Iminosugars: Effects of Stereochemistry, Ring Size, and N-Substituents on Glucosidase Activities
Abstract
1. Introduction
2. Results and Discussion
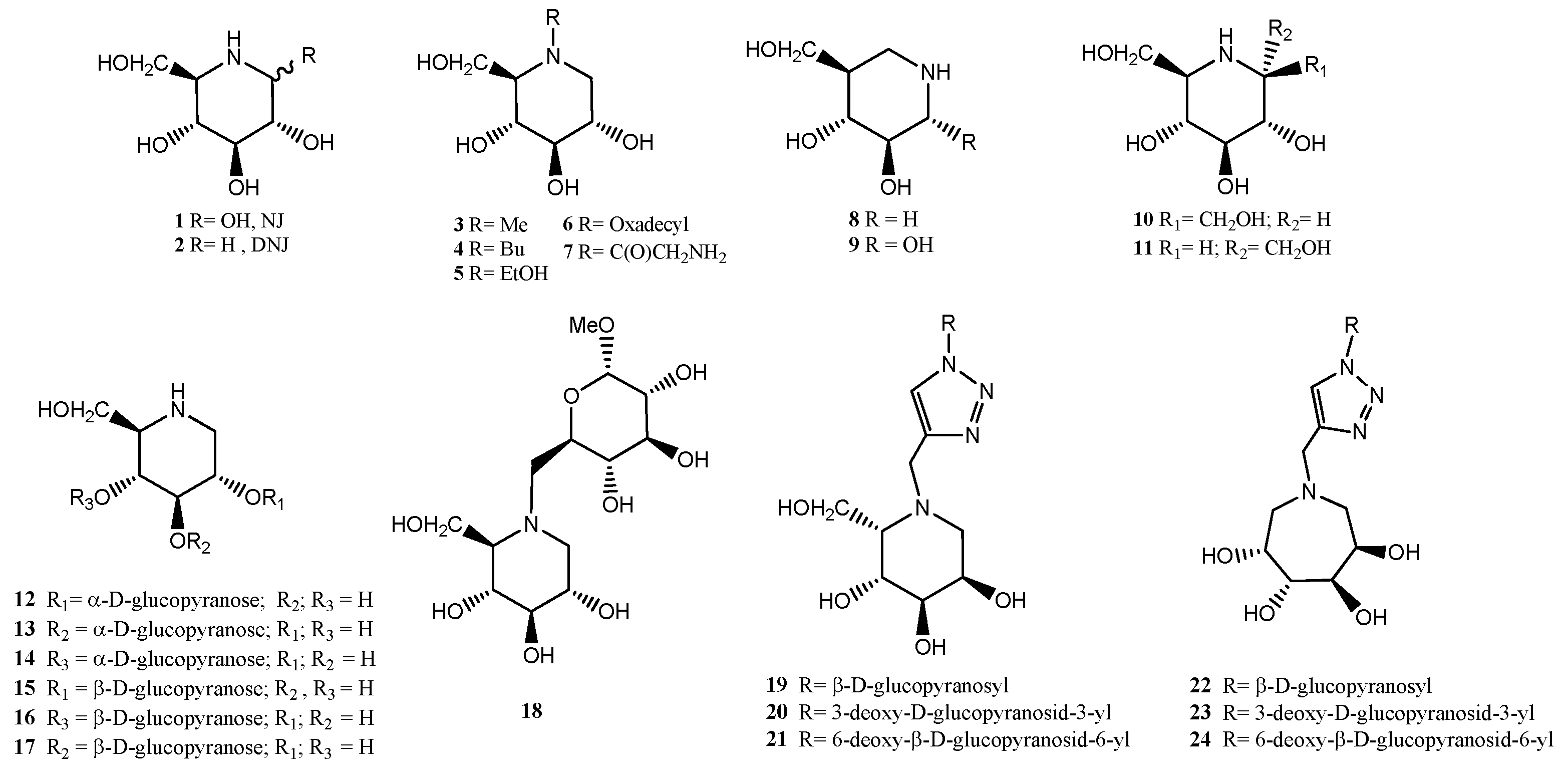
2.1. Chemistry
2.2. Biological Assays
2.2.1. Yeast α-glucosidase Activities
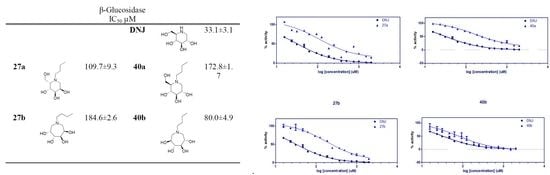
2.2.2. Almond β-glucosidase Activities:
3. Conclusions
4. Material and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Melo, E.B.; Gomes, A.S.; Carvalho, I. α-and β-Glucosidase inhibitors: Chemical structure and biological activity. Tetrahedron 2006, 62, 10277–10302. [Google Scholar]
- Gao, K.; Zheng, C.; Wang, T.; Zhao, H.; Wang, J.; Wang, Z.; Zhai, X.; Jia, Z.; Chen, J.; Zhou, Y.; et al. Review 1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and In Silico Target Fishing. Molecules 2016, 21, 1600. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Blechert, S. Inhibition of fucosyltransferase V by a GDP-azasugar. Bioorg. Med. Chem. Lett. 2001, 11, 1809–1811. [Google Scholar] [CrossRef]
- Jakobsen, P.; Lundbeck, J.M.; Kristiansen, M.; Breinholt, J.; Demuth, H.; Pawlas, J.; Candela, M.P.T.; Andersen, B.; Westergaard, N.; Lundgren, K.; et al. Imino sugars: Potential inhibitors of liver glycogen phosphorylase. Bioorg. Med. Chem. 2001, 9, 733–744. [Google Scholar] [CrossRef]
- Compain, P.; Martin, O.R. Carbohydrate mimetics-based glycosyltransferase inhibitors. Bioorg. Med. Chem. 2001, 9, 3077–3092. [Google Scholar] [CrossRef]
- Fedorov, A.; Shi, W.; Kicska, G.; Fedorov, E.; Tyler, P.C.; Furneaux, R.H.; Hanso, J.C.; Gainsford, G.J.; Larese, J.Z.; Schramm, V.L.; et al. Transition state structure of purine nucleoside phosphorylase and principles of atomic motion in enzymatic catalysis. Biochemistry 2001, 40, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.E.; Smith, M.D.; Pickering, L.; Fleet, G.W.J. An approach to combinatorial library generation of galactofuranose mimics as potential inhibitors of mycobacterial cell wall biosynthesis: Synthesis of a peptidomimetic of uridine 5′-diphosphogalactofuranose (UDP-galf). Tetrahedron Lett. 1999, 40, 8689–8692. [Google Scholar] [CrossRef]
- Campo, V.L.; Aragão-Leoneti, V.; Carvalho, I. Glycosidases and diabetes: Metabolic changes, mode of action and therapeutic perspectives. In Carbohydrate Chemistry; Amélia Pilar, R., Thisbe, L., Eds.; Royal Society of Chemistry: Cambridge, UK, 2013; Volume 9, pp. 181–203. [Google Scholar]
- Rosenbloom, B.E.; Weinreb, N.J. Gaucher disease: A comprehensive review. Crit. Rev. Oncog. 2013, 18, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xiong, D.; Song, C.; Tai, G.; Ye, X. Synthesis of N-dialkylphosphoryl iminosugar derivatives and their immunosuppressive activities. Org. Biomol. Chem. 2015, 13, 9364–9368. [Google Scholar] [CrossRef] [PubMed]
- Warfield, K.; Ramstedt, U. Iminosugars as Antibacterial Compounds and Uses Thereof for Treating Bacterial Infections. PCT Int. App. WO 2014143999 A1, 18 September 2014. [Google Scholar]
- Alonzi, D.S.; Scott, K.A.; Dwek, R.A.; Zitzmann, N. Iminosugar antivirals: The therapeutic sweet spot. Biochem. Soc. Trans. 2017, 45, 571–582. [Google Scholar] [CrossRef]
- Chang, J.; Block, T.M.; Guo, J. Antiviral therapies targeting host ER alpha-glucosidases: Current status and future directions. Antivir. Res. 2013, 99, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Fowler, P.A.; Haines, A.H.; Taylor, R.J.K.; Chrystal, E.J.T.; Gravestock, M.B. Synthesis and biological activity of acyclic analogues of nojirimycin. J. Chem. Soc. Perkin Trans. 1994, 1, 2229–2235. [Google Scholar] [CrossRef]
- Wetherilla, L.F.; Wassona, C.W.; Swinscoea, G.; Kealya, D.; Fosterb, R.; Griffinc, S.; Macdonald, A. Alkyl-imino sugars inhibit the pro-oncogenic ion channel function of humanpapillomavirus (HPV) E5. Antivir. Res. 2018, 158, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.R.; Mansfield, K.; You, J.E.; Tennant, B.C.; Kim, Y.H. Natural iminosugar derivatives of 1-deoxynojirimycin inhibit glycosylation of hepatitis viral envelope proteins. J. Microbiol. 2007, 45, 431–440. [Google Scholar] [PubMed]
- Ouzounov, S.; Mehta, A.; Dwek, R.A.; Block, T.M.; Jordan, R. The combination of interferon α-2b and n-butyl deoxynojirimycin has a greater than additive antiviral effect upon production of infectious bovine viral diarrhea virus (BVDV) in vitro: Implications for hepatitis C virus (HCV) therapy. Antivir. Res. 2002, 55, 425–435. [Google Scholar] [CrossRef]
- Miller, J.L.; Spiro, S.G.; Dowall, S.D.; Taylor, I.; Rule, A.; Alonzi, D.S.; Sayce, A.C.; Wright, E.; Bentley, E.M.; Thom, R.; et al. In Vivo Efficacy of Iminosugars in a Lethal Ebola Virus Guinea Pig Model. PLoS ONE 2016, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Warfield, K.L.; Warren, T.K.; Qiu, X.; Wells, J.; Mire, C.; Geisbert, J.B.; Stuthman, K.S.; Garza, N.L.; Tongeren, S.A.V.; Shurtleff, A.C.; et al. Assessment of the potential for host-targeted iminosugars UV-4 and UV-5 activity against filovirus infections in vitro and in vivo. Antivir. Res. 2017, 138, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, B.E.; Sayce, A.C.; Warfield, K.L.; Miller, J.L.; Zitzmann, N. Iminosugars: Promising therapeutics for influenza infection. Crit. Rev. Microbiol. 2017, 43, 521–545. [Google Scholar] [CrossRef] [PubMed]
- Treston, A.M.; Warfield, K.L. Methods of Treating Zika Virus Infection. PCT Int. App. WO 2017201052 A1, 23 November 2017. [Google Scholar]
- Miller, J.L.; Tyrrell, B.E.; Zitzmann, N. Mechanisms of Antiviral Activity of Iminosugars Against Dengue Virus. In Dengue and Zika: Control and Antiviral Treatment Strategies; Rolf Hilgenfeld, R., Vasudevan, S.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1062, pp. 277–301. [Google Scholar]
- Sayce, A.C.; Alonzi, D.S.; Killingbeck, S.S.; Tyrrell, B.E.; Hill, M.L.; Caputo, A.T.; Iwaki, R.; Kinami, K.; Ide, D.; Kiappes, J.L.; et al. Iminosugars Inhibit Dengue Virus Production viaInhibition of ER Alpha-Glucosidases Not Glycolipid Processing Enzymes. PLoS Negl. Trop. Dis. 2010, 3, e0004524. [Google Scholar] [CrossRef]
- Tan, A.; Broek, L.V.D.; Boeckel, S.V.; Ploegh, H.; Bolscher, J.J. Chemical modification of the glucosidase inhibitor 1-deoxynojirimycin. Structure-activity relationships. Biol. Chem. 1991, 266, 14504–14510. [Google Scholar]
- Collins, P.; Ferrier, R. Monosaccharides: Their Chemistry and Their Roles in Natural Products; Wiley: New York, NY, USA, 1995; pp. 37–38. [Google Scholar]
- Sorbera, L.A.; Castaner, J.; Bayes, M. Miglustat. Drugs Fut. 2003, 28, 229–236. [Google Scholar] [CrossRef]
- Asano, N.; Oseki, k.; Kizu, H.; Matsui, K. Nitrogen-in-the-Ring Pyranoses and Furanoses: Structural Basis of Inhibition of Mammalian Glycosidases. J. Med. Chem. 1994, 37, 3701–3706. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, L.A.G.M.; Vermaas, D.J.; van Kemenade, F.J.; Tan, M.C.C.A.; Rotteveel, F.T.M.; Zandberg, P.; Butters, T.D.; Miedema, F.; Ploegh, H.L.; van Boeckel, C.A.A. Synthesis of oxygen-substituted N-alkyl 1-deoxynojirimycin derivatives: Aza sugar α-glucosidase inhibitors showing antiviral (HIV-1) and immunosuppressive activity. Recl. Trav. Chim. Pays-Bas 1994, 113, 507–516. [Google Scholar] [CrossRef]
- Hines, J.; Chang, H.; Gerdeman, M.S.; Warn, D.E. Isotope edited NMR studies of glycosidases: Design and synthesis of a novel glycosidase inhibitor. Bioorg. Med. Chem. Lett. 1999, 9, 1255–1260. [Google Scholar] [CrossRef]
- Horne, G.; Wilson, F.X.; Tinsley, J.; Williams, D.H.; Storer, R. Iminosugars past, present and future: Medicines for tomorrow. Drug Discov. Today 2011, 16, 107–118. [Google Scholar] [CrossRef]
- Parmeggiani, C.; Catarzi, S.; Matassini, C.; D’Adamio, G. Human Acid β-Glucosidase Inhibition by Carbohydrate Derived Iminosugars: Towards New Pharmacological Chaperones for Gaucher Disease. ChemBioChem 2015, 16, 2054–2064. [Google Scholar] [CrossRef]
- Trapero, A.; Llebaria, A. Glucocerebrosidase inhibitors for the treatment of Gaucher disease. Future Med. Chem. 2013, 5, 573–590. [Google Scholar] [CrossRef]
- Désiré, J.; Mondon, M.; Fontelle, N.; Nakagawa, S.; Hirokami, Y.; Adachi, I.; Iwaki, R.; Fleet, G.W.J.; Alonzi, D.S.; Twigg, G.; et al. N-and C-alkylation of seven-membered iminosugars generates potent glucocerebrosidase inhibitors and F508del-CFTR correctors. Org. Biomol. Chem. 2014, 12, 8977–8996. [Google Scholar] [CrossRef]
- Shih, T.L.; Liang, M.T.; Wu, K.D.; Lin, C.H. Synthesis of polyhydroxy 7-and N-alkyl-azepanes as potent glycosidase inhibitors. Carbohydr. Res. 2011, 346, 183–190. [Google Scholar] [CrossRef]
- Taghzouti, H.; Goumain, S.; Harakat, D.; Portella, C.; Behr, J.B.; Plantier-Royon, R. Synthesis of 2-carboxymethyl polyhydroxyazepanes and their evaluation as glycosidase inhibitors. Bioorg. Chem. 2015, 58, 11–17. [Google Scholar] [CrossRef]
- Nash, R.J.; Kato, A.; Yu, C.Y.; Fleet, G.W. Iminosugars as therapeutic agents: Recent advances and promising trends. Future Med. Chem. 2011, 2011 3, 513–521. [Google Scholar] [CrossRef]
- Brás, N.F.; Cerqueira, N.M.; Ramos, M.J.; Fernandes, P.A. Glycosidase inhibitors: A patent review (2008–2013). Expert Opin. Ther. Pat. 2014, 24, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Asano, N. Iminosugars: The Potential of Carbohydrate Analogs. In Carbohydrate Chemistry: State of the Art and Challenges for Drug Development; Cipolla, L., Ed.; University of Milano-Bicocca: Milano, Italy, 2015; Chapter 11; pp. 279–301. [Google Scholar] [CrossRef]
- Wadood, A.; Ghufran, M.; Khan, A.; Azam, S.S.; Jelani, M.; Uddin, R. Selective glycosidase inhibitors: A patent review (2012–present). Int. J. Biol. Macromol. 2018, 111, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Asano, N.; Oseki, K.; Kaneko, E.; Matsui, K. Enzymic synthesis of alpha- and beta-D-glucosides of 1-deoxynojirimycin and their glycosidase inhibitory activities. Carbohydr. Res. 1994, 258, 255–266. [Google Scholar] [CrossRef]
- Yoshikuni, Y.; Ezure, Y.; Seto, T.; Mori, K.; Watanabe, M.; Enomoto, H. Synthesis and alpha-glucosidase-inhibiting activity of a new alpha-glucosidase inhibitor, 4-O-alpha-D-glucopyranosylmoranoline and its N-substituted derivatives. Chem. Pharm. Bull. 1989, 37, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.M.; Begovic, M.E.; Rhinehart, B.L.; Heineke, E.W.; Ducep, J.B.; Kastner, P.R.; Marshall, F.N.; Danzin, C. New potent α-glucohydrolase inhibitor MDL 73945 with long duration of action in rats. Diabetes 1991, 40, 825–830. [Google Scholar] [CrossRef]
- Zamoner, L.O.B.; Aragão-Leoneti, V.; Mantoani, S.P.; Rugen, M.D.; Nepogodiev, S.A.; Field, R.A.; Carvalho, I. CuAAC click chemistry with N-propargyl 1,5-dideoxy-1,5-imino-Dgulitol and N-propargyl 1,6-dideoxy-1,6-imino-D-mannitol provides access to triazole-linked piperidine and azepane pseudo-disaccharide iminosugars displaying glycosidase inhibitory properties. Carbohydr. Res. 2016, 429, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Alonzi, D.S.; Dwek, R.A.; Butters, T.D. Improved cellular inhibitors for glycoprotein processinga-glucosidases:biological characterisation of alkyl-and arylalkyl-N-substituted deoxynojirimycins. Tetrahedron Asymmetry 2009, 20, 897–901. [Google Scholar] [CrossRef]
- Poitout, L.; Le Merrer, Y.; Depezay, J.C.L. Polyhydroxylated piperidines and azepanes from D-mannitol synthesis of 1-deoxynojirimycin and analogues. Tetrahedron Lett. 1994, 35, 3293–3296. [Google Scholar] [CrossRef]
- Le Merrer, Y.; Poitout, L.; Depezay, J.C.; Dosbaa, I.; Geoffroy, S.; Foglietti, M. Synthesis of azasugars as potent inhibitors of glycosidases. Bioorg. Med. Chem. 1997, 5, 519–533. [Google Scholar] [CrossRef]
- Wilkinson, B.L.; Bornaghi, L.F.; Lopez, M.; Healy, P.C.; Poulsen, S.; Houston, T.A. Synthesis of N-Propargyl Imino-Sugar Scaffolds for Compound Library Generation using Click Chemistry. Aust. J. Chem. 2010, 63, 821–829. [Google Scholar] [CrossRef]
- Jurczak, J.; Bauer, T.; Chmielewski, M.A. general approach to the synthesis of 2,3-di-O-protected derivatives of D-glyceraldehyde. Carbohydr. Res. 1987, 164, 493–498. [Google Scholar] [CrossRef]
- Aragão-Leoneti, V.; Carvalho, I. Simple and efficient synthesis of 2,5-anhydro-D-glucitol. Tetrahedron Lett. 2013, 54, 1087–1089. [Google Scholar] [CrossRef]
- Jung, M.E.; Lyster, M.A. Quantitative dealkylation of alkyl ethers via treatment with trimethylsilyl iodide. A new method for ether hydrolysis. J. Org. Chem. 1977, 42, 3761–3764. [Google Scholar] [CrossRef]
- Kasai, K.; Okada, K.; Saito, S.; Tokutake, M.; Tobe, K. Preparation of N-substituted-hexahydro-3,4,5,6-tetrahydroxyazepine as Glycosidase Inhibitors. Jpn. Kokai Tokkyo Koho JP 2001002648 A, 9 January 2001. [Google Scholar]
- Qian, X.; Morís-Varas, F.; Fitzgerald, M.C.; Wong, C.-H. C2-Symmetrical Tetrahydroxyazepanes as Inhibitors of Glycosidases and HIV/FIV Proteases. Bioorg. Med. Chem. 1996, 4, 2055–2069. [Google Scholar] [CrossRef]
- Li, H.; Liu, T.; Zhang, Y.; Favre, S.; Pierre, C.B.; Vogel, T.D.B.; Oikonomakos, N.G.; Marrot, J.; Blériot, Y. New Synthetic Seven-Membered 1-Azasugars Displaying Potent Inhibition Towards Glycosidases and Glucosylceramide Transferase. ChemBioChem 2008, 9, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Cendret, V.; Legigan, T.; Mingot, A.; Thibaudeau, S.; Adachi, I.; Forcella, M.; Parenti, P.; Bertrand, J.; Becq, F.; Norez, C.; et al. Synthetic deoxynojirimycin derivatives bearing a thiolated, fluorinated or unsaturated N-alkyl chain: Identification of potent α-glucosidase and trehalase inhibitors as well as F508del-CFTR correctors. Org. Biomol. Chem. 2015, 13, 10734–10744. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Wu, B.; Wang, B.; Li, T.H.; Zhang, P.F.; Guo, L.N.; Wang, W.J.; Zhao, W.; Wang, P.G. Facile and stereo-controlled synthesis of 2-deoxynojirimycin, Miglustat and Miglitol. Tetrahedron Lett. 2011, 52, 3802–3804. [Google Scholar] [CrossRef]
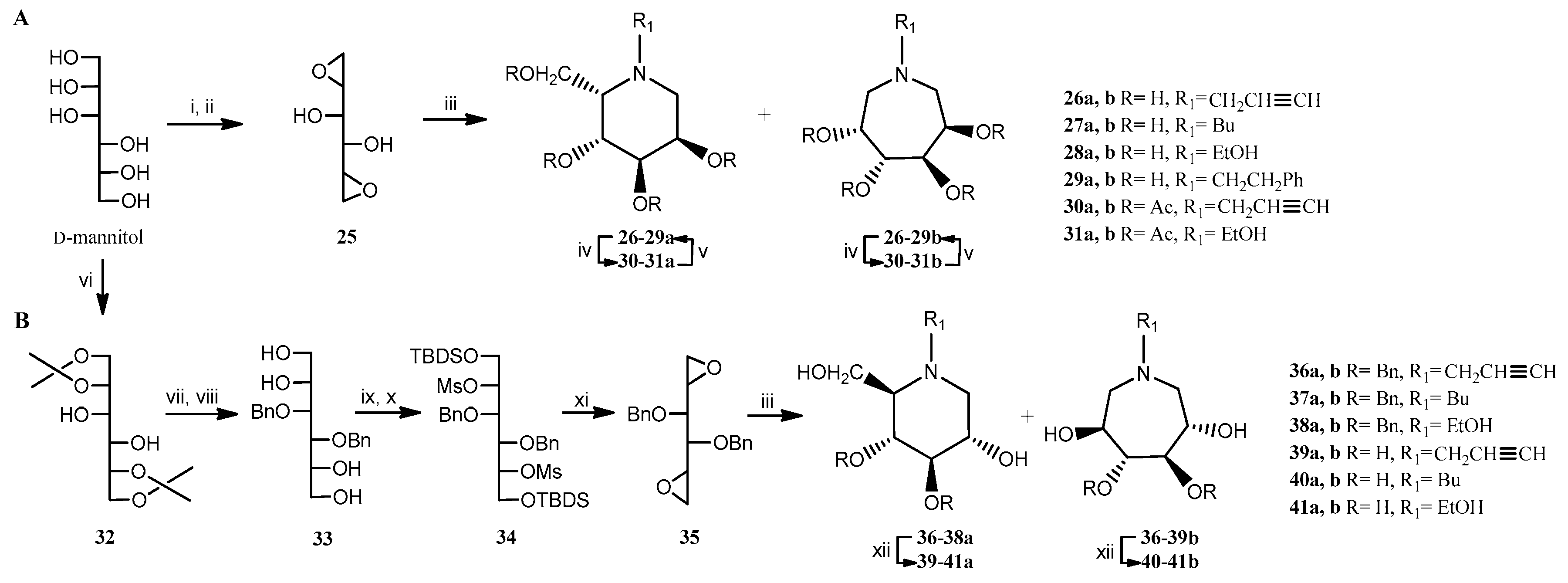
| Primary Amine for Aminocyclization Reaction | Yield (%) | |||
|---|---|---|---|---|
| Polyhydroxy-Piperidine | Polyhydroxy-Azepane | |||
| 1-deoxy-L-gulo-nojirimycin 26-29a | 1-deoxy-D-gluco-nojirimycin (DNJ) 39-41a | D-manno-azepane 26-29b | L-ido-azepane 39-41b | |
| Propargylamine | 32 | 40 | 35 | 37 |
| Butylamine | 20 | 33 | 24 | 38 |
| Ethanolamine | 17 | 22 | 21 | 28 |
| Phenethylamine* | 4 | - | 5 | - |
| Iminosugars with Inverted Configuration at C-2 and C-5 with Respect to Glucose | Iminosugars Preserving Glucose Stereochemistry | ||||||
|---|---|---|---|---|---|---|---|
| Inhibition (µM) | Inhibition (µM) | ||||||
| α-Glucosidase | β-Glucosidase | α-Glucosidase | β-Glucosidase | ||||
| - | - | - | - | DNJ |  | 134.4 ± 2.1 | 33.1 ± 3.1 |
| 26a |  | NI | 1716 ± 12.8 | 39a |  | 2527 ± 82.2 | 635.7 ± 8.5 |
| 26b |  | NI | NI | 39b | 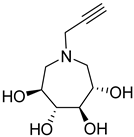 | NI | 3437 ± 70.6 |
| 27a |  | NI | 109.7 ± 9.3 | 40a | 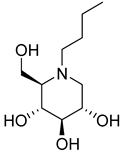 | NI | 172.8 ± 1.7 |
| 27b | 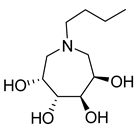 | 2031 ± 17.1 | 184.6 ± 2.6 | 40b |  | NI | 80.0 ± 4.9 |
| 28a |  | NI | NI | 41a | 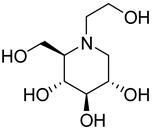 | 41.3 ± 10.1 | 4.0 ± 1.5 |
| 28b | 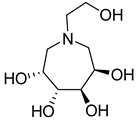 | NI | NI | 41b | 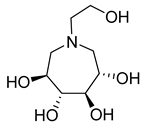 | 138.8 ± 1.2 | 4.0 ± 1.4 |
| 29a |  | NI | NI | - | - | - | - |
| 29b |  | NI | NI | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamoner, L.O.B.; Aragão-Leoneti, V.; Carvalho, I. Iminosugars: Effects of Stereochemistry, Ring Size, and N-Substituents on Glucosidase Activities. Pharmaceuticals 2019, 12, 108. https://doi.org/10.3390/ph12030108
Zamoner LOB, Aragão-Leoneti V, Carvalho I. Iminosugars: Effects of Stereochemistry, Ring Size, and N-Substituents on Glucosidase Activities. Pharmaceuticals. 2019; 12(3):108. https://doi.org/10.3390/ph12030108
Chicago/Turabian StyleZamoner, Luís O. B., Valquiria Aragão-Leoneti, and Ivone Carvalho. 2019. "Iminosugars: Effects of Stereochemistry, Ring Size, and N-Substituents on Glucosidase Activities" Pharmaceuticals 12, no. 3: 108. https://doi.org/10.3390/ph12030108
APA StyleZamoner, L. O. B., Aragão-Leoneti, V., & Carvalho, I. (2019). Iminosugars: Effects of Stereochemistry, Ring Size, and N-Substituents on Glucosidase Activities. Pharmaceuticals, 12(3), 108. https://doi.org/10.3390/ph12030108