Recent Insights in Barium-131 as a Diagnostic Match for Radium-223: Cyclotron Production, Separation, Radiolabeling, and Imaging †
Abstract
1. Introduction
2. Results and Discussion
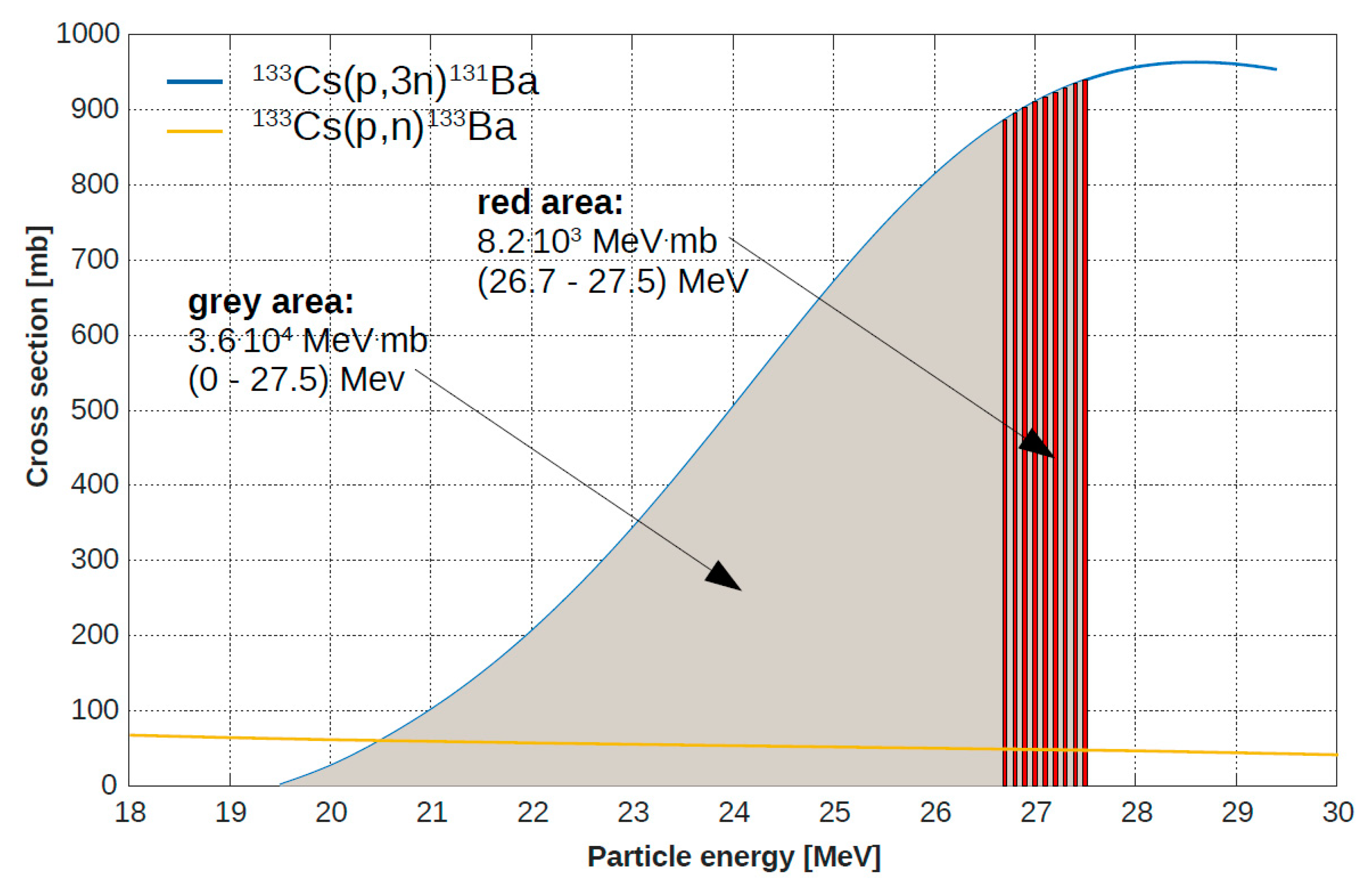
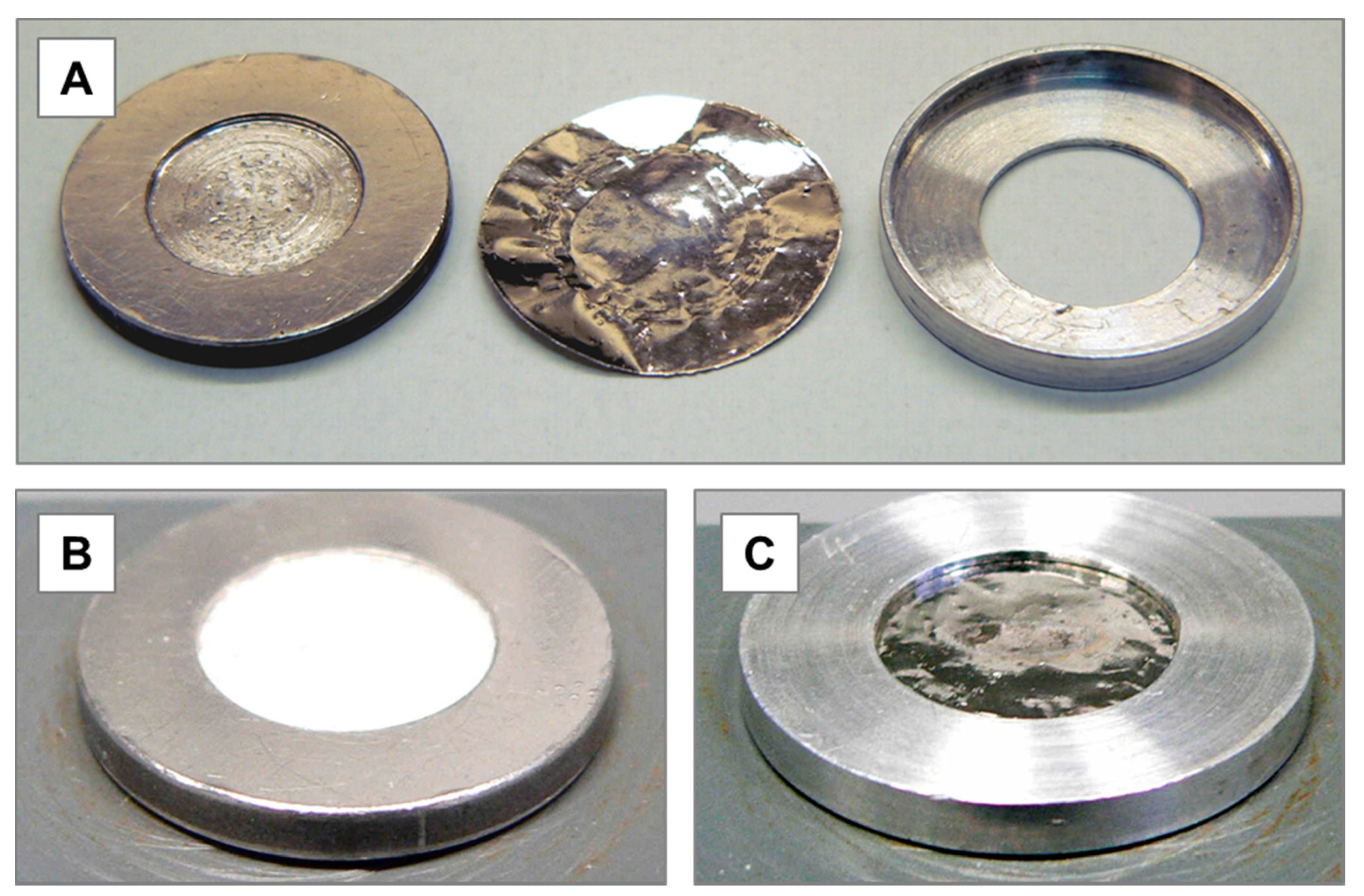
2.1. Target Fabrication and Irradiation
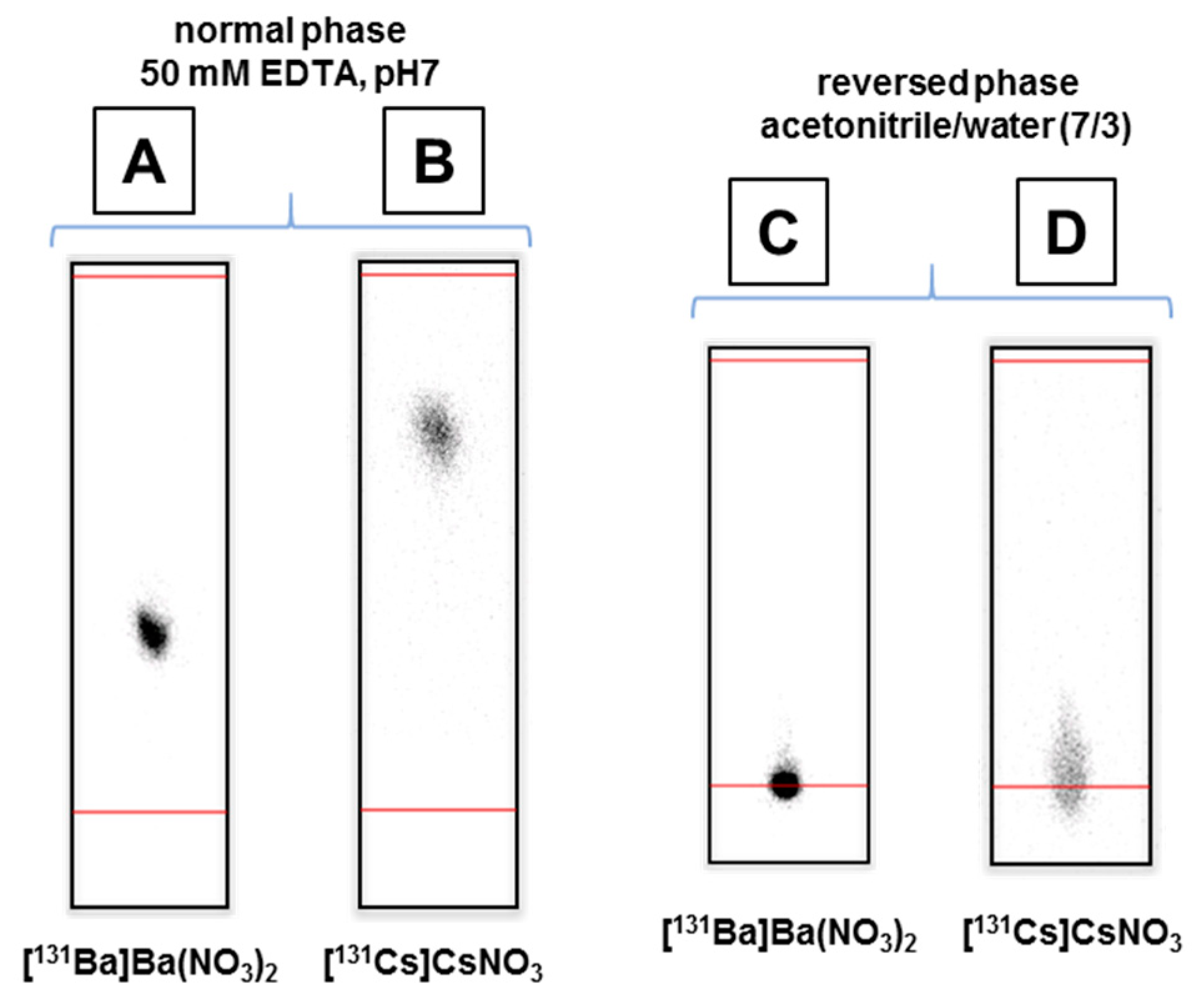
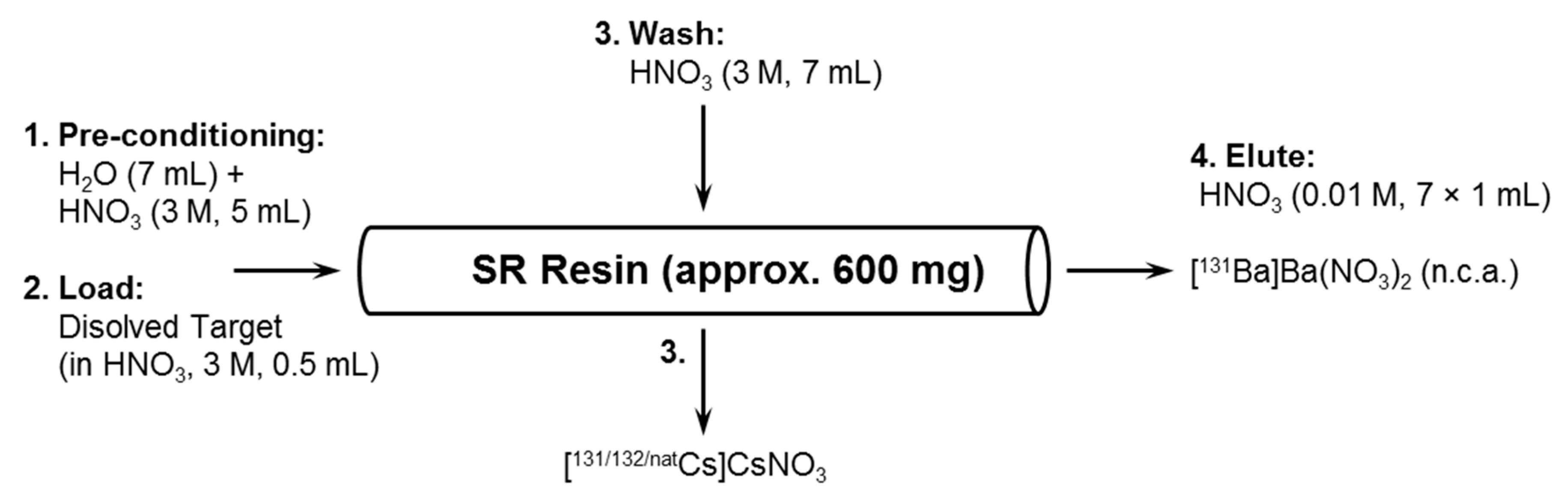
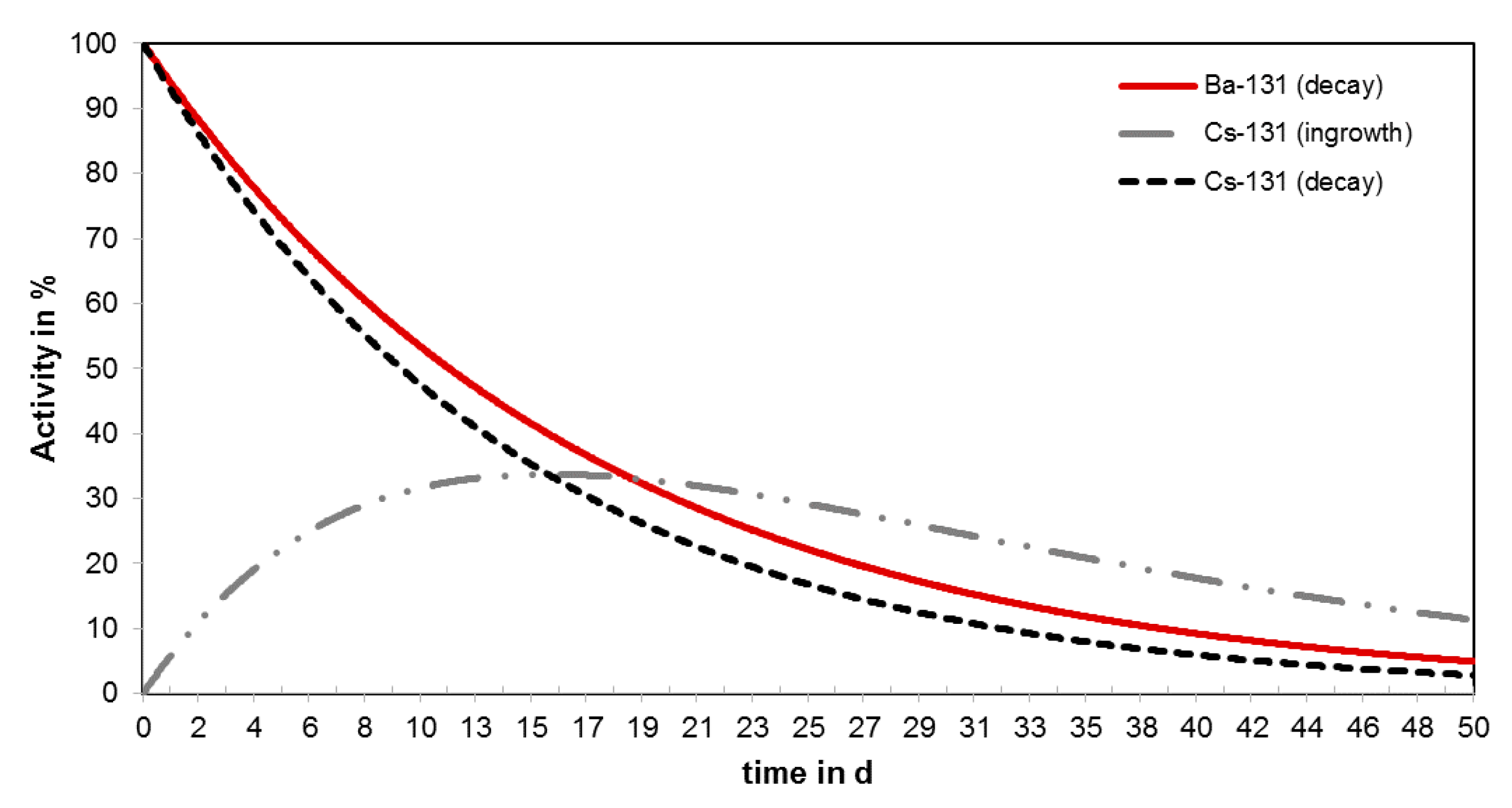
2.2. Barium-131 Purification
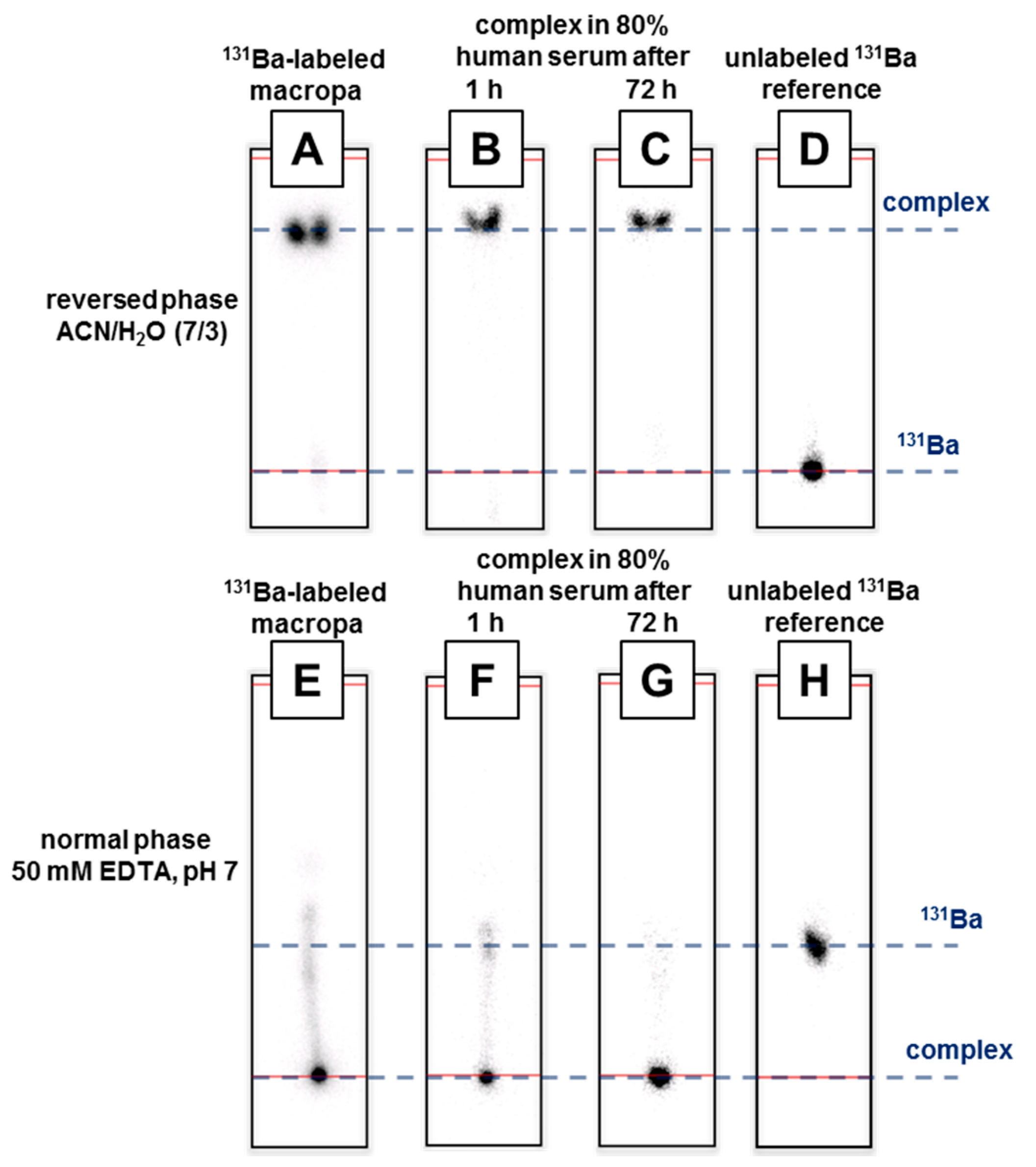
2.3. Radiolabeling
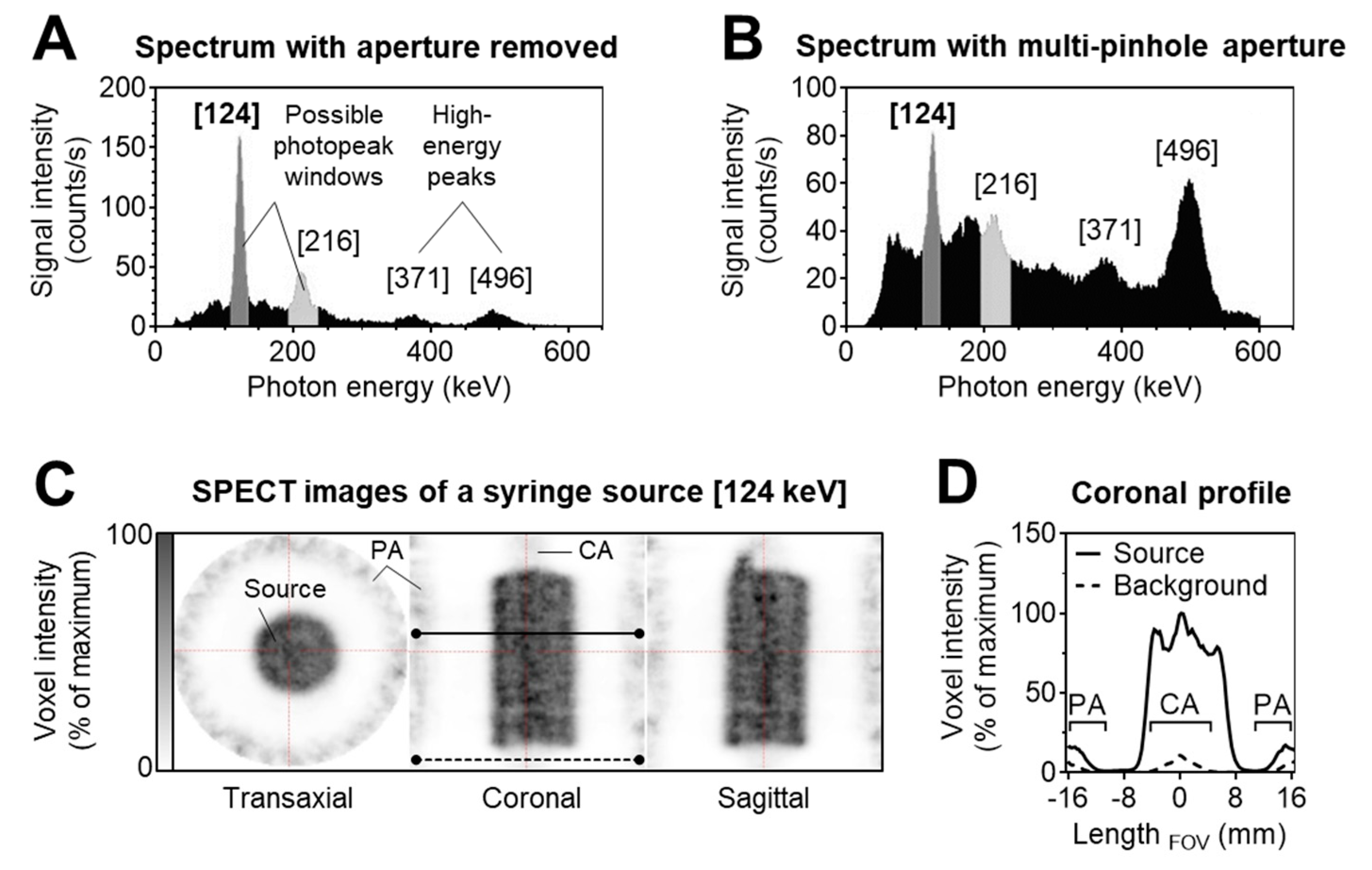
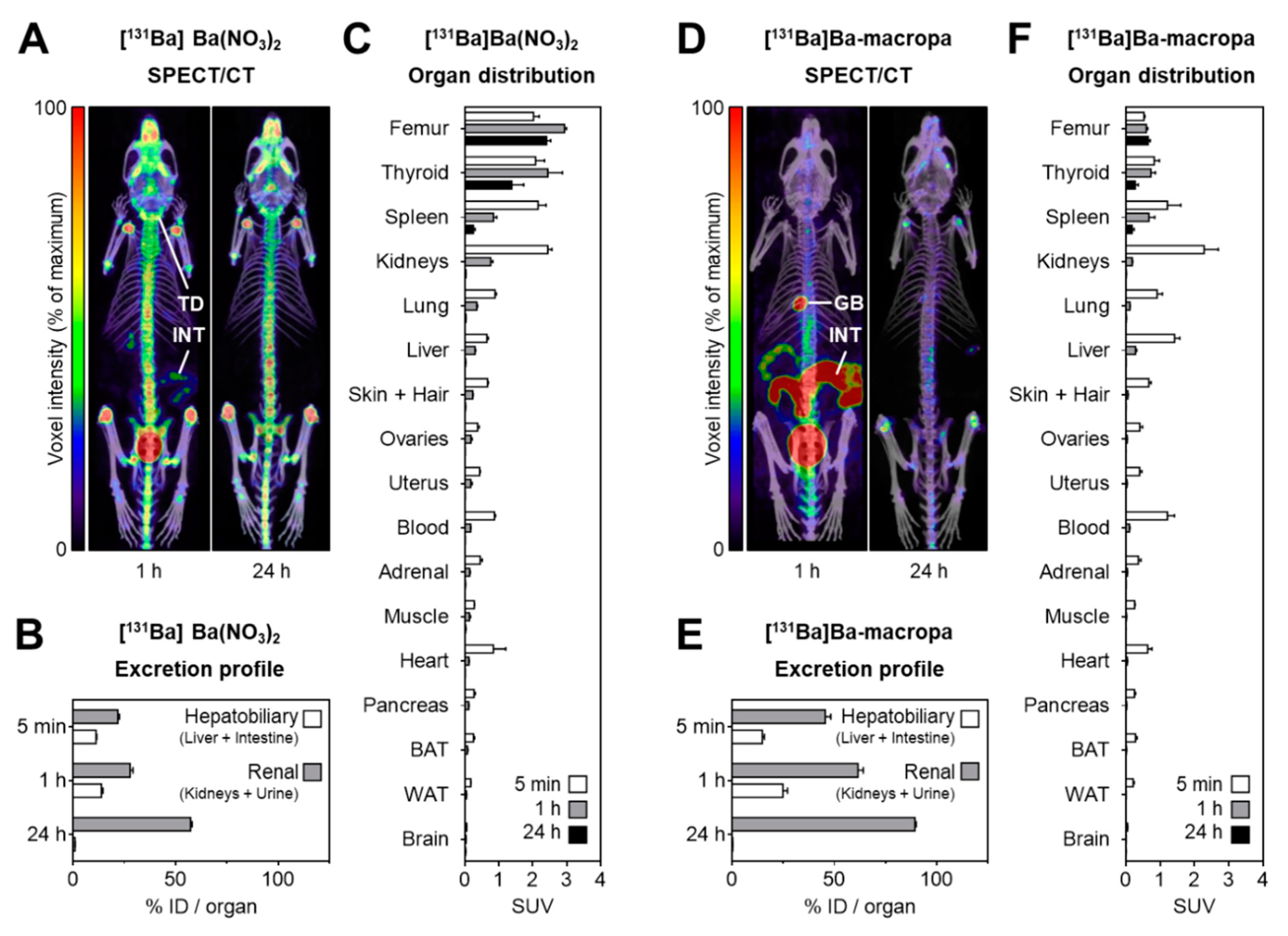
2.4. SPECT Imaging
3. Materials and Methods
3.1. Target Preparation
3.2. Irradiation Conditions
3.3. Determination and Quantification of Radionuclides
3.4. Barium-131 and Cesium-131 Separation
3.5. Radiolabeling and Reaction Control
3.6. Animal Experiments
3.7. SPECT Imaging
3.8. Biodistribution
3.9. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khazov, Y.; Mitropolsky, I.; Rodionov, A. Nuclear Data Sheets for A = 131. Nucl. Data Sheets 2006, 107, 2715–2930. [Google Scholar] [CrossRef]
- Spencer, R.P.; Lange, R.C.; Treves, S. Use of Ba-135m and Ba-131 as Bone-Scanning Agents. J. Nucl. Med. 1971, 12, 216–221. [Google Scholar]
- Spencer, R.P.; Lange, R.C.; Treves, S. 131Ba: An Intermediate-Lived Radionuclide for Bone Scanning. J. Nucl. Med. 1970, 11, 95–96. [Google Scholar]
- Lange, R.C.; Treves, S.; Spencer, R.P. Oral Presentation: 135mBa and 131Ba as Bone Scanning Agents. J. Nucl. Med. 1970, 11, 340–341. [Google Scholar]
- Mahlstedt, J.; Prignitz, I.; Joseph, K. Barium 131—A New Nuclide for Bone Scintigraphy. First Clinical Results. Strahlenther. Sonderb. 1972, 72, 402–406. [Google Scholar]
- Love, C.; Din, A.S.; Tomas, M.B.; Kalapparambath, T.P.; Palestro, C.J. Radionuclide Bone Imaging: An Illustrative Review. Radiographics 2003, 23, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Ram, P.C.; Fordham, E.W. An Historical Survey of Bone Scanning. Semin. Nucl. Med. 1979, 9, 190–196. [Google Scholar] [CrossRef]
- Salutsky, M.L.; Kirby, H.W. The Radiochemistry of Radium; National Bureau of Standards, U.S. Department of Commerce: Springfield, VA, USA, 1964; p. 210. [Google Scholar]
- Harrison, G.E.; Carr, T.E.F.; Sutton, A. Distribution of Radioactive Calcium, Strontium, Barium and Radium Following Intravenous Injection into a Healthy Man. Int. J. Radiat. Biol. 1967, 13, 235–247. [Google Scholar] [CrossRef]
- Poeppel, T.D.; Handkiewicz-Junak, D.; Andreeff, M.; Becherer, A.; Bockisch, A.; Fricke, E.; Geworski, L.; Heinzel, A.; Krause, B.J.; Krause, T.; et al. Eanm Guideline for Radionuclide Therapy with Radium-223 of Metastatic Castration-Resistant Prostate Cancer. Eur. J. Nucl. Med. Mol. Imag. 2018, 45, 824–845. [Google Scholar] [CrossRef]
- Braun, J.; Lemmel, E.M.; Manger, B.; Rau, R.; Sörensen, H.; Sieper, J. Therapie der ankylosierenden Spondylitis (AS) mit Radiumchlorid (224SpondylAT®). Z. Rheumatol. 2001, 60, 74–83. [Google Scholar] [CrossRef]
- Wick, R.R.; Nekolla, E.A.; Gaubitz, M.; Schulte, T.L. Increased risk of myeloid leukaemia in patients with ankylosing spondylitis following treatment with radium-224. Rheumatology 2008, 47, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Cooks, T.; Tal, M.; Raab, S.; Efrati, M.; Reitkopf, S.; Lazarov, E.; Etzyoni, R.; Schmidt, M.; Arazi, L.; Kelson, I.; et al. Intratumoral 224Ra-Loaded Wires Spread Alpha-Emitters Inside Solid Human Tumors in Athymic Mice Achieving Tumor Control. Anticancer Res. 2012, 32, 5315–5321. [Google Scholar] [PubMed]
- Confino, H.; Hochman, I.; Efrati, M.; Schmidt, M.; Umansky, V.; Kelson, I.; Keisari, Y. Tumor ablation by intratumoral Ra-224-loaded wires induces anti-tumor immunity against experimental metastatic tumors. Cancer Immunol. Immunother. 2015, 64, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Neirincky, R.D. Production of 133mBa for medical purposes. Int. J. Appl. Radiat. Isot. 1977, 28, 323–325. [Google Scholar] [CrossRef]
- Reissig, F.; Hübner, R.; Steinbach, J.; Pietzsch, H.-J.; Mamat, C. Facile Preparation of radium-doped, functionalized nanoparticles as carriers for targeted alpha therapy. Inorg. Chem. Front. 2019, 6, 1341–1349. [Google Scholar] [CrossRef]
- Vasiliev, A.N.; Severin, A.; Lapshina, E.; Chernykh, E.; Ermolaev, S.; Kalmykov, S. Hydroxyapatite particles as carriers for 223Ra. J. Radioanal. Nucl. Chem. 2017, 311, 1503–1509. [Google Scholar] [CrossRef]
- Suchánková, P.; Kukleva, E.; Štamberg, K.; Nykl, P.; Vlk, M.; Kozempel, J. Study of 223Ra uptake mechanism on hydroxyapatite and titanium dioxide nanoparticles as a function of pH. RSC Adv. 2020, 10, 3659–3666. [Google Scholar] [CrossRef]
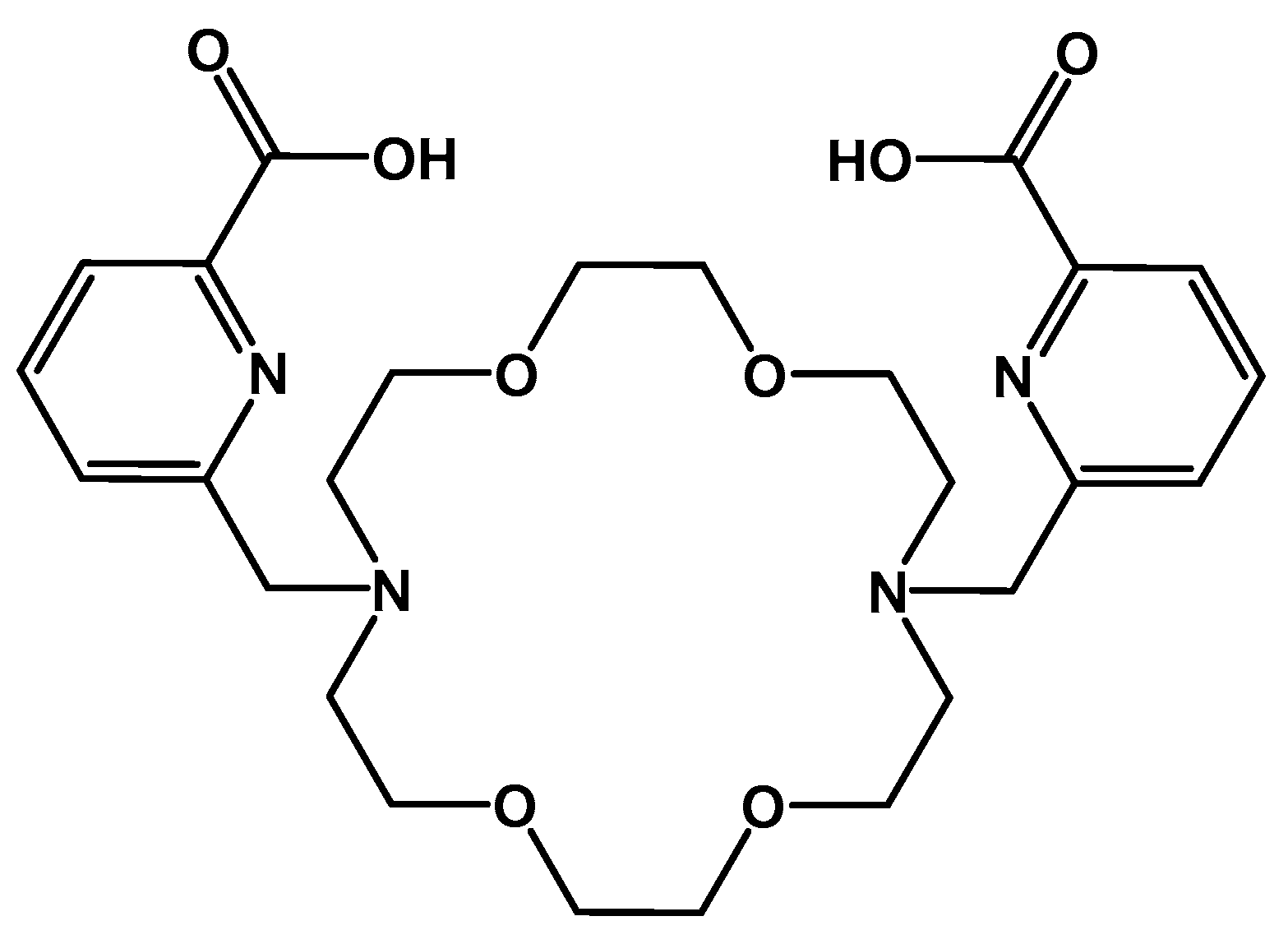
- Bauer, D.; Gott, M.; Steinbach, J.; Mamat, C. Chelation of heavy group 2 (radio)metals by p-tert-butylcalix[4]arene-1,3-crown-6 and logK determination via NMR. Spectrochim. Acta A 2018, 199, 50–56. [Google Scholar] [CrossRef]
- Bauer, D.; Blumberg, M.; Köckerling, M.; Mamat, C. A comparative evaluation of calix[4]arene-1,3-crown-6 as a ligand for selected divalent cations of radiopharmaceutical interest. RSC Adv. 2019, 9, 32357–32366. [Google Scholar] [CrossRef]
- Steinberg, J.; Bauer, D.; Reissig, F.; Köckerling, M.; Pietzsch, H.-J.; Mamat, C. Modified Calix[4]crowns as Molecular Receptors for Barium. ChemistryOpen 2018, 7, 432–438. [Google Scholar] [CrossRef]
- Abou, D.; Thiele, N.A.; Longtine, M.; Villmer, A.L.; Wilson, J.J.; Thorek, D.L.J. Chelation of Radium-223 for Targeted Radionuclide Alpha Particle Therapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46 (Suppl. S1), S286. [Google Scholar]
- Thiele, N.A.; MacMillan, S.N.; Wilson, J.J. Rapid Dissolution of BaSO4 by Macropa, an 18-Membered Macrocycle with High Affinity for Ba2+. J. Am. Chem. Soc. 2018, 140, 17071–17078. [Google Scholar] [PubMed]
- Abou, D.; Thiele, N.; Villmer, A.; Gustche, N.; Escorcia, F.; Wilson, J.; Thorek, D. MACROPA highly stable chelator of Radium-223 and functionalization attempts for targeted treatment of cancer. J. Nucl. Med. 2020, 61 (Suppl. S1), 587. [Google Scholar]
- Ramamoorthy, N.; Iyer, M.G.; Mani, R.S. Studies on preparation of a 131Ba–131Cs generator. J. Radioanal. Chem. 1978, 42, 93–103. [Google Scholar]
- Meikrantz, D.H.; Snyder, J.R. Methods Producing Cesium-131. U.S. Patent US8270554B2, 18 September 2012. [Google Scholar]
- Moran, B.J.; Rice, S.R.; Chhabra, A.M.; Amin, N.; Braccioforte, M.; Agarwal, M. Long-term biochemical outcomes using cesium-131 in prostate brachytherapy. Brachytherapy 2019, 18, 800–805. [Google Scholar]
- Moran, B.J.; Braccioforte, M.H. Cesium-131 prostate prachytherapy: An early experience. Brachytherapy 2007, 6, 80. [Google Scholar]
- Konik, A.; Auer, B.; De Beenhouwer, J.; Kalluri, K.; Zeraatkar, N.; Furenlid, L.R.; King, M.A. Primary, scatter, and penetration characterizations of parallel-hole and pinhole collimators for I-123 SPECT. Phys. Med. Biol. 2019, 64, 245001. [Google Scholar]
- Jiang, W.; Ulmert, D.; Simons, B.W.; Abou, D.S.; Thorek, D.L.J. The impact of age on radium-223 distribution and an evaluation of molecular imaging surrogates. Nucl. Med. Biol. 2018, 62–63, 1–8. [Google Scholar]
- Newton, D.; Ancill, A.K.; Naylor, K.E.; Eastell, R. Long-term Retention of Injected Barium-133 in Man. Radiat. Prot. Dosim. 2001, 97, 231–240. [Google Scholar]
- Marshall, J.H.; Onkelinx, C. Radial Diffusion and Power Function Retention of Alkaline Earth Radio-isotopes in Adult Bone. Nature 1968, 217, 742–743. [Google Scholar]
- Barden, A.O. Radium-226 Concentrations in Human Thyroid and Other Selected Tissues Measured by the Radon Emanation Technique. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2007. [Google Scholar]
- Kaminskyi, O.V.; Kopylova, O.V.; Afanasyev, D.Y.; Mazurenko, O.V.; Berezovskyi, S.Y. Pilot study of parathyroid glands in adult and pediatric subjects exposed to ionizing radiation after the ChNPP accident, methodology of parathyroid diagnostic ultrasound. Probl. Radiat. Med. Radiobiol. 2017, 22, 382–394. [Google Scholar] [CrossRef]
- Roca-Sabio, A.; Mato-Iglesias, M.; Esteban-Gómez, D.; Tóth, E.; de Blas, A.; Platas-Iglesias, C.; Rodríguez-Blas, T. Macrocyclic Receptor Exhibiting Unprecedented Selectivity for Light Lanthanides. J. Am. Chem. Soc. 2009, 131, 3331–3341. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reissig, F.; Bauer, D.; Ullrich, M.; Kreller, M.; Pietzsch, J.; Mamat, C.; Kopka, K.; Pietzsch, H.-J.; Walther, M. Recent Insights in Barium-131 as a Diagnostic Match for Radium-223: Cyclotron Production, Separation, Radiolabeling, and Imaging. Pharmaceuticals 2020, 13, 272. https://doi.org/10.3390/ph13100272
Reissig F, Bauer D, Ullrich M, Kreller M, Pietzsch J, Mamat C, Kopka K, Pietzsch H-J, Walther M. Recent Insights in Barium-131 as a Diagnostic Match for Radium-223: Cyclotron Production, Separation, Radiolabeling, and Imaging. Pharmaceuticals. 2020; 13(10):272. https://doi.org/10.3390/ph13100272
Chicago/Turabian StyleReissig, Falco, David Bauer, Martin Ullrich, Martin Kreller, Jens Pietzsch, Constantin Mamat, Klaus Kopka, Hans-Jürgen Pietzsch, and Martin Walther. 2020. "Recent Insights in Barium-131 as a Diagnostic Match for Radium-223: Cyclotron Production, Separation, Radiolabeling, and Imaging" Pharmaceuticals 13, no. 10: 272. https://doi.org/10.3390/ph13100272
APA StyleReissig, F., Bauer, D., Ullrich, M., Kreller, M., Pietzsch, J., Mamat, C., Kopka, K., Pietzsch, H.-J., & Walther, M. (2020). Recent Insights in Barium-131 as a Diagnostic Match for Radium-223: Cyclotron Production, Separation, Radiolabeling, and Imaging. Pharmaceuticals, 13(10), 272. https://doi.org/10.3390/ph13100272