Cytotoxicity Effects of Water-Soluble Multi-Walled Carbon Nanotubes Decorated with Quaternized Hyperbranched Poly(ethyleneimine) Derivatives on Autotrophic and Heterotrophic Gram-Negative Bacteria
Abstract
1. Introduction
2. Results and Discussion
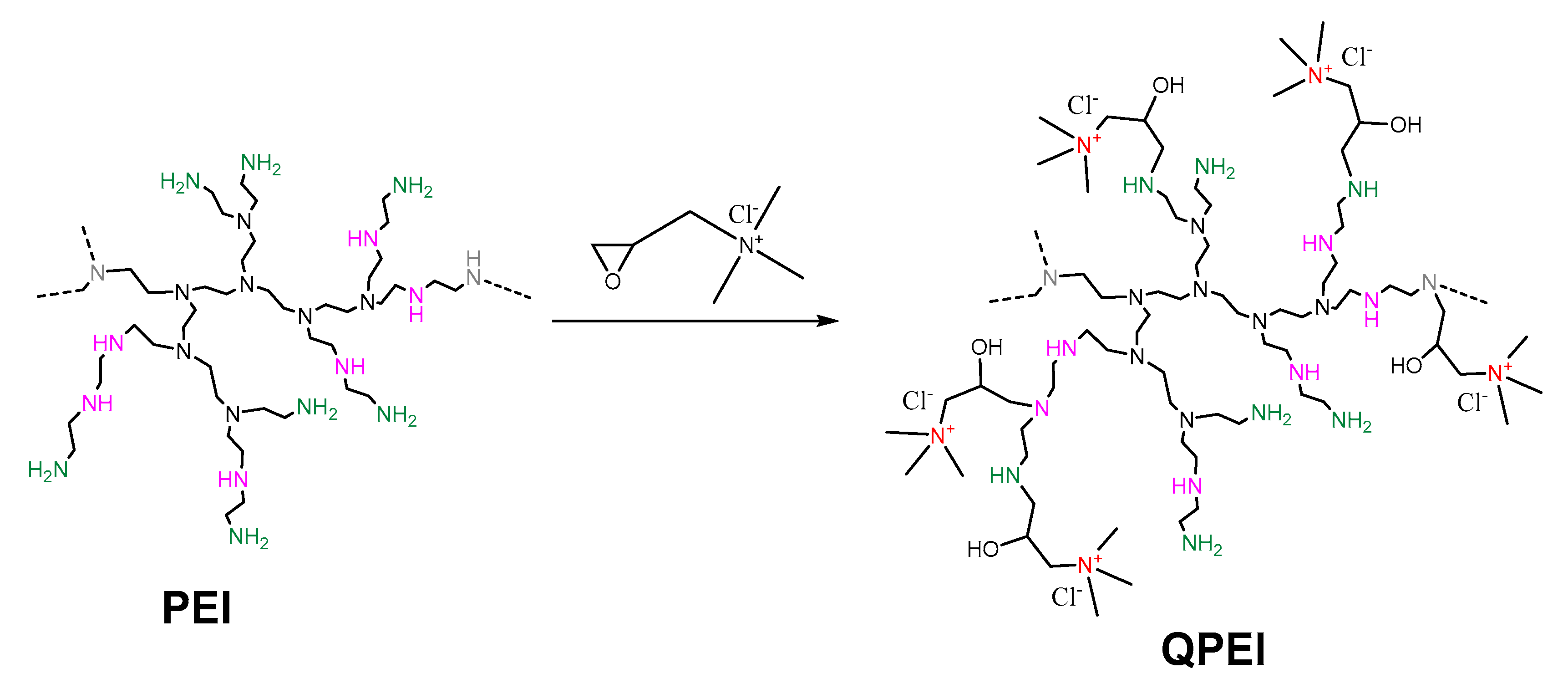
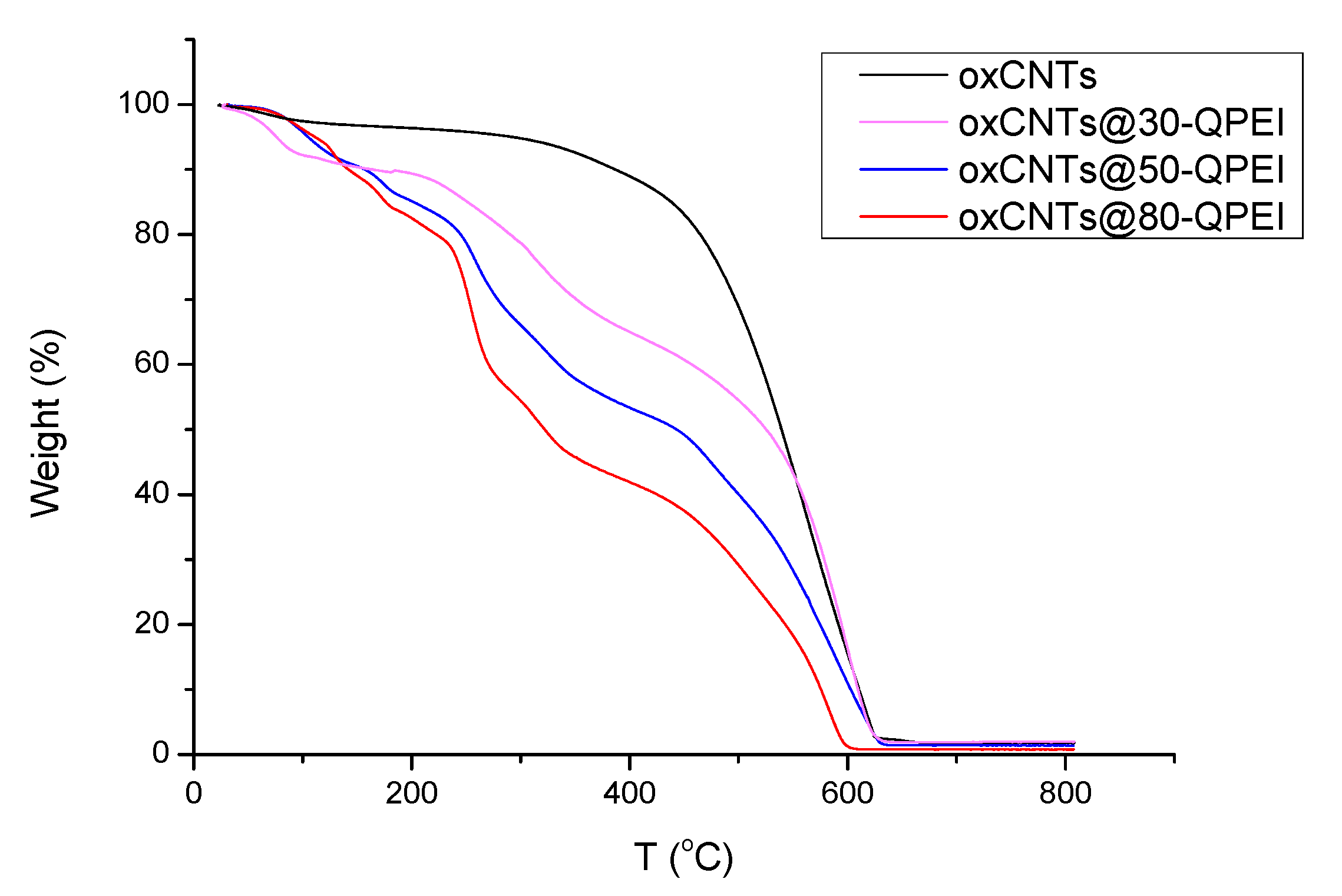
2.1. Synthesis and Characterization of QPEI-Functionalized oxCNTs
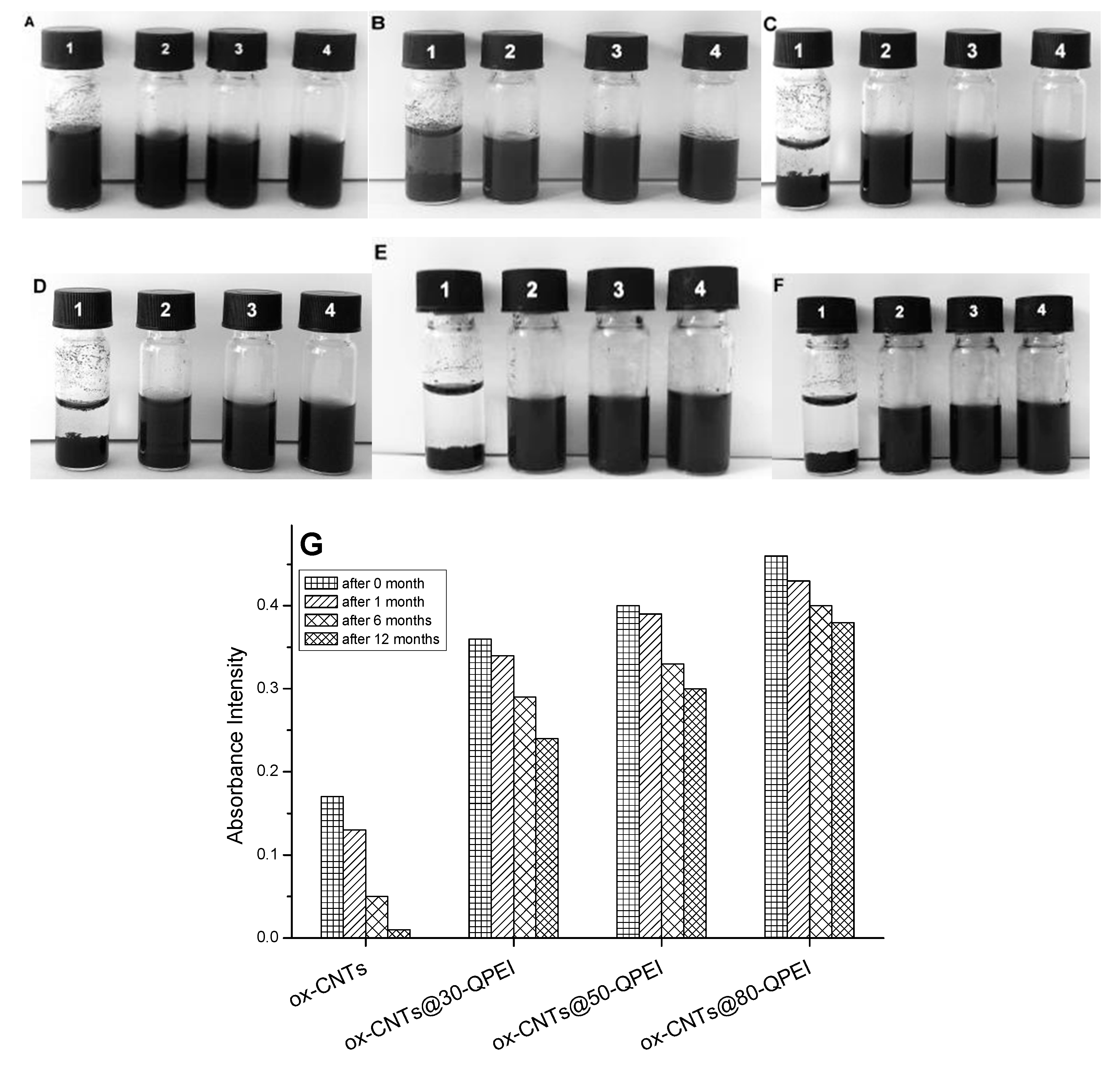
2.2. Colloidal Stability of the CNTs Dispersions
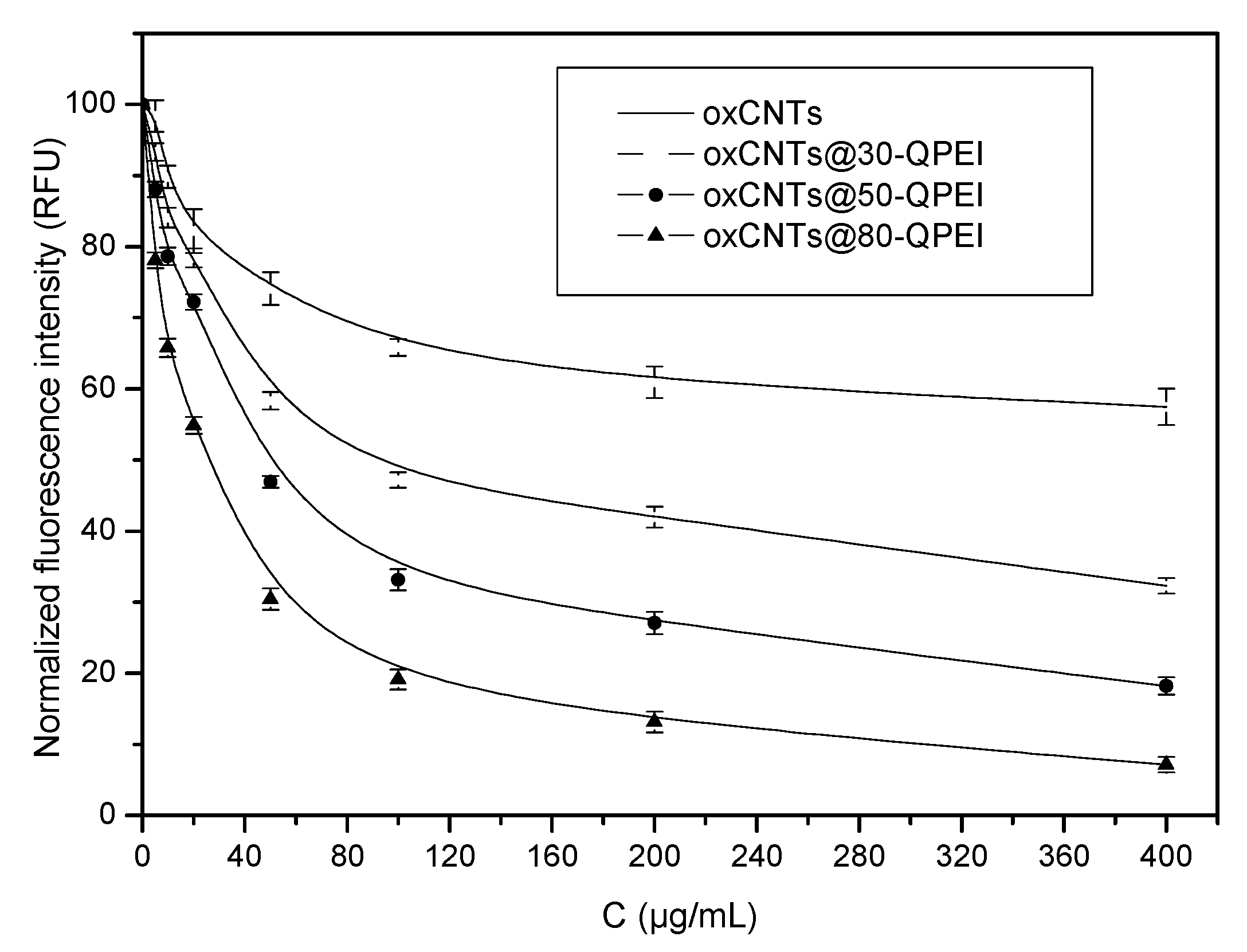
2.3. Characterization of the QPEI-Functionalized oxCNTs Dispersions
2.4. Evaluation of Antibacterial and Anti-Cyanobacterial Activity
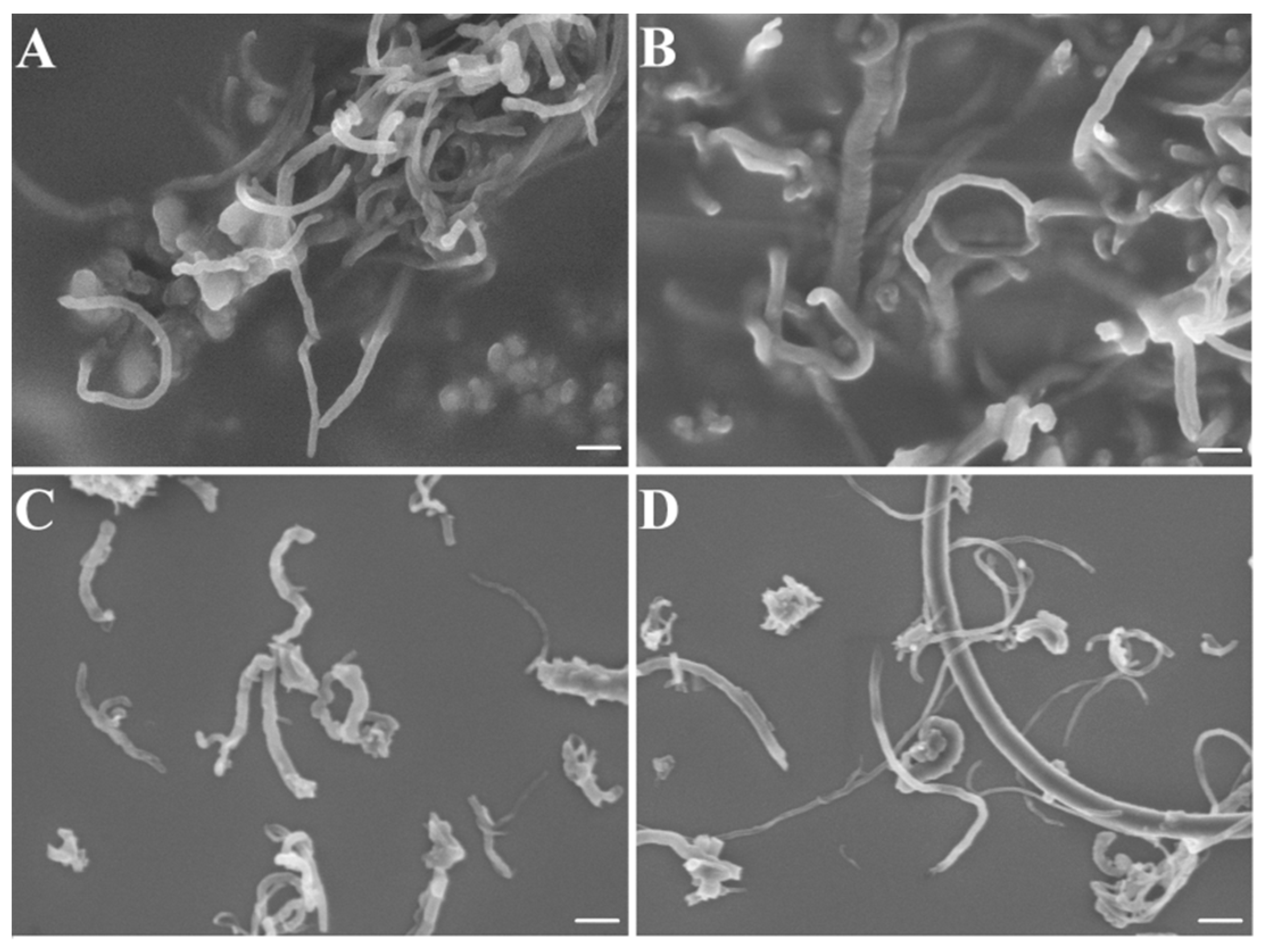
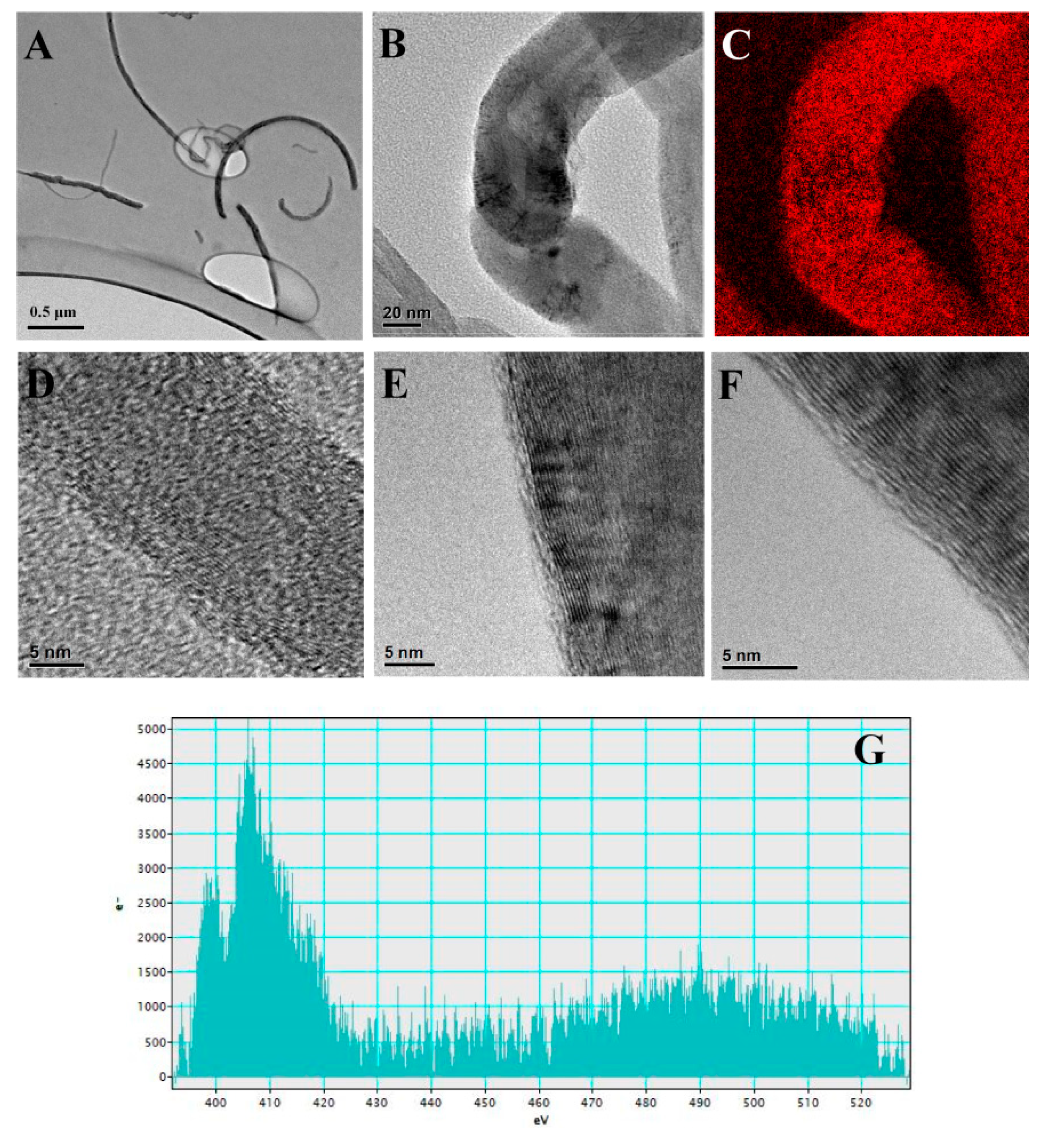
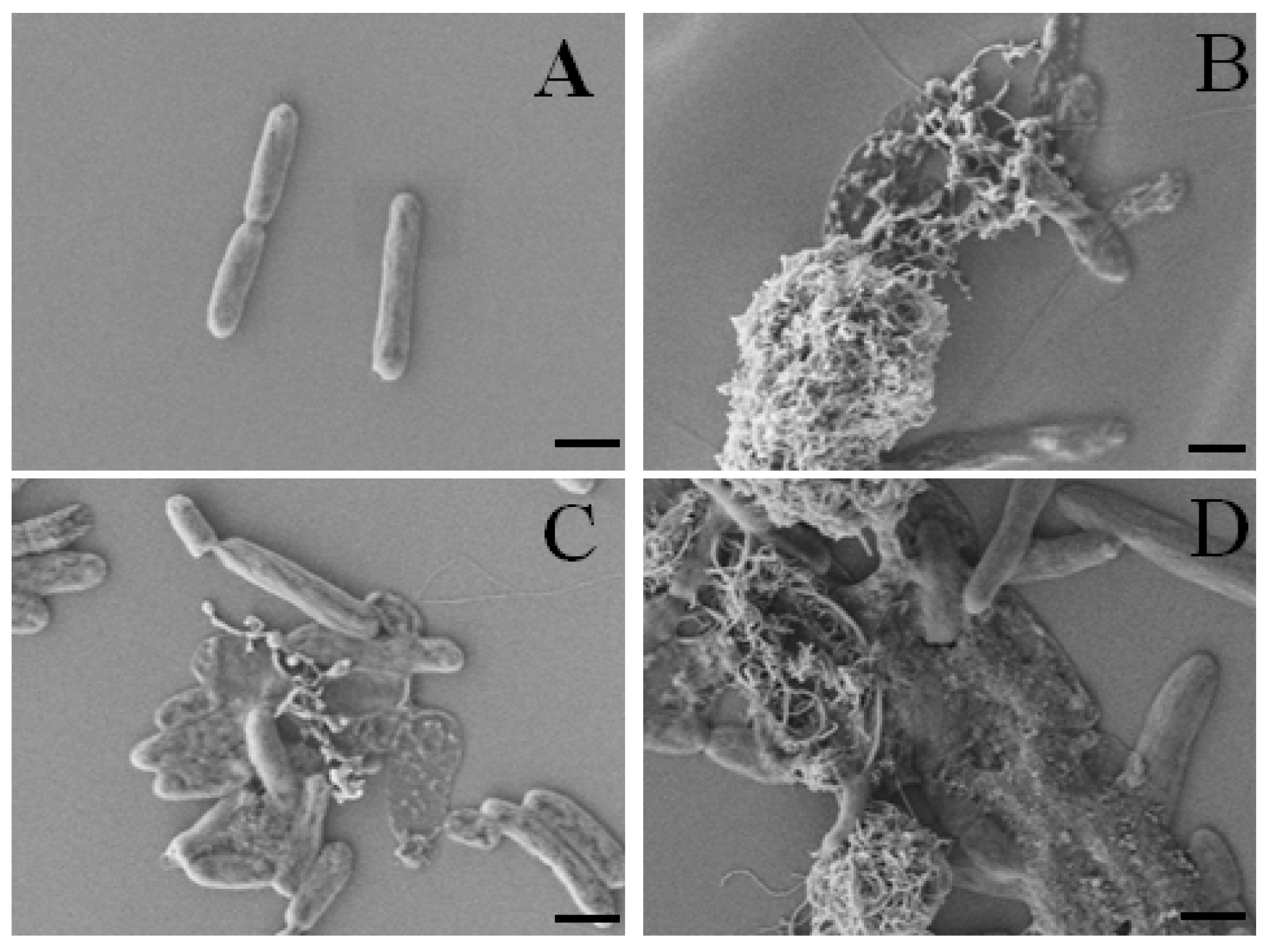
2.4.1. Cytotoxicity Effects of oxCNTs@QPEIs on Escherichia coli XL1-Blue Bacteria
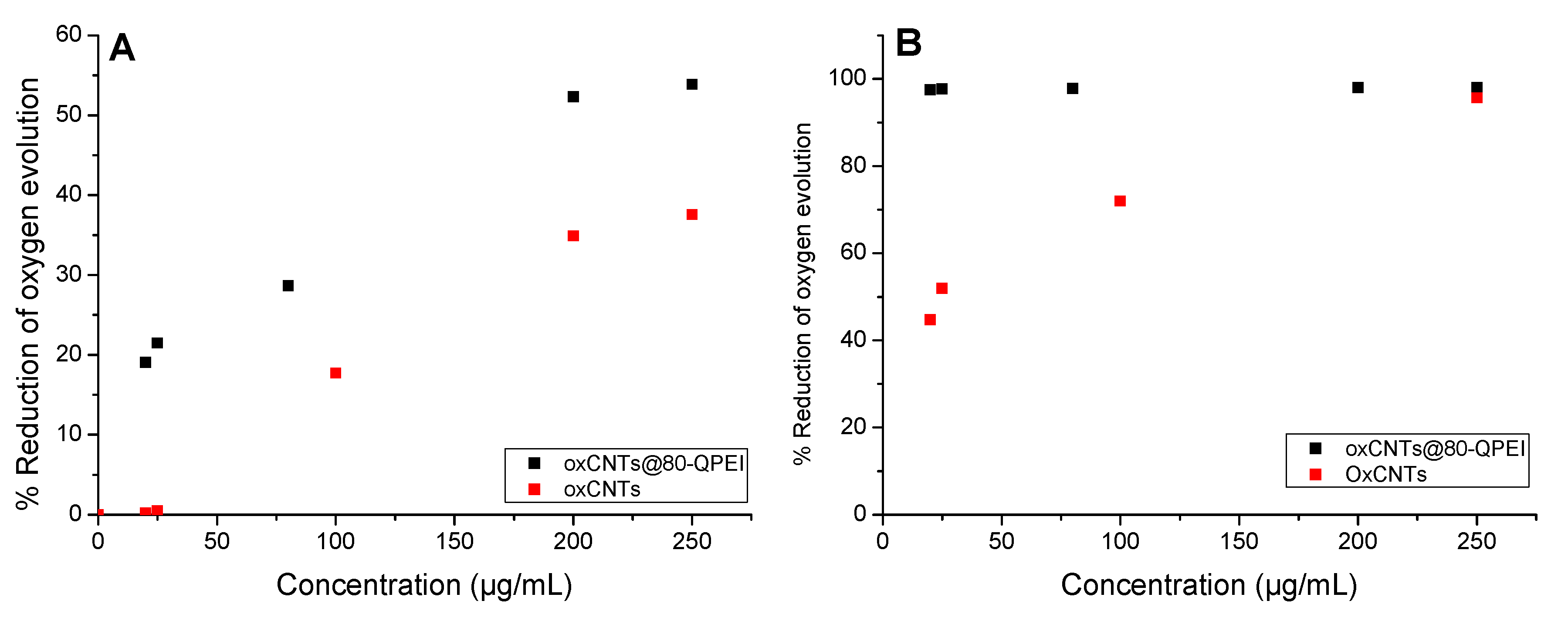
2.4.2. Cytotoxicity Effects of oxCNTs@QPEIs on Synechococcus sp. PCC 7942 Cyanobacteria
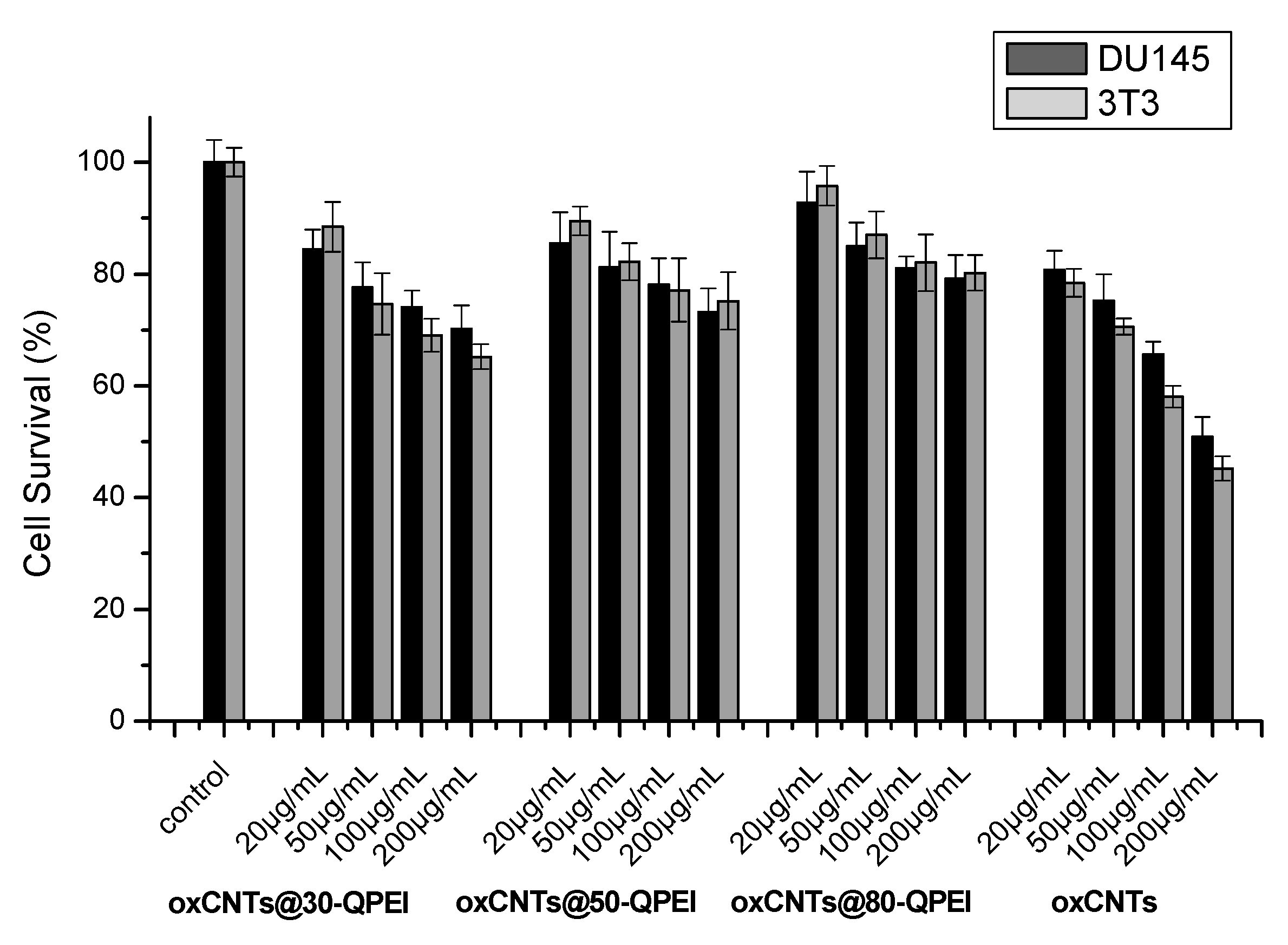
2.5. Cell Viability Assay
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of Quaternized Hyperbranched Poly(ethyleneimine) Derivatives
3.3. Preparation of QPEI-Functionalized oxCNTs
3.4. Characterization of QPEI-Functionalized oxCNTs
3.5. Preparation and Characterization of QPEI-Functionalized oxCNTs Aqueous Dispersions
3.6. Escherichia coli Growth Inhibition Assay
3.7. SEM Analysis of the Cellular Morphology
3.8. Synechococcus sp. PCC7942 Cyanobacteria Growth Inhibition Assay
3.9. Measurements of Photosystem I and II Electron Transport Activities
3.10. Cell Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dresselhaus, M.S.; Dresselhaus, G.; Avouris, P. Carbon Nanotubes: Synthesis, Structure, Properties and Applications; Springer: Berlin, Germany, 2001. [Google Scholar]
- Novoselov, K.S.; Fal, V.; Colombo, L.; Gellert, P.; Schwab, M.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Sun, H.; Qu, X. Antibacterial applications of graphene-based nanomaterials: Recent achievements and challenges. Adv. Drug Deliv. Rev. 2016, 105, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Breuer, O.; Uttandaraman, S. Big returns from small fibers: A review of polymer/carbon nanotube composites. Polym. Compos. 2004, 25, 630–645. [Google Scholar] [CrossRef]
- Soleyman, R.; Hirbod, S.; Adeli, M. Advances in the biomedical application of polymer-functionalized carbon nanotubes. Biomater. Sci. 2015, 3, 695–711. [Google Scholar] [CrossRef]
- Baia, Y.; Park, I.S.; Lee, S.J.; Bae, T.S.; Watari, F.; Uo, M.; Lee, M.H. Aqueous dispersion of surfactant-modified multiwalled carbon nanotubes and their application as an antibacterial agent. Carbon 2011, 49, 3663–3671. [Google Scholar] [CrossRef]
- Sun, J.-T.; Hong, C.-Y.; Pan, C.-Y. Surface modification of carbon nanotubes with dendrimers or hyperbranched polymers. Polym. Chem. 2011, 2, 998–1007. [Google Scholar] [CrossRef]
- Tuncel, D. Non-covalent interactions between carbon nanotubes and conjugated polymers. Nanoscale 2011, 3, 3545–3554. [Google Scholar] [CrossRef]
- Bilalis, P.; Katsigiannopoulos, D.; Avgeropoulos, A.; Sakellariou, G. Non-covalent functionalization of carbon nanotubes with polymers. RSC Adv. 2014, 4, 2911–2934. [Google Scholar] [CrossRef]
- Ata, M.S.; Poon, R.; Syed, A.M.; Milne, J.; Zhitomirsky, I. New developments in non-covalent surface modification, dispersion and electrophoretic deposition of carbon nanotubes. Carbon 2018, 130, 584–598. [Google Scholar] [CrossRef]
- Star, A.; Stoddart, J.F. Dispersion and Solubilization of Single-Walled Carbon Nanotubes with a Hyperbranched Polymer. Macromolecules 2002, 35, 7516–7520. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Majoral, J.-P. Dendrimers and nanotubes: A fruitful association. Chem. Soc. Rev. 2010, 39, 2034–2047. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-L.; Chen, M.-L.; Chen, X.-W.; Wang, J.-H. Functionalization of MWNTs with Hyperbranched PEI for Highly Selective Isolation of BSA. Macromol. Biosci. 2010, 10, 906–915. [Google Scholar] [CrossRef]
- Fréchet, J.M.J.; Tomalia, D.A. Dendrimers and Other Dendritic Polymers; J Wiley & Sons: Chichester, UK, 2001. [Google Scholar]
- Pedziwiatr-Werbicka, E.; Milowska, K.; Dzmitruk, V.; Ionov, M.; Shcharbin, D.; Bryszewska, M. Dendrimers and hyperbranched structures for biomedical applications. Eur. Polym. J. 2019, 119, 61–73. [Google Scholar] [CrossRef]
- Paleos, C.M.; Tsiourvas, D.; Sideratou, Z. Triphenylphosphonium decorated liposomes and dendritic polymers: Prospective second generation drug delivery systems for targeting mitochondria. Mol. Pharm. 2016, 13, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Yudovin-Farber, I.; Golenser, J.; Beyth, N.; Weiss, E.I.; Domb, A.J. Quaternary ammonium polyethyleneimine: Antibacterial activity. J. Nanomater. 2010, 2010. [Google Scholar] [CrossRef]
- Mammen, M.; Choi, S.-K.; Whitesides, G.M. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. 1998, 37, 2755–2794. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial activity of carbon-based nanoparticles. Adv. Pharm. Bull. 2015, 5, 19–23. [Google Scholar]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the antimicrobial properties of carbon nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef]
- Maas, M. Carbon Nanomaterials as Antibacterial Colloids. Materials 2016, 9, 617. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef] [PubMed]
- Mocan, T.; Matea, C.T.; Pop, T.; Mosteanu, O.; Buzoianu, A.D.; Suciu, S.; Puia, C.; Zdrehus, C.; Iancu, C.; Mocan, L. Carbon nanotubes as anti-bacterial agents. Cell. Mol. Life Sci. 2017, 74, 3467–3479. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial effects of carbon nanotubes: Size does matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Mamouni, J.; Tang, Y.; Yang, L. Antimicrobial activity of single-walled carbon nanotubes: Length effect. Langmuir 2010, 26, 16013–16019. [Google Scholar] [CrossRef]
- Kang, S.; Mauter, M.S.; Elimelech, M. Physicochemical determinants of multiwalled carbon nanotube bacterial cytotoxicity. Environ. Sci. Technol. 2008, 42, 7528–7534. [Google Scholar] [CrossRef]
- Arias, L.R.; Yang, L.J. Inactivation of bacterial pathogens by carbon nanotubes in suspensions. Langmuir 2009, 25, 3003–3012. [Google Scholar] [CrossRef]
- Baek, S.; Joo, S.H.; Su, C.; Toborek, M. Antibacterial effects of graphene- and carbon-nanotube-based nanohybrids on Escherichia coli: Implications for treating multidrug-resistant bacteria. J. Environ. Manag. 2019, 247, 214–223. [Google Scholar] [CrossRef]
- Xia, L.; Xu, M.; Cheng, G.; Yang, L.; Guo, Y.; Li, D.; Fang, D.; Zhang, Q.; Liu, H. Facile construction of Ag nanoparticles encapsulated into carbon nanotubes with robust antibacterial activity. Carbon 2018, 130, 775–781. [Google Scholar] [CrossRef]
- Atiyah, A.A.; Haider, A.J.; Dhahi, R.M. Cytotoxicity properties of functionalised carbon nanotubes on pathogenic bacteria. IET Nanobiotechnol. 2019, 13, 597–601. [Google Scholar] [CrossRef]
- Baia, Y.; Park, I.S.; Lee, S.J.; Wen, P.S.; Bae, T.S.; Lee, M.H. Effect of AOT-assisted multi-walled carbon nanotubes on antibacterial activity. Colloids Surf. B 2012, 89, 101–107. [Google Scholar] [CrossRef]
- Deng, R.; Zhu, Y.; Hou, J.; White, J.C.; Gardea-Torresdey, J.L.; Lin, D. Antagonistic toxicity of carbon nanotubes and pentachlorophenol to Escherichia coli: Physiological and transcriptional responses. Carbon 2019, 145, 658–667. [Google Scholar] [CrossRef]
- Trompeta, A.-F.A.; Preiss, I.; Ben-Ami, F.; Benayahu, Y.; Charitidis, C.A. Toxicity testing of MWCNTs to aquatic organisms. RSC Adv. 2019, 9, 36707–36716. [Google Scholar] [CrossRef]
- Ganguly, P.; Breen, A.; Pillai, S.C. Toxicity of nanomaterials: Exposure, pathways, assessment, and recent advances. ACS Biomater. Sci. Eng. 2018, 4, 2237–2275. [Google Scholar] [CrossRef]
- Wang, X.P.; Han, H.Y.; Liu, X.Q.; Gu, X.X.; Chen, K.; Lu, D.L. Multi-walled carbon nanotubes can enhance root elongation of wheat (Triticum aestivum) plants. J. Nanopart. Res. 2012, 14, 841–850. [Google Scholar] [CrossRef]
- Schwab, F.; Bucheli, T.D.; Lukhele, L.P.; Magrez, A.; Nowack, B.; Sigg, L.; Knauer, K. Are carbon nanotube effects on green algae caused by shading and agglomeration? Environ. Sci. Technol. 2011, 45, 6136–6144. [Google Scholar] [CrossRef]
- Wei, L.; Thakkar, M.; Chen, Y.; Ntim, S.A.; Mitra, S.; Zhang, X. Cytotoxicity effects of water dispersible oxidized multiwalled carbon nanotubes on marine alga Dunaliella Tertiolecta. Aquat. Toxicol. 2010, 100, 194–201. [Google Scholar] [CrossRef]
- Timofeeva, A.V.; Tashlitsky, V.N.; Tkachev, A.G.; Baratova, L.A.; Koksharova, O.A. Nanocomplexes on the basis of taunit associated with biocides as effective anti-cyanobacterial agents. Russ. J. Plant Physiol. 2017, 64, 833–838. [Google Scholar] [CrossRef]
- Sideratou, Z.; Tsiourvas, D.; Paleos, C.M. Quaternized poly(propylene imine) dendrimers as novel pH-sensitive controlled-release systems. Langmuir 2000, 16, 1766–1769. [Google Scholar] [CrossRef]
- Sapalidis, A.; Sideratou, Z.; Panagiotaki, K.N.; Sakellis, E.; Kouvelos, E.P.; Papageorgiou, S.; Katsaros, F. Fabrication of antibacterial PVA nanocomposite films containing dendritic polymer functionalized multi-walled carbon nanotubes. Front. Mater. 2018, 5. [Google Scholar] [CrossRef]
- Cao, X.; Li, Z.; Song, X.; Cui, X.; Cao, P.; Liu, H.; Cheng, F.; Chen, Y. Core-shell type multiarm star poly(ε-caprolactone) with high molecular weight hyperbranched polyethylenimine as core: Synthesis, characterization and encapsulation properties. Eur. Polym. J. 2008, 44, 1060–1070. [Google Scholar] [CrossRef]
- Bellamy, L. The Infra-Red Spectra of Complex Molecules; Springer: Amsterdam, The Netherlands, 1975. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D. Organic/inorganic hybrid nanospheres based on hyperbranched poly(ethyleneimine) encapsulated into silica for the sorption of toxic metal ions and polycyclic aromatic hydrocarbons from water. J. Hazard. Mater. 2009, 170, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Pigorsch, E. Spectroscopic characterisation of cationic quaternary ammonium starches. Starke 2009, 61, 129–138. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Bitter, J.L.; Fairbrother, D.H. Chemical and structural characterization of carbon nanotube surfaces. Anal. Bioanal. Chem. 2010. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Jiang, G.; Che, J.; Qi, X.; Xu, R.; Chan-Park, M.B. Deposition of Silver Nanoparticles on Multiwalled Carbon Nanotubes Grafted with Hyperbranched Poly(amidoamine) and Their Antimicrobial Effects. J. Phys. Chem. C 2008, 112, 18754–18759. [Google Scholar] [CrossRef]
- Murugan, E.; Vimala, G. Effective functionalization of multiwalled carbon nanotube with amphiphilic poly(propyleneimine) dendrimer carrying silver nanoparticles for better dispersability and antimicrobial activity. J. Colloid Interface Sci. 2011, 357, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, Z.; Yan, D.; Lu, H. Deposition of Fe–Ni nanoparticles on polyethyleneimine-decorated graphene oxide and application in catalytic dehydrogenation of ammonia borane. J. Mater. Chem. 2012, 22, 13506–13516. [Google Scholar] [CrossRef]
- Zhang, N.; Xie, J.; Guers, M.; Varadan, V.K. Chemical bonding of multiwalled carbon nanotubes to SU-8 via ultrasonic irradiation. Smart Mater. Struct. 2003, 12, 260–263. [Google Scholar] [CrossRef]
- Schierz, A.; Zänker, H. Aqueous suspensions of carbon nanotubes: Surface oxidation, colloidal stability and uranium sorption. Environ. Pollut. 2009, 157, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Etika, K.C.; Cox, M.A.; Grunlan, J.C. Tailored dispersion of carbon nanotubes in water with pH-responsive polymers. Polymer 2010, 51, 1761–1770. [Google Scholar] [CrossRef]
- Yu, J.; Grossiord, N.; Koning, C.E.; Loos, J. Controlling the dispersion of multi-wall carbon nanotubes in aqueous surfactant solution. Carbon 2007, 45, 618–623. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, M.; Gong, X.; Diao, G. Universal water-soluble cyclodextrin polymer–carbon nanomaterials with supramolecular recognition. Carbon 2013, 61, 154–163. [Google Scholar] [CrossRef]
- Moon, Y.K.; Lee, J.; Lee, J.K.; Kim, T.K.; Kim, S.H. Synthesis of length-controlled aerosol carbon nanotubes and their dispersion stability in aqueous solution. Langmuir 2009, 25, 1739–1743. [Google Scholar] [CrossRef] [PubMed]
- Schwyzer, I.; Kaegi, R.; Sigg, L.; Nowack, B. Colloidal stability of suspended and agglomerate structures of settled carbon nanotubes in different aqueous matrices. Water Res. 2013, 47, 3910–3920. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, B.; Gao, D.; Guan, M.; Zheng, L.; Ouyang, H.; Chai, Z.; Zhao, Y.; Feng, W. Broad-Spectrum Antibacterial Activity of Carbon Nanotubes to Human Gut Bacteria. Small 2013, 9, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Mao, Y.; Ding, L. Carbon nanotubes as antimicrobial agents for water disinfection and pathogen control. J. Water Health 2018, 16, 171–180. [Google Scholar] [CrossRef]
- Zardini, H.Z.; Amiri, A.; Shanbedi, M.; Maghrebi, M.; Baniadam, M. Enhanced antibacterial activity of amino acids-functionalized multi walled carbon nanotubes by a simple method. Colloids Surf. B 2012, 92, 196–202. [Google Scholar] [CrossRef]
- Zardini, H.Z.; Davarpanah, M.; Shanbedi, M.; Amiri, A.; Maghrebi, M.; Ebrahimi, L. Microbial toxicity of ethanolamines—Multiwalled carbon nanotubes. J. Biomed. Mater. Res. Part A 2014, 102, 1774–1781. [Google Scholar] [CrossRef]
- Gottenbos, B.; van der Mei, H.C.; Klatter, F.; Nieuwenhuis, P.; Busscher, H.J. In Vitro and In Vivo antimicrobial activity of covalently coupled quaternary ammonium silane coatings on silicone rubber. Biomaterials 2002, 23, 1417–1423. [Google Scholar] [CrossRef]
- Tamayo-Belda, M.; González-Pleiter, M.; Pulido-Reyes, G.; Martin-Betancor, K.; Leganés, F.; Rosal, R.; Fernández-Piñas, F. Mechanism of the toxic action of cationic G5 and G7 PAMAM dendrimers in the cyanobacterium Anabaena sp. PCC7120. Environ. Sci. Nano 2019, 6, 863–878. [Google Scholar] [CrossRef]
- Chen, C.Z.S.; Beck-Tan, N.C.; Dhurjati, P.; van Dyk, T.K.; LaRossa, R.A.; Cooper, S.L. Quaternary ammonium functionalized poly(propylene imine) dendrimers as effective antimicrobials: Structure-activity studies. Biomacromolecules 2000, 1, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.; Flores, E. The Cyanobacteria: Molecular Biology, Genomics and Evolution; Caister Academic Press: Norfolk, UK, 2008. [Google Scholar]
- Wieg, C.; Pflugmacher, S. Ecotoxicological effects of selected cyanobacterial secondary metabolites a short review. Toxicol. Appl. Pharmacol. 2005, 203, 201–218. [Google Scholar]
- Vernon, L.P.; Shaw, E.R. Photoreduction of 2,6-dichlorophenolindophenol by diphenylcarbazide: A Photosystem 2 reaction catalyzed by tris-washed chloroplasts and subchloroplast fragments. Plant Physiol. 1969, 44, 1645–1649. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Papageorgiou, G.C. Rapid permeabilization of anacystis nidulans to electrolytes. Meth. Enzymol. 1988, 167, 259–262. [Google Scholar]
- Kumazawa, S.; Mitsui, A. Photosynthetic activities of a synchronously grown aerobic N2-fixing unicellular cyanobacterium, Synechococcus sp. Miami BG 043511. J. Gen. Microbiol. 1992, 138, 467–472. [Google Scholar] [CrossRef]
- Zheng, M.; Diner, B.A. Solution Redox Chemistry of Carbon Nanotubes. J. Am. Chem. Soc. 2004, 126, 15490–15494. [Google Scholar] [CrossRef]
- Brand, J.; Baszynski, T.; Crane, F.L.; Krogmann, D.W. Selective inhibition of photosynthetic reactions by polycations. J. Biol. Chem. 1972, 247, 2814–2819. [Google Scholar]
- Schreiber, U.; Klughammer, C.; Neubauer, C. Measuring P700 absorbance changes around 830 nm with a new type of pulse modulation system. Z. Naturforsch. C 1988, 43, 686–698. [Google Scholar] [CrossRef]
- Sekar, N.; Umasankar, Y.; Ramasamy, R.P. Photocurrent generation by immobilized cyanobacteria via direct electron transport in photo-bioelectrochemical cells. Phys. Chem. Chem. Phys. 2014, 16, 7862–7871. [Google Scholar] [CrossRef]
- Goldbeck, J.C.; Victoria, F.N.; Motta, A.; Savegnago, L.; Jacob, R.G.; Perin, G.; Lenardᾶo, E.J.; da Silva, W.P. Bioactivity and morphological changes of bacterial cells after exposure to 3-(p-chlorophenyl)thio citronellal. LWT 2014, 59, 813–819. [Google Scholar] [CrossRef]
- Stamatakis, K.; Papageorgiou, G.C. The osmolality of the cell suspension regulates phycobilisome-to-photosystem I excitation transfers in Cyanobacteria. Biochim. Biophys. Acta 2001, 1506, 172–181. [Google Scholar] [CrossRef][Green Version]
- Moran, P. Formulae for determination of chlorophyllous pigments extracted with N,N- Dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Trebst, A.; Pistorius, E. Photosynthetische reaktionen in UV-bestrahlten chloroplasten. Z. Naturforsch. 1965, 20b, 885–889. [Google Scholar] [CrossRef][Green Version]





| Samples | IC-50 (μg/mL) |
|---|---|
| oxCNTs@30-QPEI | 93.2 |
| oxCNTs@50-QPEI | 50.1 |
| oxCNTs@80-QPEI | 28.4 |
| Samples | IC-50 (μg/mL) |
|---|---|
| oxCNTs@30-QPEI | 12.4 |
| oxCNTs@50-QPEI | ≤10 |
| oxCNTs@80-QPEI | <10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heliopoulos, N.S.; Kythreoti, G.; Lyra, K.M.; Panagiotaki, K.N.; Papavasiliou, A.; Sakellis, E.; Papageorgiou, S.; Kouloumpis, A.; Gournis, D.; Katsaros, F.K.; et al. Cytotoxicity Effects of Water-Soluble Multi-Walled Carbon Nanotubes Decorated with Quaternized Hyperbranched Poly(ethyleneimine) Derivatives on Autotrophic and Heterotrophic Gram-Negative Bacteria. Pharmaceuticals 2020, 13, 293. https://doi.org/10.3390/ph13100293
Heliopoulos NS, Kythreoti G, Lyra KM, Panagiotaki KN, Papavasiliou A, Sakellis E, Papageorgiou S, Kouloumpis A, Gournis D, Katsaros FK, et al. Cytotoxicity Effects of Water-Soluble Multi-Walled Carbon Nanotubes Decorated with Quaternized Hyperbranched Poly(ethyleneimine) Derivatives on Autotrophic and Heterotrophic Gram-Negative Bacteria. Pharmaceuticals. 2020; 13(10):293. https://doi.org/10.3390/ph13100293
Chicago/Turabian StyleHeliopoulos, Nikolaos S., Georgia Kythreoti, Kyriaki Marina Lyra, Katerina N. Panagiotaki, Aggeliki Papavasiliou, Elias Sakellis, Sergios Papageorgiou, Antonios Kouloumpis, Dimitrios Gournis, Fotios K. Katsaros, and et al. 2020. "Cytotoxicity Effects of Water-Soluble Multi-Walled Carbon Nanotubes Decorated with Quaternized Hyperbranched Poly(ethyleneimine) Derivatives on Autotrophic and Heterotrophic Gram-Negative Bacteria" Pharmaceuticals 13, no. 10: 293. https://doi.org/10.3390/ph13100293
APA StyleHeliopoulos, N. S., Kythreoti, G., Lyra, K. M., Panagiotaki, K. N., Papavasiliou, A., Sakellis, E., Papageorgiou, S., Kouloumpis, A., Gournis, D., Katsaros, F. K., Stamatakis, K., & Sideratou, Z. (2020). Cytotoxicity Effects of Water-Soluble Multi-Walled Carbon Nanotubes Decorated with Quaternized Hyperbranched Poly(ethyleneimine) Derivatives on Autotrophic and Heterotrophic Gram-Negative Bacteria. Pharmaceuticals, 13(10), 293. https://doi.org/10.3390/ph13100293








