Localization of 99mTc-GRP Analogs in GRPR-Expressing Tumors: Effects of Peptide Length and Neprilysin Inhibition on Biological Responses
Abstract
:1. Introduction
2. Results
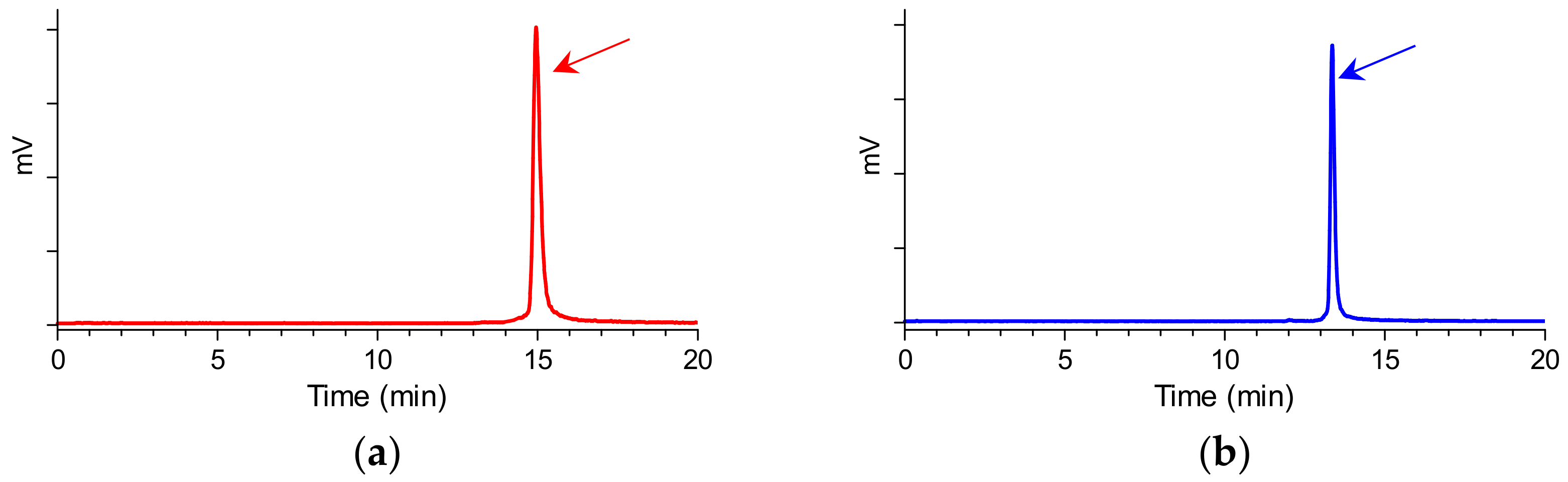
2.1. Peptides and Radioligands
2.2. In Vitro Assays
2.2.1. Receptor Autoradiography in Human Tumor Samples
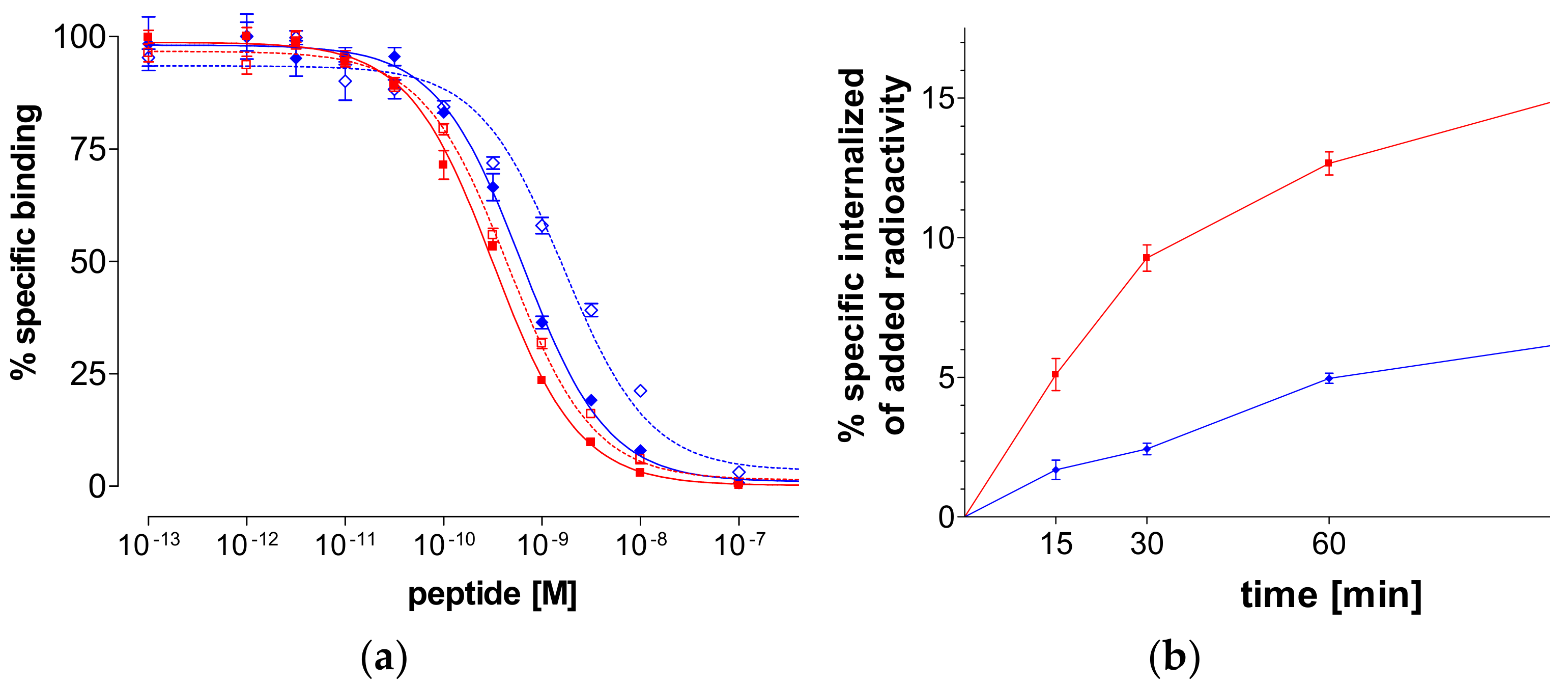
2.2.2. Binding Affinity for the Human GRPR
2.2.3. Internalization of 99mTc-N4-GRP(14–27) and 99mTc-N4-GRP(18–27) in PC-3 Cells
2.3. In Vivo Comparison of 99mTc-N4-GRP(14–27) and 99mTc-N4-GRP(18–27)
2.3.1. Stability of 99mTc-N4-GRP(14–27) and 99mTc-N4-GRP(18–27) in Mice
2.3.2. Biodistribution in PC-3 Xenograft-Bearing Mice
3. Discussion
4. Materials and Methods
4.1. Peptides and Reagents
Preparation and Quality Control of 99mTc-N4-GRP(14–27) and 99mTc-N4-GRP(18–27)
4.2. In Vitro Assays
4.2.1. Cell Lines and Culture
4.2.2. Receptor Autoradiography
4.2.3. Competition Binding in PC-3 Cell-Membranes
4.2.4. Internalization Assay in PC-3 Cells
4.3. Animal Studies
4.3.1. In Vivo Stability Tests
4.3.2. Induction of PC-3 Xenografts in SCID Mice
4.3.3. Biodistribution in PC-3 Xenograft-Bearing SCID Mice
Author Contributions
Funding
Conflicts of Interest
References
- Kroog, G.S.; Jensen, R.T.; Battey, J.F. Mammalian bombesin receptors. Med. Res. Rev. 1995, 15, 389–417. [Google Scholar] [CrossRef]
- Jensen, R.T.; Battey, J.F.; Spindel, E.R.; Benya, R.V. International union of pharmacology. LXVIII. Mammalian bombesin receptors: Nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol. Rev. 2008, 60, 1–42. [Google Scholar] [CrossRef]
- Markwalder, R.; Reubi, J.C. Gastrin-releasing peptide receptors in the human prostate: Relation to neoplastic transformation. Cancer Res. 1999, 59, 1152–1159. [Google Scholar] [PubMed]
- Körner, M.; Waser, B.; Rehmann, R.; Reubi, J.C. Early over-expression of GRP receptors in prostatic carcinogenesis. Prostate 2014, 74, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Beer, M.; Montani, M.; Gerhardt, J.; Wild, P.J.; Hany, T.F.; Hermanns, T.; Muntener, M.; Kristiansen, G. Profiling gastrin-releasing peptide receptor in prostate tissues: Clinical implications and molecular correlates. Prostate 2012, 72, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Wenger, S.; Schmuckli-Maurer, J.; Schaer, J.C.; Gugger, M. Bombesin receptor subtypes in human cancers: Detection with the universal radioligand 125I-[d-Tyr6,beta-Ala11,Phe13,Nle14]bombesin(6-14). Clin. Cancer Res. 2002, 8, 1139–1146. [Google Scholar]
- Halmos, G.; Wittliff, J.L.; Schally, A.V. Characterization of bombesin/gastrin-releasing peptide receptors in human breast cancer and their relationship to steroid receptor expression. Cancer Res. 1995, 55, 280–287. [Google Scholar] [PubMed]
- Gugger, M.; Reubi, J.C. Gastrin-releasing peptide receptors in non-neoplastic and neoplastic human breast. Am. J. Pathol. 1999, 155, 2067–2076. [Google Scholar] [CrossRef]
- Mattei, J.; Achcar, R.D.; Cano, C.H.; Macedo, B.R.; Meurer, L.; Batlle, B.S.; Groshong, S.D.; Kulczynski, J.M.; Roesler, R.; Dal Lago, L.; et al. Gastrin-releasing peptide receptor expression in lung cancer. Arch. Pathol. Lab. Med. 2014, 138, 98–104. [Google Scholar] [CrossRef]
- Guinee, D.G., Jr.; Fishback, N.F.; Koss, M.N.; Abbondanzo, S.L.; Travis, W.D. The spectrum of immunohistochemical staining of small-cell lung carcinoma in specimens from transbronchial and open-lung biopsies. Am. J. Clin. Pathol. 1994, 102, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.; Ramos-Alvarez, I.; Moody, T.W.; Jensen, R.T. Bombesin related peptides/receptors and their promising therapeutic roles in cancer imaging, targeting and treatment. Expert. Opin. Ther. Targets 2016, 20, 1055–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maina, T.; Nock, B.A. From bench to bed: New gastrin-releasing peptide receptor-directed radioligands and their use in prostate cancer. PET Clin. 2017, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Gonzalez, N.; Sancho, V.; Mantey, S.A.; Nuche-Berenguer, B.; Pradhan, T.; Coy, D.H.; Jensen, R.T. Pharmacology and selectivity of various natural and synthetic bombesin related peptide agonists for human and rat bombesin receptors differs. Peptides 2011, 32, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Vigna, S.R.; Mantyh, C.R.; Giraud, A.S.; Soll, A.H.; Walsh, J.H.; Mantyh, P.W. Localization of specific binding sites for bombesin in the canine gastrointestinal tract. Gastroenterology 1987, 93, 1287–1295. [Google Scholar] [CrossRef]
- Chave, H.S.; Gough, A.C.; Palmer, K.; Preston, S.R.; Primrose, J.N. Bombesin family receptor and ligand gene expression in human colorectal cancer and normal mucosa. Br. J. Cancer 2000, 82, 124–130. [Google Scholar] [CrossRef]
- Fleischmann, A.; Laderach, U.; Friess, H.; Buechler, M.W.; Reubi, J.C. Bombesin receptors in distinct tissue compartments of human pancreatic diseases. Lab. Investig. 2000, 80, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Delle Fave, G.; Annibale, B.; de Magistris, L.; Severi, C.; Bruzzone, R.; Puoti, M.; Melchiorri, P.; Torsoli, A.; Erspamer, V. Bombesin effects on human GI functions. Peptides 1985, 6 (Suppl. 3), 113–116. [Google Scholar] [CrossRef]
- Bruzzone, R.; Tamburrano, G.; Lala, A.; Mauceri, M.; Annibale, B.; Severi, C.; de Magistris, L.; Leonetti, F.; Delle Fave, G. Effect of bombesin on plasma insulin, pancreatic glucagon, and gut glucagon in man. J. Clin. Endocrinol. Metab. 1983, 56, 643–647. [Google Scholar] [CrossRef]
- Delle Fave, G.; Kohn, A.; De Magistris, L.; Annibale, B.; Bruzzone, R.; Sparvoli, C.; Severi, C.; Torsoli, A. Effects of bombesin on gastrin and gastric acid secretion in patients with duodenal ulcer. Gut 1983, 24, 231–235. [Google Scholar] [CrossRef] [Green Version]
- Severi, C.; Jensen, R.T.; Erspamer, V.; D’Arpino, L.; Coy, D.H.; Torsoli, A.; Delle Fave, G. Different receptors mediate the action of bombesin-related peptides on gastric smooth muscle cells. Am. J. Physiol. 1991, 260, G683–G690. [Google Scholar] [CrossRef]
- Bitar, K.N.; Zhu, X.X. Expression of bombesin-receptor subtypes and their differential regulation of colonic smooth muscle contraction. Gastroenterology 1993, 105, 1672–1680. [Google Scholar] [CrossRef]
- Falconieri Erspamer, G.; Severini, C.; Erspamer, V.; Melchiorri, P.; Delle Fave, G.; Nakajima, T. Parallel bioassay of 27 bombesin-like peptides on 9 smooth muscle preparations. Structure-activity relationships and bombesin receptor subtypes. Regul. Pept. 1988, 21, 1–11. [Google Scholar] [CrossRef]
- Nock, B.A.; Cescato, R.; Ketani, E.; Waser, B.; Reubi, J.C.; Maina, T. [99mTc]Demomedin C, a radioligand based on human gastrin releasing peptide(18–27): Synthesis and preclinical evaluation in gastrin releasing peptide receptor-expressing models. J. Med. Chem. 2012, 55, 8364–8374. [Google Scholar] [CrossRef] [PubMed]
- Marsouvanidis, P.J.; Maina, T.; Sallegger, W.; Krenning, E.P.; de Jong, M.; Nock, B.A. 99mTc Radiotracers based on human GRP(18–27): Synthesis and comparative evaluation. J. Nucl. Med. 2013, 54, 1797–803. [Google Scholar] [CrossRef] [PubMed]
- Nock, B.A.; Nikolopoulou, A.; Galanis, A.; Cordopatis, P.; Waser, B.; Reubi, J.C.; Maina, T. Potent bombesin-like peptides for GRP-receptor targeting of tumors with 99mTc: A preclinical study. J. Med. Chem. 2005, 48, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Marsouvanidis, P.J.; Maina, T.; Sallegger, W.; Krenning, E.P.; de Jong, M.; Nock, B.A. Tumor diagnosis with new 111In-radioligands based on truncated human gastrin releasing peptide sequences: Synthesis and preclinical comparison. J. Med. Chem. 2013, 56, 8579–8587. [Google Scholar] [CrossRef]
- Roques, B.P.; Noble, F.; Dauge, V.; Fournie-Zaluski, M.C.; Beaumont, A. Neutral endopeptidase 24.11: Structure, inhibition, and experimental and clinical pharmacology. Pharmacol. Rev. 1993, 45, 87–146. [Google Scholar] [PubMed]
- Suda, H.; Aoyagi, T.; Takeuchi, T.; Umezawa, H. Letter: A thermolysin inhibitor produced by actinomycetes: Phosphoramidon. J. Antibiot. (Tokyo) 1973, 26, 621–623. [Google Scholar] [CrossRef]
- Oefner, C.; D’Arcy, A.; Hennig, M.; Winkler, F.K.; Dale, G.E. Structure of human neutral endopeptidase (neprilysin) complexed with phosphoramidon. J. Mol. Biol. 2000, 296, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Marsouvanidis, P.J.; Melis, M.; de Blois, E.; Breeman, W.A.; Krenning, E.P.; Maina, T.; Nock, B.A.; de Jong, M. In vivo enzyme inhibition improves the targeting of [177Lu]DOTA-GRP(13-27) in GRPR-positive tumors in mice. Cancer Biother. Radiopharm. 2014, 29, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Nock, B.A.; Maina, T.; Krenning, E.P.; de Jong, M. “To serve and protect”: Enzyme inhibitors as radiopeptide escorts promote tumor targeting. J. Nucl. Med. 2014, 55, 121–127. [Google Scholar] [CrossRef]
- Chatalic, K.L.; Konijnenberg, M.; Nonnekens, J.; de Blois, E.; Hoeben, S.; de Ridder, C.; Brunel, L.; Fehrentz, J.A.; Martinez, J.; van Gent, D.C.; et al. In vivo stabilization of a gastrin-releasing peptide receptor antagonist enhances pet imaging and radionuclide therapy of prostate cancer in preclinical studies. Theranostics 2016, 6, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Maina, T.; Kaloudi, A.; Valverde, I.E.; Mindt, T.L.; Nock, B.A. Amide-to-triazole switch vs. In vivo NEP-inhibition approaches to promote radiopeptide targeting of GRPR-positive tumors. Nucl. Med. Biol. 2017, 52, 57–62. [Google Scholar] [CrossRef]
- Lymperis, E.; Kaloudi, A.; Sallegger, W.; Bakker, I.L.; Krenning, E.P.; de Jong, M.; Maina, T.; Nock, B.A. Radiometal-dependent biological profile of the radiolabeled gastrin-releasing peptide receptor antagonist SB3 in cancer theranostics: Metabolic and biodistribution patterns defined by neprilysin. Bioconjug. Chem. 2018, 29, 1774–1784. [Google Scholar] [CrossRef]
- Nock, B.; Maina, T. Tetraamine-coupled peptides and resulting 99mTc-radioligands: An effective route for receptor-targeted diagnostic imaging of human tumors. Curr. Top. Med. Chem. 2012, 12, 2655–2667. [Google Scholar] [CrossRef] [PubMed]
- Mather, S.J.; Nock, B.A.; Maina, T.; Gibson, V.; Ellison, D.; Murray, I.; Sobnack, R.; Colebrook, S.; Wan, S.; Halberrt, G.; et al. GRP receptor imaging of prostate cancer using [99mTc]Demobesin 4: A first-in-man study. Mol. Imaging Biol. 2014, 16, 888–895. [Google Scholar] [CrossRef] [PubMed]
- De Castiglione, R.; Gozzini, L. Bombesin receptor antagonists. Crit. Rev. Oncol. Hematol. 1996, 24, 117–151. [Google Scholar] [CrossRef]
- Maina, T.; Nock, B.A.; Kulkarni, H.; Singh, A.; Baum, R.P. Theranostic prospects of gastrin-releasing peptide receptor-radioantagonists in oncology. PET Clin. 2017, 12, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Kassis, A.I. Therapeutic radionuclides: Biophysical and radiobiologic principles. Semin. Nucl. Med. 2008, 38, 358–366. [Google Scholar] [CrossRef]
- Wild, D.; Frischknecht, M.; Zhang, H.; Morgenstern, A.; Bruchertseifer, F.; Boisclair, J.; Provencher-Bolliger, A.; Reubi, J.C.; Maecke, H.R. Alpha- versus beta-particle radiopeptide therapy in a human prostate cancer model (213Bi-DOTA-Pesin and 213Bi-AMBA versus 177Lu-DOTA-Pesin). Cancer Res. 2011, 71, 1009–1018. [Google Scholar] [CrossRef]
- Linder, K.E.; Metcalfe, E.; Arunachalam, T.; Chen, J.; Eaton, S.M.; Feng, W.; Fan, H.; Raju, N.; Cagnolini, A.; Lantry, L.E.; et al. In vitro and in vivo metabolism of Lu-AMBA, a GRP-receptor binding compound, and the synthesis and characterization of its metabolites. Bioconjug. Chem. 2009, 20, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Shipp, M.A.; Tarr, G.E.; Chen, C.Y.; Switzer, S.N.; Hersh, L.B.; Stein, H.; Sunday, M.E.; Reinherz, E.L. CD10/neutral endopeptidase 24.11 hydrolyzes bombesin-like peptides and regulates the growth of small cell carcinomas of the lung. Proc. Natl. Acad. Sci. USA 1991, 88, 10662–10666. [Google Scholar] [CrossRef] [PubMed]
- Reile, H.; Armatis, P.E.; Schally, A.V. Characterization of high-affinity receptors for bombesin/gastrin releasing peptide on the human prostate cancer cell lines PC-3 and DU-145: Internalization of receptor bound 125I-(Tyr4)bombesin by tumor cells. Prostate 1994, 25, 29–38. [Google Scholar] [CrossRef] [PubMed]


| Peptide Conjugate | IC50s in nM | ||
|---|---|---|---|
| GRPR 1 | NMBR 2 | BB3R 3 | |
| Universal ligand 4 | 1.5 ± 0.1 (3) | 1.5 ± 0.2 (3) | 3.5 ± 0.7 (3) |
| N4-GRP(14–27) | 4.2 ± 1.0 (3) | 72 ± 7.6 (3) | >1000 (3) |
| N4-GRP(18–27) | 2.4 ± 1.0 (3) | 106 ± 13 (2) | >1000 (3) |
| Demobesin 3 | 0.5 (2) | 1.6 (2) | >100 (3) |
| Tissue | 1 h 1 | 4 h 1 | 24 h 1 | 4 h block 2 |
|---|---|---|---|---|
| Blood | 1.54 ± 0.17 | 0.10 ± 0.03 | 0.07 ± 0.01 | 0.08 ± 0.02 |
| Liver | 5.02 ± 0.46 | 3.75 ± 1.11 | 3.23 ± 0.78 | 6.12 ± 2.89 |
| Heart | 0.70 ± 0.07 | 0.11 ± 0.04 | 0.07 ± 0.01 | 0.28 ± 0.13 |
| Kidneys | 14.82 ± 2.22 | 4.83 ± 2.12 | 3.10 ± 0.73 | 4.85 ± 2.66 |
| Stomach | 1.02 ± 0.29 | 1.42 ± 0.93 | 0.78 ± 0.33 | 0.43 ± 0.18 |
| Intestines | 7.21 ± 0.52 | 7.61 ± 2.23 | 1.73 ± 0.23 | 1.45 ± 0.44 |
| Muscle | 0.29 ± 0.02 | 0.03 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.02 |
| Lungs | 1.96 ± 0.33 | 0.49 ± 0.30 | 0.18 ± 0.04 | 0.65 ± 0.22 |
| Pancreas | 37.85 ± 1.95 | 35.24 ± 4.70 | 13.41 ± 0.77 | 0.83 ± 0.24 |
| Tumor | 10.20 ± 0.72 | 8.41 ± 4.16 | 4.50 ± 0.69 | 0.62 ± 0.24 |
| Tissue | 99mTc-N4-GRP(14–27) | 99mTc-N4-GRP(18–27) | ||
|---|---|---|---|---|
| 4 h 1 | 4 h PA 1,2 | 4 h1 | 4 h PA 1,2 | |
| Blood | 0.10 ± 0.03 | 0.23 ± 0.08 | 0.13 ± 0.04 | 0.21 ± 0.04 |
| Liver | 3.75 ± 1.11 | 4.99 ± 1.53 | 1.02 ± 0.17 | 2.13 ± 0.37 |
| Heart | 0.11 ± 0.04 | 0.89 ± 0.06 | 0.14 ± 0.10 | 0.47 ± 0.27 |
| Kidneys | 4.83 ± 2.12 | 11.68 ± 1.61 | 6.01 ± 1.38 | 7.66 ± 1.39 |
| Stomach | 1.42 ± 0.93 | 3.82 ± 1.10 | 1.12 ± 0.75 | 4.02 ± 0.66 |
| Intestines | 7.61 ± 2.23 | 21.35 ± 1.68 | 7.28 ± 0.60 | 16.23 ± 3.90 |
| Muscle | 0.03 ± 0.01 | 0.06 ± 0.02 | 0.03 ± 0.01 | 0.06 ± 0.01 |
| Lungs | 0.49 ± 0.30 | 1.06 ± 0.19 | 0.28 ± 0.09 | 0.84 ± 0.26 |
| Pancreas | 35.24 ± 4.70 | 110.32 ± 8.76 | 32.18 ± 5.91 | 95.39 ± 20.34 |
| Tumor | 8.41 ± 4.16 | 38.19 ± 4.79 | 7.08 ± 1.29 | 28.37 ± 8.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaloudi, A.; Lymperis, E.; Kanellopoulos, P.; Waser, B.; de Jong, M.; Krenning, E.P.; Reubi, J.C.; Nock, B.A.; Maina, T. Localization of 99mTc-GRP Analogs in GRPR-Expressing Tumors: Effects of Peptide Length and Neprilysin Inhibition on Biological Responses. Pharmaceuticals 2019, 12, 42. https://doi.org/10.3390/ph12010042
Kaloudi A, Lymperis E, Kanellopoulos P, Waser B, de Jong M, Krenning EP, Reubi JC, Nock BA, Maina T. Localization of 99mTc-GRP Analogs in GRPR-Expressing Tumors: Effects of Peptide Length and Neprilysin Inhibition on Biological Responses. Pharmaceuticals. 2019; 12(1):42. https://doi.org/10.3390/ph12010042
Chicago/Turabian StyleKaloudi, Aikaterini, Emmanouil Lymperis, Panagiotis Kanellopoulos, Beatrice Waser, Marion de Jong, Eric P. Krenning, Jean Claude Reubi, Berthold A. Nock, and Theodosia Maina. 2019. "Localization of 99mTc-GRP Analogs in GRPR-Expressing Tumors: Effects of Peptide Length and Neprilysin Inhibition on Biological Responses" Pharmaceuticals 12, no. 1: 42. https://doi.org/10.3390/ph12010042
APA StyleKaloudi, A., Lymperis, E., Kanellopoulos, P., Waser, B., de Jong, M., Krenning, E. P., Reubi, J. C., Nock, B. A., & Maina, T. (2019). Localization of 99mTc-GRP Analogs in GRPR-Expressing Tumors: Effects of Peptide Length and Neprilysin Inhibition on Biological Responses. Pharmaceuticals, 12(1), 42. https://doi.org/10.3390/ph12010042






