Development and Characterization of Chitosan Microparticles-in-Films for Buccal Delivery of Bioactive Peptides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Cell Line
2.2. Formulation of Chitosan Microparticles Loaded with the Antihypertensive Peptide
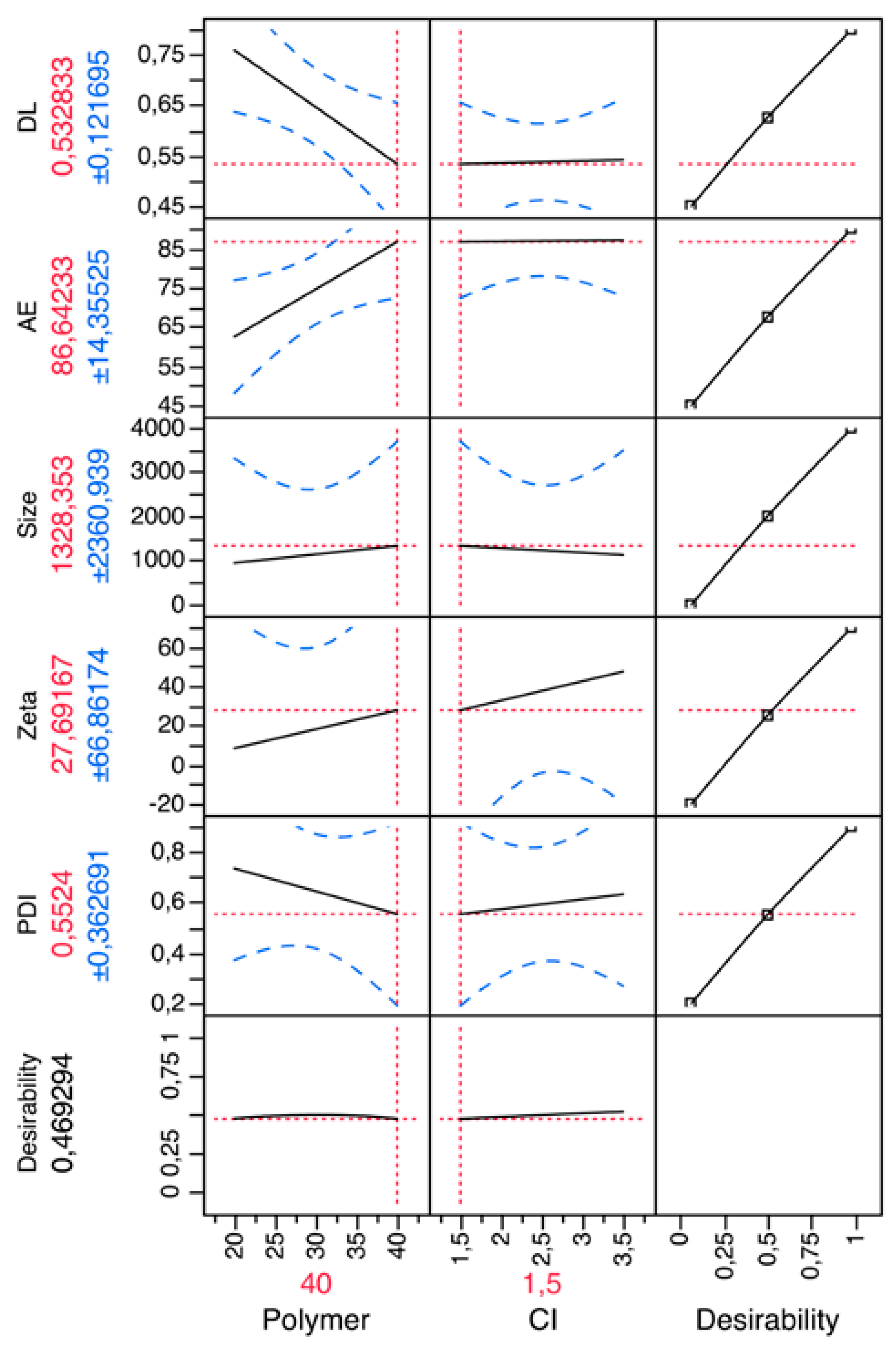
Preliminary Optimization and Factorial Design
2.3. Characterization of Chitosan Microparticles
2.4. Association Efficiency and Loading Degree
2.5. Preparation of Chitosan Oral Films
Chitosan Films Experimental Design Testing
2.6. Chitosan Films Characterization
2.6.1. Film Appearance
2.6.2. Film Weight and Thickness
2.6.3. Determination of the Mechanical Properties
2.6.4. Swelling and Erosion Studies
2.7. Chitosan Films with Chitosan Microparticles
2.8. Cell Culture
Cell Viability Studies
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Chitosan Microparticles
3.2. Chitosan Films Characterization
3.3. In Vitro Cell Viability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Batista, P.; Castro, P.M.; Madureira, A.R.; Sarmento, B.; Pintado, M. Recent insights in the use of nanocarriers for the oral delivery of bioactive proteins and peptides. Peptides 2018, 101, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Giovino, C.; Ayensu, I.; Tetteh, J.; Boateng, J.S. An integrated buccal delivery system combining chitosan films impregnated with peptide loaded PEG-b-PLA nanoparticles. Colloids Surf. B Biointerfaces 2013, 112, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Cholkar, K.; Mitra, A.K. Recent developments in protein and peptide parenteral delivery approaches. Ther. Deliv. 2014, 5, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wu, J.; Shi, J.; Farokhzad, O.C. Nanotechnology for protein delivery: Overview and perspectives. J. Control. Release 2016, 240, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, C.G.; Matos, C.M.; Teixeira, J.A.; Balcão, V.M. Nanocarrier possibilities for functional targeting of bioactive peptides and proteins: State-of-the-art. J. Drug Target. 2012, 20, 114–141. [Google Scholar] [CrossRef] [PubMed]
- Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a source of peptides with remarkable biological activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef]
- Tavares, T.; del Mar Contreras, M.; Amorim, M.; Pintado, M.; Recio, I.; Malcata, F.X. Novel whey-derived peptides with inhibitory effect against angiotensin-converting enzyme: In vitro effect and stability to gastrointestinal enzymes. Peptides 2011, 32, 1013–1019. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef]
- Morishita, M.; Peppas, N.A. Is the oral route possible for peptide and protein drug delivery? Drug Discov. Today 2006, 11, 905–910. [Google Scholar] [CrossRef]
- Castro, P.M.; Fonte, P.; Sousa, F.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Oral films as breakthrough tools for oral delivery of proteins/peptides. J. Control. Release 2015, 211, 63–73. [Google Scholar] [CrossRef]
- Rossi, S.; Sandri, G.; Caramella, C.M. Buccal drug delivery: A challenge already won? Drug Discov. Today Technol. 2005, 2, 59–65. [Google Scholar] [CrossRef]
- Mahato, R.I.; Narang, A.S.; Thoma, L.; Miller, D.D. Emerging trends in oral delivery of peptide and protein drugs. Crit. Rev.™ Ther. Drug Carr. Syst. 2003, 20. [Google Scholar] [CrossRef]
- Shaji, J.; Patole, V. Protein and Peptide Drug Delivery: Oral Approaches. Indian J. Pharm. Sci. 2008, 70, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Giovino, C.; Ayensu, I.; Tetteh, J.; Boateng, J.S. Development and characterisation of chitosan films impregnated with insulin loaded PEG-b-PLA nanoparticles (NPs): A potential approach for buccal delivery of macromolecules. Int. J. Pharm. 2012, 428, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.M.; Fonte, P.; Oliveira, A.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Optimization of two biopolymer-based oral films for the delivery of bioactive molecules. Mater. Sci. Eng. C 2017, 76, 171–180. [Google Scholar] [CrossRef]
- Andrade, F.; Antunes, F.; Vanessa Nascimento, A.; Baptista da Silva, S.; das Neves, J.; Ferreira, D.; Sarmento, B. Chitosan formulations as carriers for therapeutic proteins. Curr. Drug Discov. Technol. 2011, 8, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Stevens, H.N.; Eccleston, G.M.; Auffret, A.D.; Humphrey, M.J.; Matthews, K.H. Development and mechanical characterization of solvent-cast polymeric films as potential drug delivery systems to mucosal surfaces. Drug Dev. Ind. Pharm. 2009, 35, 986–996. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.-S.; Ngo, D.-N.; Kang, K.-H.; Je, J.-Y.; Pham, H.N.-D.; Byun, H.-G.; Kim, S.-K. Biological effects of chitosan and its derivatives. Food Hydrocoll. 2015, 51, 200–216. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765. [Google Scholar]
- Caetano, L.; Almeida, A.; Gonçalves, L. Effect of Experimental Parameters on Alginate/Chitosan Microparticles for BCG Encapsulation. Mar. Drugs 2016, 14, 90. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, N.; Sharma, P.K. Buccal film: An advance technology for oral drug delivery. Adv. Biol. Res. 2014, 8, 260–267. [Google Scholar]
- FDA, Food and Drug Administration. GRAS Notice Inventory. Available online: https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/ (accessed on 17 January 2019).
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Araújo, F.; Shrestha, N.; Shahbazi, M.-A.; Fonte, P.; Mäkilä, E.M.; Salonen, J.J.; Hirvonen, J.T.; Granja, P.L.; Santos, H.A.; Sarmento, B. The impact of nanoparticles on the mucosal translocation and transport of GLP-1 across the intestinal epithelium. Biomaterials 2014, 35, 9199–9207. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.M.; Baptista, P.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Combination of PLGA nanoparticles with mucoadhesive guar-gum films for buccal delivery of antihypertensive peptide. Int. J. Pharm. 2018, 547, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Cardelle-Cobas, A.; Madureira, A.R.; Costa, E.; Barros, R.; Tavaria, F.K.; Pintado, M.E. Development of Oral Strips Containing Chitosan as Active Ingredient: A Product for Buccal Health. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 906–918. [Google Scholar] [CrossRef]
- Nielsen, H.M.; Rassing, M.R. TR146 cells grown on filters as a model of human buccal epithelium: IV. Permeability of water, mannitol, testosterone and β-adrenoceptor antagonists. Comparison to human, monkey and porcine buccal mucosa. Int. J. Pharm. 2000, 194, 155–167. [Google Scholar] [CrossRef]
- Portero, A.; Remuñán-López, C.; Nielsen, H.M. The potential of chitosan in enhancing peptide and protein absorption across the TR146 cell culture model—An in vitro model of the buccal epithelium. Pharm. Res. 2002, 19, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2017, 110, 97–109. [Google Scholar] [CrossRef]
- Jain, A.; Thakur, K.; Sharma, G.; Kush, P.; Jain, U.K. Fabrication, characterization and cytotoxicity studies of ionically cross-linked docetaxel loaded chitosan nanoparticles. Carbohydr. Polym. 2016, 137, 65–74. [Google Scholar] [CrossRef]
- Shah, U.; Joshi, G.; Sawant, K. Improvement in antihypertensive and antianginal effects of felodipine by enhanced absorption from PLGA nanoparticles optimized by factorial design. Mater. Sci. Eng. C 2014, 35, 153–163. [Google Scholar] [CrossRef]
- Oliveira, P.M.; Matos, B.N.; Pereira, P.A.; Gratieri, T.; Faccioli, L.H.; Cunha-Filho, M.S.; Gelfuso, G.M. Microparticles prepared with 50–190 kDa chitosan as promising non-toxic carriers for pulmonary delivery of isoniazid. Carbohydr. Polym. 2017, 174, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Walke, S.; Srivastava, G.; Nikalje, M.; Doshi, J.; Kumar, R.; Ravetkar, S.; Doshi, P. Fabrication of chitosan microspheres using vanillin/TPP dual crosslinkers for protein antigens encapsulation. Carbohydr. Polym. 2015, 128, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Sipoli, C.C.; Santana, N.; Shimojo, A.A.M.; Azzoni, A.; de la Torre, L.G. Scalable production of highly concentrated chitosan/TPP nanoparticles in different pHs and evaluation of the in vitro transfection efficiency. Biochem. Eng. J. 2015, 94, 65–73. [Google Scholar] [CrossRef]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly (lactide-co-glycolide)(PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Tao, C.; Huang, J.; Lu, Y.; Zou, H.; He, X.; Chen, Y.; Zhong, Y. Development and characterization of GRGDSPC-modified poly (lactide-co-glycolide acid) porous microspheres incorporated with protein-loaded chitosan microspheres for bone tissue engineering. Colloids Surf. B Biointerfaces 2014, 122, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhao, S.; Li, Z.; Wang, Y.; Xu, B.; Fang, D.; Wang, F.; Zhang, Z.; He, L.; Song, X. Enhanced and Extended Anti-Hypertensive Effect of VP5 Nanoparticles. Int. J. Mol. Sci. 2016, 17, 1977. [Google Scholar] [CrossRef] [PubMed]
- Dammak, I.; Bittante, A.M.Q.B.; Lourenço, R.V.; do Amaral Sobral, P.J. Properties of gelatin-based films incorporated with chitosan-coated microparticles charged with rutin. Int. J. Biol. Macromol. 2017, 101, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, X.; Chen, E.; Wu, S.; McClements, D.J.; Liu, S.; Li, B.; Li, Y. Preparation, characterization, and properties of chitosan films with cinnamaldehyde nanoemulsions. Food Hydrocoll. 2016, 61, 662–671. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010, 122, 161–166. [Google Scholar] [CrossRef]
- Park, S.-I.; Zhao, Y. Incorporation of a high concentration of mineral or vitamin into chitosan-based films. J. Agric. Food Chem. 2004, 52, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Frindy, S.; Primo, A.; el kacem Qaiss, A.; Bouhfid, R.; Lahcini, M.; Garcia, H.; Bousmina, M.; El Kadib, A. Insightful understanding of the role of clay topology on the stability of biomimetic hybrid chitosan-clay thin films and CO2-dried porous aerogel microspheres. Carbohydr. Polym. 2016, 146, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Chonkar, A.D.; Rao, J.V.; Managuli, R.S.; Mutalik, S.; Dengale, S.; Jain, P.; Udupa, N. Development of fast dissolving oral films containing lercanidipine HCl nanoparticles in semicrystalline polymeric matrix for enhanced dissolution and ex vivo permeation. Eur. J. Pharm. Biopharm. 2016, 103, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, K.C.; Tello, F.; Bierhalz, A.C.; Romo, M.G.G.; Flores, H.E.M.; Grosso, C.R. Protein adsorption onto alginate-pectin microparticles and films produced by ionic gelation. J. Food Eng. 2015, 154, 17–24. [Google Scholar] [CrossRef]
- Irfan, M.; Rabel, S.; Bukhtar, Q.; Qadir, M.I.; Jabeen, F.; Khan, A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm. J. 2016, 24, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Guan, Y.-X.; Yao, S.-J.; Zhu, Z.-Q. Preparation of ibuprofen-loaded chitosan films for oral mucosal drug delivery using supercritical solution impregnation. Int. J. Pharm. 2014, 473, 434–441. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. American Guidance for Industry: Orally Disintegrating Tablets; US Food and Drug Administration: Washington, DC, USA, 2008.
- Dahiya, M.; Saha, S.; Shahiwala, A.F. A review on mouth dissolving films. Curr. Drug Deliv. 2009, 6, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Zhu, A.; Chen, D.; Jiang, X.; Xu, X.; Zhang, L.; Tian, W. In vitro degradation of starch/PVA films and biocompatibility evaluation. J. Appl. Polym. Sci. 2010, 115, 346–357. [Google Scholar] [CrossRef]


| Caption | Size (µm) | Polydispersity Index | Zeta Potential (mV) | Association Efficiency (%) | Loading Capacity (%) |
|---|---|---|---|---|---|
| CH MPs | 2.544 ± 0.97 | 0.66 ± 0.18 | 50.38 ± 7.18 | - | - |
| CH MPs + Peptide | 2.582 ± 0.87 | 0.45 ± 0.18 | 60.97 ± 9.20 | 76.16 ± 1.96 | 0.46 ± 0.01 |
| Caption | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| CH MPs | 2.12 ± 0.93 | 0.71 ± 0.09 | 20.06 ± 0.68 |
| CH MPs + Peptide | 2.29 ± 0.81 | 0.77 ± 0.09 | 20.27 ± 0.72 |
| Erosion (%) | Swelling (%) | Disintegration Time (s) |
|---|---|---|
| 20.03 ± 1.3 | 257 ± 56 | 30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, P.; Castro, P.; Madureira, A.R.; Sarmento, B.; Pintado, M. Development and Characterization of Chitosan Microparticles-in-Films for Buccal Delivery of Bioactive Peptides. Pharmaceuticals 2019, 12, 32. https://doi.org/10.3390/ph12010032
Batista P, Castro P, Madureira AR, Sarmento B, Pintado M. Development and Characterization of Chitosan Microparticles-in-Films for Buccal Delivery of Bioactive Peptides. Pharmaceuticals. 2019; 12(1):32. https://doi.org/10.3390/ph12010032
Chicago/Turabian StyleBatista, Patrícia, Pedro Castro, Ana Raquel Madureira, Bruno Sarmento, and Manuela Pintado. 2019. "Development and Characterization of Chitosan Microparticles-in-Films for Buccal Delivery of Bioactive Peptides" Pharmaceuticals 12, no. 1: 32. https://doi.org/10.3390/ph12010032
APA StyleBatista, P., Castro, P., Madureira, A. R., Sarmento, B., & Pintado, M. (2019). Development and Characterization of Chitosan Microparticles-in-Films for Buccal Delivery of Bioactive Peptides. Pharmaceuticals, 12(1), 32. https://doi.org/10.3390/ph12010032







