H.; de Holanda e Silva, K.G.; Barradas, T.N. Chitosan-Based Films Containing Nanoemulsions of Methyl Salicylate: Formulation Development, Physical-Chemical and in Vitro Drug Release Characterization. Int. J. Biol. Macromol. 2020, 164, 2558–2568. [Google Scholar] [CrossRef]
- Dehghan-Baniani, D.; Chen, Y.; Wang, D.; Bagheri, R.; Solouk, A.; Wu, H. Injectable in Situ Forming Kartogenin-Loaded Chitosan Hydrogel with Tunable Rheological Properties for Cartilage Tissue Engineering. Colloids Surf. B Biointerfaces 2020, 192, 111059. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Singh, A.; Singh, A.V. Synthesis and Characterization of Pectin-Chitosan Conjugate for Biomedical Application. Int. J. Biol. Macromol. 2020, 153, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmed, S. A Review on Chitosan and Its Nanocomposites in Drug Delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Situ, W.; Xiang, T.; Liang, Y. Chitosan-Based Particles for Protection of Proteins during Storage and Oral Administration. Int. J. Biol. Macromol. 2018, 117, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an Environment Friendly Biomaterial – a Review on Recent Modifications and Applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, Characterization, and Potential Application of Chitosan, Chitosan Derivatives, and Chitosan Metal Nanoparticles in Pharmaceutical Drug Delivery. Drug Des. Devel. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.M.; Raquel, A.; Sarmento, B.; Pintado, M. Recent Insights in the Use of Nanocarriers for the Oral Delivery of Bioactive Proteins and Peptides. Peptides 2018. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of Nanoparticle Systems in Drug Delivery Technology. Saudi Pharm. J. 2017, 26, 64–70. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Xiong, X.Y.; Tian, Y.; Li, Z.L.; Gong, Y.C.; Li, Y.P. A Review of Biodegradable Polymeric Systems for Oral Insulin Delivery. Drug Deliv. 2015, 23, 1882–1891. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel Hydrophilic Chitosan-Polyethylene Oxide Nanoparticles as Protein Carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan Nanoparticles Preparation and Applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Brunel, F.; Véron, L.; David, L.; Domard, A.; Delair, T. A Novel Synthesis of Chitosan Nanoparticles in Reverse Emulsion. Langmuir 2008, 24, 11370–11377. [Google Scholar] [CrossRef]
- Chandra Hembram, K.; Prabha, S.; Chandra, R.; Ahmed, B.; Nimesh, S. Advances in Preparation and Characterization of Chitosan Nanoparticles for Therapeutics. Artif. Cells Nanomed. Biotechnol. 2016, 44, 305–314. [Google Scholar] [CrossRef]
- Naskar, S.; Sharma, S.; Kuotsu, K. Chitosan-Based Nanoparticles: An Overview of Biomedical Applications and Its Preparation. J. Drug Deliv. Sci. Technol. 2019, 49, 66–81. [Google Scholar] [CrossRef]
- Orellano, M.S.; Longo, G.S.; Porporatto, C.; Correa, N.M.; Falcone, R.D. Role of Micellar Interface in the Synthesis of Chitosan Nanoparticles Formulated by Reverse Micellar Method. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124876. [Google Scholar] [CrossRef]
- El-Shabouri, M.H. Positively Charged Nanoparticles for Improving the Oral Bioavailability of Cyclosporin-A. Int. J. Pharm. 2002, 249, 101–108. [Google Scholar] [CrossRef]
- Kumar, S.; Dilbaghi, N.; Saharan, R.; Bhanjana, G. Nanotechnology as Emerging Tool for Enhancing Solubility of Poorly Water-Soluble Drugs. Bionanoscience 2012, 2, 227–250. [Google Scholar] [CrossRef]
- Anu Bhushani, J.; Anandharamakrishnan, C. Electrospinning and Electrospraying Techniques: Potential Food Based Applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Yilmaz, A.; Akman, P.K.; Bozkurt, F.; Dertli, E.; Basahel, A.; Al-Sasi, B.; Taylan, O.; Sagdic, O. Electrospraying Method for Fabrication of Essential Oil Loaded-Chitosan Nanoparticle Delivery Systems Characterized by Molecular, Thermal, Morphological and Antifungal Properties. Innov. Food Sci. Emerg. Technol. 2019, 52, 166–178. [Google Scholar] [CrossRef]
- Jalvo, B.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antibacterial Surfaces Prepared by Electrospray Coating of Photocatalytic Nanoparticles. Chem. Eng. J. 2018, 334, 1108–1118. [Google Scholar] [CrossRef]
- Pawar, A.; Thakkar, S.; Misra, M. A Bird’s Eye View of Nanoparticles Prepared by Electrospraying: Advancements in Drug Delivery Field. J. Control. Release 2018, 286, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kawakami, K. One-Step Preparation of Chitosan Solid Nanoparticles by Electrospray Deposition. Int. J. Pharm. 2010, 397, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Abyadeh, M.; Sadroddiny, E.; Ebrahimi, A.; Esmaeili, F.; Landi, F.S.; Amani, A. Electrosprayed Chitosan Nanoparticles: Facile and Efficient Approach for Bacterial Transformation. Int. Nano Lett. 2017, 7, 291–295. [Google Scholar] [CrossRef]
- Abyadeh, M.; Aghajani, M.; Gohari Mahmoudabad, A.; Amani, A. Preparation and Optimization of Chitosan/PDNA Nanoparticles Using Electrospray. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 931–937. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Qin, W.; Dai, J.; Zhang, Q.; Lee, K.J.; Liu, Y. Physicochemical Properties of Gelatin Films Containing Tea Polyphenol-Loaded Chitosan Nanoparticles Generated by Electrospray. Mater. Des. 2020, 185, 108277. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Torres-Chávez, P.I.; Ramírez-Wong, B.; Rascón-Chu, A.; Plascencia-Jatomea, M.; Barreras-Urbina, C.G.; Rangel-Vázquez, N.A.; Rodríguez-Félix, F. Micro- and Nanoparticles by Electrospray: Advances and Applications in Foods. J. Agric. Food Chem. 2015, 63, 4699–4707. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Recent Advances of Polysaccharide-Based Nanoparticles for Oral Insulin Delivery. Int. J. Biol. Macromol. 2018, 120, 775–782. [Google Scholar] [CrossRef]
- Tavernini, L.; Ottone, C.; Illanes, A.; Wilson, L. Entrapment of Enzyme Aggregates in Chitosan Beads for Aroma Release in White Wines. Int. J. Biol. Macromol. 2020, 154, 1082–1090. [Google Scholar] [CrossRef]
- Bugnicourt, L.; Ladavière, C. Interests of Chitosan Nanoparticles Ionically Cross-Linked with Tripolyphosphate for Biomedical Applications. Prog. Polym. Sci. 2016, 60, 1–17. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.W.; Han, E.J.; Ahn, G. Alginate-Based Nanomaterials: Fabrication Techniques, Properties, and Applications. Chem. Eng. J. 2019, 391, 123823. [Google Scholar] [CrossRef]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan Based Self-Assembled Nanoparticles in Drug Delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Chakraborty, S.; Bhattacharya, S.; Mishra, R.; Kundu, P.P. PH-Sensitive Chitosan/Alginate Core-Shell Nanoparticles for Efficient and Safe Oral Insulin Delivery. Int. J. Biol. Macromol. 2015, 72, 640–648. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Mukherjee, D.; Mishra, R.; Kundu, P.P. Preparation of Polyurethane–Alginate/Chitosan Core Shell Nanoparticles for the Purpose of Oral Insulin Delivery. Eur. Polym. J. 2017, 92, 294–313. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Zhu, W.; Liang, Z.; Zeng, Q. Self-Assembly PH-Sensitive Chitosan/Alginate Coated Polyelectrolyte Complexes for Oral Delivery of Insulin. J. Microencapsul. 2019, 36, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Pereira De Sousa, I.; Moser, T.; Steiner, C.; Fichtl, B.; Bernkop-Schnürch, A. Insulin Loaded Mucus Permeating Nanoparticles: Addressing the Surface Characteristics as Feature to Improve Mucus Permeation. Int. J. Pharm. 2016, 500, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Formulation and Characterisation of Insulin-Loaded Chitosan Nanoparticles Capable of Inducing Glucose Uptake in Skeletal Muscle Cells in Vitro. J. Drug Deliv. Sci. Technol. 2020, 57, 101738. [Google Scholar] [CrossRef]
- Erel, G.; Kotmakçı, M.; Akbaba, H.; Sözer Karadağlı, S.; Kantarcı, A.G. Nanoencapsulated Chitosan Nanoparticles in Emulsion-Based Oral Delivery System: In Vitro and in Vivo Evaluation of Insulin Loaded Formulation. J. Drug Deliv. Sci. Technol. 2016, 36, 161–167. [Google Scholar] [CrossRef]
- He, Z.; Santos, J.L.; Tian, H.; Huang, H.; Hu, Y.; Liu, L.; Leong, K.W.; Chen, Y.; Mao, H.-Q. Scalable Fabrication of Size-Controlled Chitosan Nanoparticles for Oral Delivery of Insulin. Biomaterials 2017, 130, 28–41. [Google Scholar] [CrossRef]
- Wiessner, J.H.; Hwang, K.J. Binding of Insulin to the External Surface of Liposomes. Effect of Surface Curvature, Temperature, and Lipid Composition. BBA Biomembr. 1982, 689, 490–498. [Google Scholar] [CrossRef]
- Al-Remawi, M.; Elsayed, A.; Maghrabi, I.; Hamaidi, M.; Jaber, N. Chitosan/Lecithin Liposomal Nanovesicles as an Oral Insulin Delivery System. Pharm. Dev. Technol. 2017, 22, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.; Leong, K.H.; Nyamathulla, S.; Onuki, Y.; Takayama, K.; Chung, L.Y. Optimization of PH-Responsive Carboxymethylated Iota-Carrageenan/Chitosan Nanoparticles for Oral Insulin Delivery Using Response Surface Methodology. React. Funct. Polym. 2017, 119, 145–155. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P.; Guo, Z. Cationic Chitosan Derivatives as Potential Antifungals: A Review of Structural Optimization and Applications. Carbohydr. Polym. 2020, 236, 116002. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.-C.; Chen, C.-H.; Lin, C.-W.; Ho, Y.-C.; Mi, F.-L. Development of Mutlifunctional Nanoparticles Self-Assembled from Trimethyl Chitosan and Fucoidan for Enhanced Oral Delivery of Insulin. Int. J. Biol. Macromol. 2019, 126, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Jafary Omid, N.; Bahari Javan, N.; Dehpour, A.-R.; Partoazar, A.; Rafiee Tehrani, M.; Dorkoosh, F. In-Vitro and in-Vivo Cytotoxicity and Efficacy Evaluation of Novel Glycyl-Glycine and Alanyl-Alanine Conjugates of Chitosan and Trimethyl Chitosan Nano-Particles as Carriers for Oral Insulin Delivery. Int. J. Pharm. 2018, 535, 293–307. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Trimethyl Chitosan and Its Applications in Drug Delivery. J. Mater. Sci. Mater. Med. 2009, 20, 1057–1079. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.; Zhu, X.; Shan, W.; Li, L.; Zhong, J.; Zhang, Z.; Huang, Y. Efficient Mucus Permeation and Tight Junction Opening by Dissociable “Mucus-Inert” Agent Coated Trimethyl Chitosan Nanoparticles for Oral Insulin Delivery. J. Control. Release 2016, 222, 67–77. [Google Scholar] [CrossRef]
- Sheng, J.; Han, L.; Qin, J.; Ru, G.; Li, R.; Wu, L.; Cui, D.; Yang, P.; He, Y.; Wang, J. N -Trimethyl Chitosan Chloride-Coated PLGA Nanoparticles Overcoming Multiple Barriers to Oral Insulin Absorption. ACS Appl. Mater. Interfaces 2015, 7, 15430–15441. [Google Scholar] [CrossRef]
- Sheng, J.; He, H.; Han, L.; Qin, J.; Chen, S.; Ru, G.; Li, R.; Yang, P.; Wang, J.; Yang, V.C. Enhancing Insulin Oral Absorption by Using Mucoadhesive Nanoparticles Loaded with LMWP-Linked Insulin Conjugates. J. Control. Release 2016, 233, 181–190. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl Chitosan: Properties and Biomedical Applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, M.; Cheng, X.; Kong, M.; Liu, Y.; Feng, C.; Chen, X. Positive/Negative Surface Charge of Chitosan Based Nanogels and Its Potential Influence on Oral Insulin Delivery. Carbohydr. Polym. 2015, 136, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Krauland, A.H.; Alonso, M.J. Chitosan/Cyclodextrin Nanoparticles as Macromolecular Drug Delivery System. Int. J. Pharm. 2007, 340, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Li, L.; Zhang, Y.; Chen, K.; Wang, H.; Gong, R. Carboxymethyl-β-Cyclodextrin Grafted Chitosan Nanoparticles as Oral Delivery Carrier of Protein Drugs. React. Funct. Polym. 2017, 117, 10–15. [Google Scholar] [CrossRef]
- Song, M.; Wang, H.; Chen, K.; Zhang, S.; Yu, L.; Elshazly, E.H.; Ke, L.; Gong, R. Oral Insulin Delivery by Carboxymethyl-β-Cyclodextrin-Grafted Chitosan Nanoparticles for Improving Diabetic Treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S774–S782. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, S.; Gupta, P.K.; Singh, A.; Teja, B.V.; Dwivedi, P.; Gupta, G.K.; Trivedi, R.; Mishra, P.R. Vitamin B12 Functionalized Layer by Layer Calcium Phosphate Nanoparticles: A Mucoadhesive and PH Responsive Carrier for Improved Oral Delivery of Insulin. Acta Biomater. 2016, 31, 288–300. [Google Scholar] [CrossRef]
- Wang, J.; Kong, M.; Zhou, Z.; Yan, D.; Yu, X.; Cheng, X.; Feng, C.; Liu, Y.; Chen, X. Mechanism of Surface Charge Triggered Intestinal Epithelial Tight Junction Opening upon Chitosan Nanoparticles for Insulin Oral Delivery. Carbohydr. Polym. 2017, 157, 596–602. [Google Scholar] [CrossRef]
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and Chitosan Coating Nanoparticles for the Treatment of Brain Disease. Int. J. Pharm. 2019, 560, 282–293. [Google Scholar] [CrossRef]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a Coating Material for Nanoparticles Intended for Biomedical Applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Lopes, M.; Shrestha, N.; Correia, A.; Shahbazi, M.A.; Sarmento, B.; Hirvonen, J.; Veiga, F.; Seiça, R.; Ribeiro, A.; Santos, H.A. Dual Chitosan/Albumin-Coated Alginate/Dextran Sulfate Nanoparticles for Enhanced Oral Delivery of Insulin. J. Control. Release 2016, 232, 29–41. [Google Scholar] [CrossRef]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting Liposomes for Oral Drug Delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, T.I.; El-Refaie, W.M. Bioadhesive Chitosan-Coated Cationic Nanoliposomes With Improved Insulin Encapsulation and Prolonged Oral Hypoglycemic Effect in Diabetic Mice. J. Pharm. Sci. 2018, 107, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Moghassemi, S.; Parnian, E.; Hakamivala, A.; Darzianiazizi, M.; Vardanjani, M.M.; Kashanian, S.; Larijani, B.; Omidfar, K. Uptake and Transport of Insulin across Intestinal Membrane Model Using Trimethyl Chitosan Coated Insulin Niosomes. Mater. Sci. Eng. C 2015, 46, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Fonte, P.; Andrade, F.; Araújo, F.; Andrade, C.; Das Neves, J.; Sarmento, B. Chitosan-Coated Solid Lipid Nanoparticles for Insulin Delivery. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2012; Volume 508, pp. 295–314. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| Barriers against Oral Insulin Administration | ||||
|---|---|---|---|---|
| Physical Barriers | Chemical Barriers | Enzymatic Barriers | ||
| Mucus layer | Epithelial layer (Trans-cellular transportation) | Tight junctions (Para-cellular transportation) | Stomach: highly acidic (pH 1–3.7) ↓ Denaturation and degradation of insulin. Intestine: neutral and slightly alkaline (pH 6–8). | Insulin breakdowns by the protease’s enzymes found in the GIT [24]. Stomach: pepsin. Intestine: mainly trypsin, chymotrypsin |
| Viscous, hydrophilic, negatively charged layer ↓ Permitting only hydrophilic net-neutral molecules to pass ↓ | Highly limited to lipophilic drugs with molecular weight less than 700 Da as the membrane is mainly consisting of phospholipid bilayers [30]. | Regulate the transportation of molecules in between the epithelial cells. | ||
| Hydrophobic drugs and proteins are unable to cross, while cationic compounds exhibit low diffusion rate than neutral ones [31,35]. | Insulin is hydrophilic protein with high molecular weight 5800 Da. | Selectively permeable to small hydrophilic molecules [30,34,36]. | This variation in pH values may cause pH-induced oxidation and deamination of the protein [37,38]. | cytosolic and membrane-bound enzymes in the microvilli of intestinal enterocytes [39,40]. |
| Nanocarrier | Preparation Method | Particle Size (nm) | Zeta Potential (mV) | Entrapment Efficiency (%) | In Vitro Insulin Release | Dose (IU/kg) | In Vivo Observation | Reference |
| Chitosan (CS) MW (25–65 kDa), 83–86% Deacetylation Degree(DD) + Alginate (ALG) MW (1.03 × 105 g/mol) | Polyelectrolyte complexation | 216 | +3.89 | 78.3 | A burst release with max. of 26.7% of insulin release was found in pH 1.2, followed by a sustained and prolonged insulin release (79–84%) through 24 h. | Oral: 50–100 SC: 5 | Insulin-loaded CS/ALG NPs (50 and 100 IU/kg) showed reduction in the blood glucose level to 143 and 104 mg/dL, respectively, with sustained effect up to 9 h. | [93] |
| Medium MW, 75%, 85% deacetylated Chitosan + TPP ratio 6:1 | Ionic gelation method | Nanoparticle 356.5 ± 43.4 (Microemultion) 99.1 ± 28.7 | Nanoparticle 46.5 (Microemultion) 13.1 | - | At pH 2.5 after 2 h, insulin release from microemulsion was 48.1%. At pH 6.8 after 2 h, the release was 51.2% and after 3 h it was 66.1%. | Oral: 50 SC: 1 | Plasma glucose level reduced to 68.7% after 3 h and it maintained at 66.4% of the initial blood glucose level after 8 h. | [98] |
| Chitosan 25 kDa, + Chondroitin sulphate (ChS) 20–30 KDa + Polyethylene glycol 5000 Da (PEG) | Ionic gelation | 510–670 | −1 to −5 | 2.18 ± 0.70 | In simulated intestinal fluid (SIF) buffer, insulin release profile showed a gradual release of the protein reaching 65% in 4 h, followed by a plateau. | - | - | [96] |
| 90 KDa MW, 85% deacetylated chitosan + TPP | Flash nanocomplexation using multi-inlet vortex mixer | 46.2 ± 2.7 | 9.4 ± 1.2 | 91.0 ± 1.7 | The amount of released insulin at pH 2.5 was about 16%, while negligible amount at pH 6.6, and a sustained release of insulin within a few hours at pH 7.4 | Oral: 60 or 120 SC: 10 | Gradual but distinct reduction of blood glucose levels by 51% (60 IU/kg) and 59% (120 IU/kg) within 8 h. | [99] |
| Chitosan (28 kDa) + Lecithin liposomes + L-Arginine | CS-insulin dispersion (polyelectrolyte complexation) added to lecithin liposomal dispersion | 105 ± 17 | −30 | 20 | Insulin was rapidly released in both 0.1 M HCl and phosphate buffer pH 6.8 media and complete release was achieved almost after 30 min. | Oral: 50 SC: 1 | A significant effect was observed at 2 h after oral administration as the blood glucose level was reduced by almost 17% of the initial level and the effect was prolonged for up to 8 h. | [101] |
| Low MW 50–190 kDa, ≥75.0% deacetylated chitosan + Iota-carrageenan (CMCi) | Polyelectrolyte complexation method | 613 ± 41 | 52.5 ± 0.5 | 86.9 ± 2.6 | After 2 h in simulated gastric fluid (SGF), the release of insulin from the nanoparticles was only 4.91 ± 0.24%, while in SIF, the release of insulin was 86.64 ± 2.20%. | - | - | [102] |
| Chitosan, alloxan monohydrate + Alginate + Polyurethane (PU-ALG/CS NPs) | Polyelectrolyte complexation method | 90–110 | 38.5 | 90 | There was a slight insulin release (13.7%) at pH 1.2 up to 1 h, while moderately release (up to 50%) till 10th h in pH 6.8 buffer solution, whereas sustained release of insulin was noticed at pH 7.4 from 11th h, and reached the maximum insulin release after 20th h (98.32%). | Oral: 50 and 100 SC: 5 | Blood glucose level was reduced up to 98 mg/dL for the insulin doses of 100 IU/kg, and 131 mg/dL for the 50 IU/kg dose at the 10th h. | [94] |
| Chitosan 95% DD + Alginate + Methoxypolyethylene glycol (mPEG, MW 5.0 kDa) + D, L-Lactide (LA) + Glycolide (GA) + Poly (vinyl alcohol)1788 low-viscosity (PVA) + poly (ethylene glycol)-block-poly (propylene glycol)-block-poly (ethylene glycol) (F68, Mw 8.4 kDa) | Double-emulsion (w/o/w) solvent evaporation method + Polyelectrolyte complexation | CS NP 224.4 ± 13.8 Alg NP 260.1 ± 17.1 | CS NP +13.7 ± 1.6 Alg NP −55.7 ± 6.6 | CS NP 55.2 ± 7.0 Alg NP 81.5 ± 7.4 | The insulin loaded PEC enabled a slight insulin release (only 13.91%) in SGF (pH 1.2) within the first 4 h. In contrast, rapid rising rate in the first 4 h (38.03%) at the pH 6.8 took place, and the cumulative drug release increased to 51.57% within 10 h, and reached 80.54% after 60 h. | Oral: 60 SC: 5 | The blood glucose level decreased after the oral administration of insulin-loaded PEC with the maximal blood glucose reduction of 30% at 8 h, and 20% after 12 h. Insulin concentration in plasma was increased gradually and resulted in a maximum plasma concentration (41.5 ± 4.4 μIU mL−1) at 10 h. | [95] |
| Chitosan (95% deacetylated; MW 150 kDa) + Dz13Scr | Complex coacervation | 534 ± 24 | 14.57 ± 1.1 | 79.96 ± 3.96 | Only 14.03% of cumulative insulin released at pH 2, while approximately 85% of insulin was released after 10 h at pH 6.8 phosphate buffer solution. | - | - | [97] |
| Polymer | Nanocarrier Components | Method of Preparation | Particle Size, Zeta Potential | Encapsulation Efficiency, Drug Loading | In Vitro Insulin Release | Dose | In Vivo Observation | Reference |
|---|---|---|---|---|---|---|---|---|
| Trimethyl chitosan | 400 KDa MW, >90% deacetylated TMC+TPP + Poly N-(2-hydroxypropyl) methacrylamide (HPMA) (pHPMA) | Mild electrostatic self-assembly process | 163.1 nm −3.35 mV | 54.1 ± 1.9% 26.5 ± 0.7% | Rapid insulin release at pH 2, while within 8 h, sustained release was observed at both pHs 6 and 7.4. 70% of insulin released in presence of trypsin within 4 h. | Oral: 50 IU/kg SC: 5 IU/kg | 36% decreasing of blood glucose level (BDL) at 4 h, and the effect lasted for 10 h. | [108] |
| Trimethyl chitosan | 275 KDa MW, 95% deacetylated chitosan (CS)/(TMC) + Alanyl alanine (AA) + Glycyl-glycine (GG) | Polyelectrolyte complexation | CS-GG 167.8 ± 46.1 nm 25.40 ± 4.2 mV CS-AA 185.3 ± 27.6 nm 24.62 ± 3.6 mV TMC-GG 157.3 ± 38.5 nm 34.37 ± 5.1 mV TMC-AA 197.7 ± 31.7 nm 24.35 ± 1.9 mV | CS-GG 86.52 ± 4.7% 56.81 ± 6.7% CS-AA 77.20 ± 5.9% 30.92 ± 4.6% TMC-GG 70.60 ± 7.2% 39.07 ± 2.6% TMC-AA 83.08 ± 6.2% 37.24 ± 1.6% | Burst release of insulin within the first 30 min, after that insulin has been released in a controlled manner and reached a maximum of 83.4% in CS-GG, 78.3% in CS-AA, 75.9% in TMC-GG, 73.9% in TMC-AA. | Oral: 20 IU/kg SC: 3 IU/kg | Both TMC-GG and TMC-AA nanoparticles reduced the BGL considerably compared to oral insulin. While TMC-nanoparticles decreased the BGL to only 61.3% of the initial, TMC-GG showed maximum reduction to 46.8%, followed by TMC-AA to 54% after 8 h. | [106] |
| Trimethyl chitosan | 33 KDa MW, 85% deacetylated TMC + Fucoidan (FD) MW (31.7 kDa) | Simple polyelectrolyte complex | 256.7 ± 4.9 nm 26.5 ± 1.1 mV | 56.4 ± 4.3% 8.6 ± 2.2% | At pH 2 slow insulin release at 38.3 ± 2.1% and 45.2 ± 2.7% of TMC/FD and CS/FD, respectively, while at pH 7.4 the release of insulin was faster, and more rapid 75.4 ± 2.2% and 93.4 ± 1.6%, respectively. | - | - | [105] |
| Carboxymethyl chitosan (CMCS) | 400 KDa MW, 95% deacetylated chitosan | Simple ionic gelation | CMCS/CS-NGs (−) 243 ± 3.85 nm −15.9 ± 0.45 (+) 260 ± 4.47 nm +17.2 ± 0.49 nm | CMCS/CS-NGs (−) 73 ± 6.36% 29 ± 3.61% (+) 74 ± 8.36% 27 ± 4.04% | Insulin released was 28% in SGF and approximately 87% in SIF. | Oral: 50 IU/kg SC: 5 IU/kg | At 4 h, the nanoparticles with negative charge have made BGL dropped to 82.8 mg/dL while positive ones to 138.6 mg/dL, and this effect prolonged for 11 h | [112] |
| Carboxymethyl chitosan (CMCS) | 400 KDa MW, 95% deacetylated chitosan | Simple ionic gelation | - | - | CMCSNP (−): pH 1.2 (20.7% at 2 h), pH 7 (83.4% at 2 h) CMCSNP (+): pH 1.2 (33.6% at 2 h), pH 7 (71.6% at 2 h) | Oral: 50 IU/kg SC: 5 IU/kg | [117] | |
| Carboxymethyl-β-cyclodextringrafted chitosan (CMCD-g-CS) | MW 46K with 90–95% deacetylation + carboxymethyl-β-cyclodextrin MW 1591 + TPP | Ionic gelation | 218 nm | EE 57.0 ± 1.38% | About 35.4 ± 0.025% of insulin was rapidly released in SGF (pH 1.2) after 15 min, while in SIF (pH 7.4), after 120 min the cumulative amount of insulin released increased to 82.9 ± 0.04%. | Oral: 50 IU/kg SC: 5 IU/kg | Insulin/CMCD-g-CS nanoparticles administration showed gradually enhanced hypoglycemic effect. After 12 h, the BGL was reduced to 51.22% of the initial level. The nanoparticles exhibited a relative bioavailability of 14.54%. | [115] |
| Vitamin B12-grafted chitosan | Vitamin B12-grafted chitosan (75–85% Deacetyld, 65–95 kDa) + alginate + calcium phosphate | Micro-emulsion method | 234.83 nm 32.56 mV | 75.16% 7.83% | At pH 1.2 only 9.9% insulin was released at 2 h. | Oral: 50 IU/kg SC: 5 IU/kg | BGL reduced to 197 mg/dL and maintained up to 12 h. | [116] |
| Type of Chitosan | Type of Nanoparticles | Preparation Method | Observation | Reference |
|---|---|---|---|---|
| Chitosan (30 KDa low MW with 85% DD) | Liposomes | Simple thin-film hydration technique | In vitro: at pH 1.2, lower percentage of insulin released from CS-coated liposomes (18.9 ± 0.35%) compared to (35.9 ± 0.75%) uncoated ones after 48 h. At higher pH (7.4), CS-coated liposomes gradually released almost 74% of insulin over a prolonged time of 48 h. In vivo: Blood glucose level remarkably decreased after 1 h of CS-coated liposomes administration. BGL continued lowering until reached its normal level and maintained it for 4 h (8 h from administration). | [122] |
| Trimethyl chitosan (TMC) (low MW) | Niosomes | Reversed-phase evaporation method | In vitro: the insulin release rate was significantly slower than insulin alone as after 5 h, insulin reached its maximum level (12% from TMC-coated niosomes while 63.42% from free solution). TMC-coated niosomes continued to improve insulin transport until 120 min through Caco-2 cells. Insulin permeation coefficient increased by 4 folds from coated niosomal nanoparticles more than insulin alone. | [123] |
| Chitosan (50 kDa, 85% DD) | Solid lipid nanoparticles | w/o/w emulsion method | In vitro: CS-coated solid lipid nanoparticles demonstrated better permeation-enhancing properties as compared to the uncoated ones through Caco-2 cell monolayer. In vivo: CS-coated solid lipid nanoparticles increased the hypoglycemic effect and enhanced the pharmacological availability to 17.7% compared to 5.1–8.3% of uncoated solid lipid nanoparticles. | [124] |
| TMC (85% DD, degree of trimethyl substitution 50%) | Polymeric nanoparticles (PLGA-NP) | Double-emulsion solvent evaporative method | In vitro: compared with PLGA nanoparticles, TMC-PLGA nanoparticles relatively protected insulin from enzymatic degradation in the GIT. TMC-coated nanoparticles showed stronger mucoadhesive and mucus-penetrating capacity through HT29-MTX cells. The cellular uptake of insulin of TMC-PLGA nanoparticles was dramatically higher than uncoated PLGA nanoparticles through HT29-MTX cells without a mucus layer, while the amount of insulin penetrated the mucus layer was 2 folds greater for the coated TMC-PLGA nanoparticles. In vivo: 40% of TMC-PLGA nanoparticles could attach to the lower part of the small intestine prolonging the retention time at the absorption site while most of the PLGA nanoparticles moved to the colon within 3 h. TMC-PLGA nanoparticles decreased the BGL rapidly to 70% of the initial level after 7 h, and continued to decrease over 12 h. TMC coated nanoparticles also had higher pharmacological availability of 11.82% compared to 5.93% of uncoated ones. | [109] |
| TMC (85% DD, degree of trimethyl substitution 50%) | Polymeric nanoparticles (PLGA-NP) + (LMW protamine conjugated insulin) | Ultrasound sonication, double emulsion | In vitro: the mucoadhesive TMC-coated PLGA nanoparticles gave effective protection to encapsulated insulin or insulin-LMW protamine as only 5% of insulin released after 1 h at SGF of 1.2 pH, while 40% at SGF with pepsin compared to 90% digestion of insulin or insulin-LMW protamine in enzyme-containing SGF within 5 min. Coated nanoparticles significantly improved insulin permeability through Caco-2 cells. Insulin permeation coefficient of insulin-LMW protamine coated nanoparticles was 10-fold higher than that of insulin solution. In vivo: pharmacological availability has been remarkably enhanced (17.98%) from insulin-LMW protamine coated nanoparticles, compared to 0.91 of the free insulin-LMW conjugates. | [110] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seyam, S.; Nordin, N.A.; Alfatama, M. Recent Progress of Chitosan and Chitosan Derivatives-Based Nanoparticles: Pharmaceutical Perspectives of Oral Insulin Delivery. Pharmaceuticals 2020, 13, 307. https://doi.org/10.3390/ph13100307
Seyam S, Nordin NA, Alfatama M. Recent Progress of Chitosan and Chitosan Derivatives-Based Nanoparticles: Pharmaceutical Perspectives of Oral Insulin Delivery. Pharmaceuticals. 2020; 13(10):307. https://doi.org/10.3390/ph13100307
Chicago/Turabian StyleSeyam, Salma, Norsyafikah Asyilla Nordin, and Mulham Alfatama. 2020. "Recent Progress of Chitosan and Chitosan Derivatives-Based Nanoparticles: Pharmaceutical Perspectives of Oral Insulin Delivery" Pharmaceuticals 13, no. 10: 307. https://doi.org/10.3390/ph13100307
APA StyleSeyam, S., Nordin, N. A., & Alfatama, M. (2020). Recent Progress of Chitosan and Chitosan Derivatives-Based Nanoparticles: Pharmaceutical Perspectives of Oral Insulin Delivery. Pharmaceuticals, 13(10), 307. https://doi.org/10.3390/ph13100307