Controlled-Deactivation CB1 Receptor Ligands as a Novel Strategy to Lower Intraocular Pressure
Abstract
:1. Introduction
2. Methods
2.1. Animals
2.2. Intraocular Pressure Measurements
2.3. Hippocampal Culture Preparation
2.4. Electrophysiology
3. Results
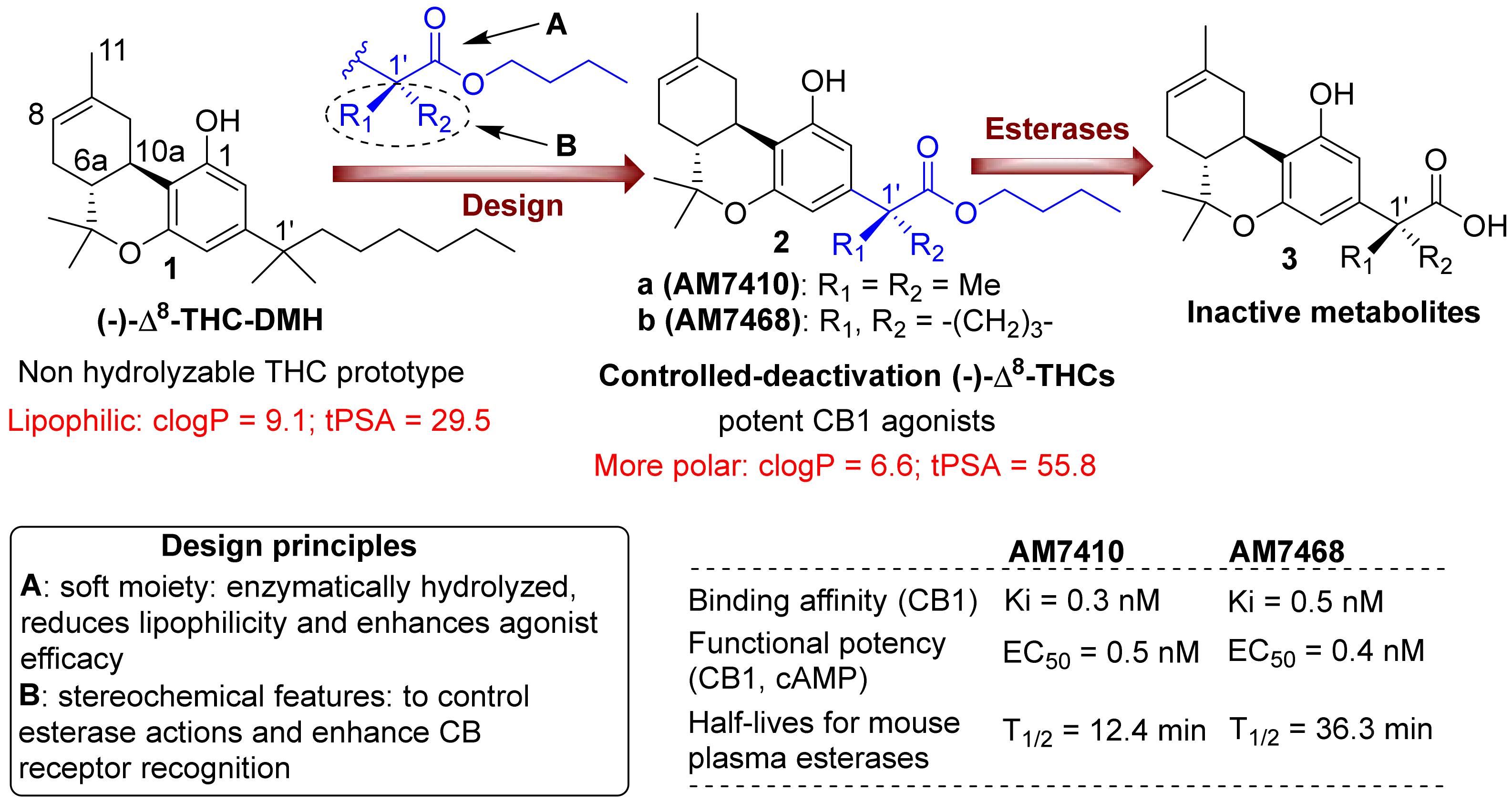
3.1. AM7410 as a Controlled-Deactivation CB1 Ligand
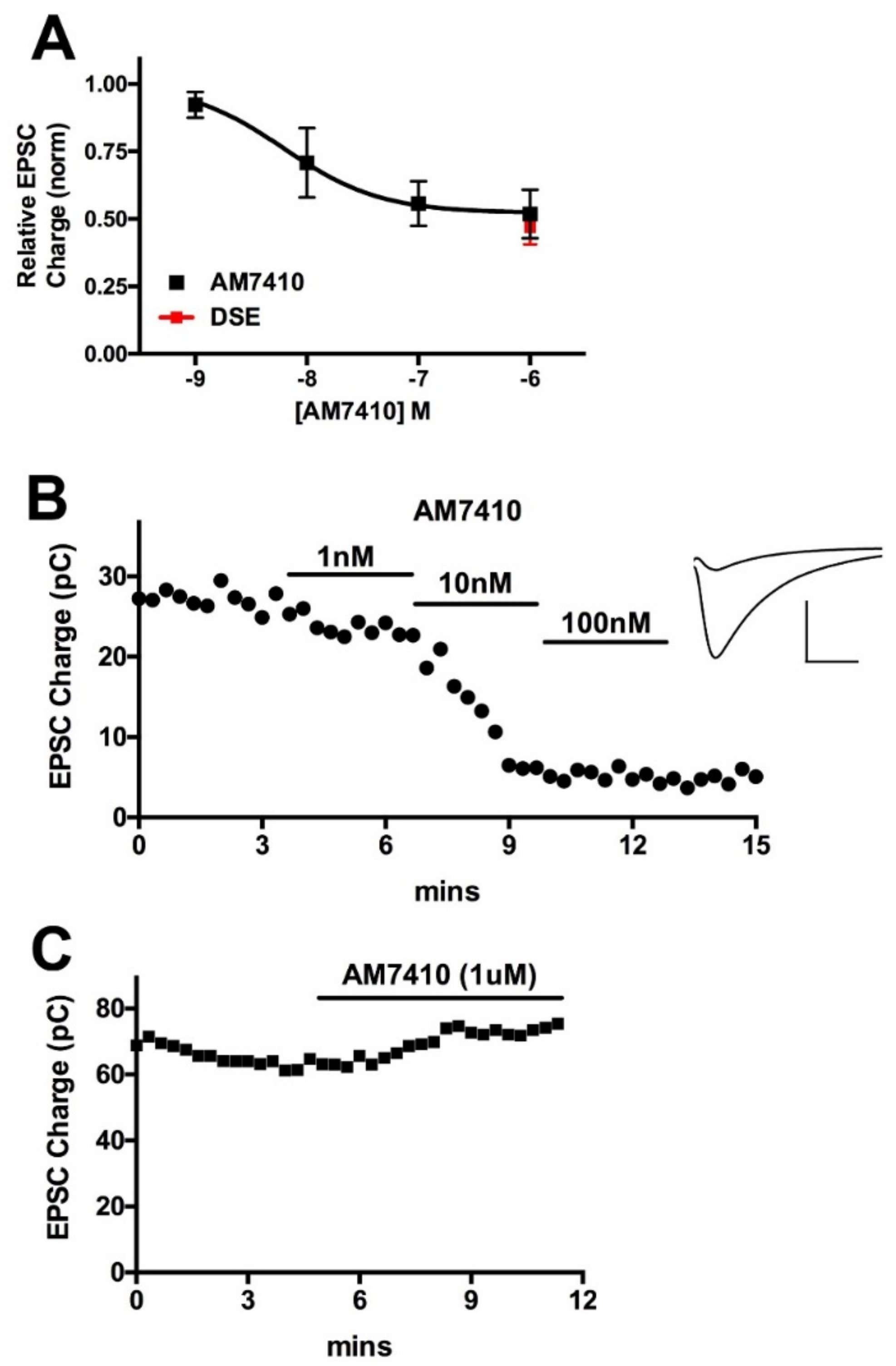
3.2. AM7410 Is a Potent and Efficacious Ligand at CB1 Receptors
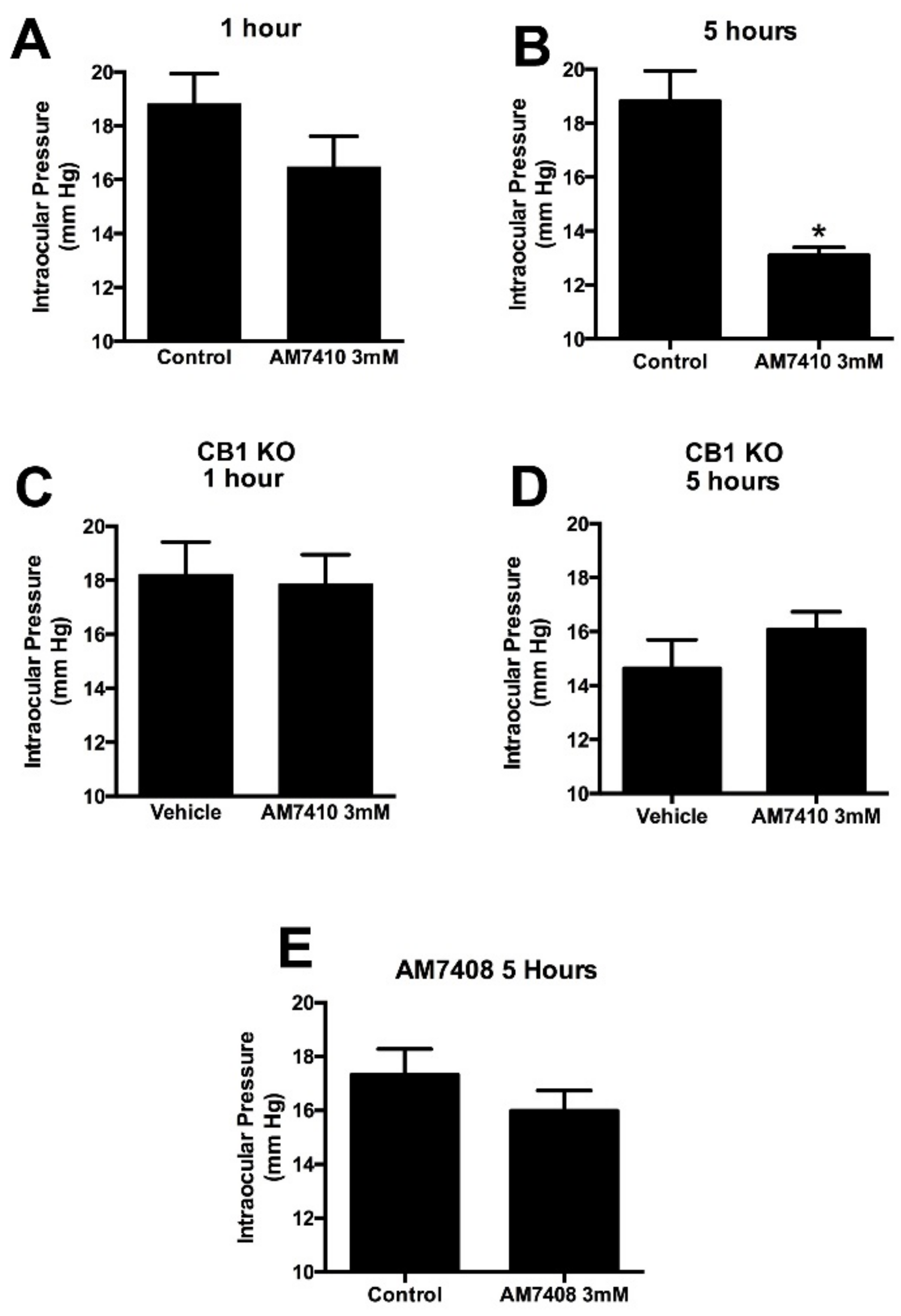
3.3. AM7410 Lowers Ocular Pressure in a Normotensive Mouse Model
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hepler, R.S.; Frank, I.R. Marihuana smoking and intraocular pressure. JAMA 1971, 217, 1392. [Google Scholar] [CrossRef] [PubMed]
- Piomelli, D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003, 4, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Hudson, B.D.; Beazley, M.; Szczesniak, A.M.; Straiker, A.; Kelly, M.E. Indirect sympatholytic actions at beta-adrenoceptors account for the ocular hypotensive actions of cannabinoid receptor agonists. J. Pharmacol. Exp. Ther. 2011, 339, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Leishman, E.; Hu, S.S.; Elghouche, A.; Daily, L.; Murataeva, N.; Bradshaw, H.; Straiker, A. Harnessing the Endocannabinoid 2-Arachidonoylglycerol to Lower Intraocular Pressure in a Murine Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3287–3296. [Google Scholar] [CrossRef] [PubMed]
- Cairns, E.A.; Toguri, J.T.; Porter, R.F.; Szczesniak, A.M.; Kelly, M.E. Seeing over the horizon—Targeting the endocannabinoid system for the treatment of ocular disease. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Jarvinen, T.; Pate, D.W.; Laine, K. Cannabinoids in the treatment of glaucoma. Pharmacol. Ther. 2002, 95, 203–220. [Google Scholar] [CrossRef]
- Panahi, Y.; Manayi, A.; Nikan, M.; Vazirian, M. The arguments for and against cannabinoids application in glaucomatous retinopathy. Biomed. Pharmacother. 2017, 86, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki, Y. Molecular design for enhancement of ocular penetration. J. Pharm. Sci. 2008, 97, 2462–2496. [Google Scholar] [CrossRef] [PubMed]
- Nikas, S.P.; Sharma, R.; Paronis, C.A.; Kulkarni, S.; Thakur, G.A.; Hurst, D.; Wood, J.T.; Gifford, R.S.; Rajarshi, G.; Liu, Y.; et al. Probing the carboxyester side chain in controlled deactivation (−)-delta(8)-tetrahydrocannabinols. J. Med. Chem. 2015, 58, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Nikas, S.P.; Paronis, C.A.; Wood, J.T.; Halikhedkar, A.; Guo, J.J.; Thakur, G.A.; Kulkarni, S.; Benchama, O.; Raghav, J.G.; et al. Controlled-deactivation cannabinergic ligands. J. Med. Chem. 2013, 56, 10142–10157. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Nikas, S.P.; Guo, J.J.; Mallipeddi, S.; Wood, J.T.; Makriyannis, A. C-ring cannabinoid lactones: A novel cannabinergic chemotype. ACS Med. Chem. Lett. 2014, 5, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Nikas, S.P.; Sharma, R.; Jiang, S.; Paronis, C.A.; Leonard, M.Z.; Zhang, B.; Honrao, C.; Mallipeddi, S.; Raghav, J.G.; et al. Novel C-Ring-Hydroxy-Substituted Controlled Deactivation Cannabinergic Analogues. J. Med. Chem. 2016, 59, 6903–6919. [Google Scholar] [CrossRef] [PubMed]
- Ledent, C.; Valverde, O.; Cossu, G.; Petitet, F.; Aubert, J.F.; Beslot, F.; Böhme, G.A.; Imperato, A.; Pedrazzini, T.; Roques, B.P.; et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 1999, 283, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Oltmanns, M.H.; Samudre, S.S.; Castillo, I.G.; Hosseini, A.; Lichtman, A.H.; Allen, R.C.; Lattanzio, F.A.; Williams, P.B. Topical WIN55212–2 alleviates intraocular hypertension in rats through a CB1 receptor mediated mechanism of action. J. Ocul. Pharmacol. Ther. 2008, 24, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Bekkers, J.M.; Stevens, C.F. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc. Natl. Acad. Sci. USA 1991, 88, 7834–7838. [Google Scholar] [CrossRef] [PubMed]
- Furshpan, E.J.; MacLeish, P.R.; O’Lague, P.H.; Potter, D.D. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: Evidence for cholinergic, adrenergic, and dual-function neurons. Proc. Natl. Acad. Sci. USA 1976, 73, 4225–4229. [Google Scholar] [CrossRef] [PubMed]
- Levison, S.W.; McCarthy, K.D. Characterization and partial purification of AIM: A plasma protein that induces rat cerebral type 2 astroglia from bipotential glial progenitors. J. Neurochem. 1991, 57, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.; Wager-Miller, J.; Mackie, K.; Straiker, A. Diacylglycerol lipasealpha (DAGLalpha) and DAGLbeta cooperatively regulate the production of 2-arachidonoyl glycerol in autaptic hippocampal neurons. Mol. Pharmacol. 2013, 84, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Straiker, A.; Mackie, K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J. Physiol. 2005, 569 Pt 2, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.I.; Nicoll, R.A. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 2001, 410, 588–592. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, S.J.; Schlamp, C.L.; Nickells, R.W. Mouse models of retinal ganglion cell death and glaucoma. Exp. Eye Res. 2009, 88, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Akaishi, T.; Odani-Kawabata, N.; Ishida, N.; Nakamura, M. Ocular hypotensive effects of anti-glaucoma agents in mice. J. Ocul. Pharmacol. Ther. 2009, 25, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, M.; Hu, S.S.; Viswanathan, S.; Bradshaw, H.; Kelly, M.E.; Straiker, A. A GPR18-based signaling system regulates IOP in murine eye. Br. J. Pharmacol. 2013, 169, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Bell, N.P.; Ramos, J.L.; Feldman, R.M. Safety, tolerability, and efficacy of fixed combination therapy with dorzolamide hydrochloride 2% and timolol maleate 0.5% in glaucoma and ocular hypertension. Clin. Ophthalmol. 2010, 4, 1331–1346. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, S.; Kulkarni, S.; Ciesielski, A.; Nikas, S.P.; Mackie, K.; Makriyannis, A.; Straiker, A. Controlled-Deactivation CB1 Receptor Ligands as a Novel Strategy to Lower Intraocular Pressure. Pharmaceuticals 2018, 11, 50. https://doi.org/10.3390/ph11020050
Miller S, Kulkarni S, Ciesielski A, Nikas SP, Mackie K, Makriyannis A, Straiker A. Controlled-Deactivation CB1 Receptor Ligands as a Novel Strategy to Lower Intraocular Pressure. Pharmaceuticals. 2018; 11(2):50. https://doi.org/10.3390/ph11020050
Chicago/Turabian StyleMiller, Sally, Shashank Kulkarni, Alex Ciesielski, Spyros P. Nikas, Ken Mackie, Alexandros Makriyannis, and Alex Straiker. 2018. "Controlled-Deactivation CB1 Receptor Ligands as a Novel Strategy to Lower Intraocular Pressure" Pharmaceuticals 11, no. 2: 50. https://doi.org/10.3390/ph11020050
APA StyleMiller, S., Kulkarni, S., Ciesielski, A., Nikas, S. P., Mackie, K., Makriyannis, A., & Straiker, A. (2018). Controlled-Deactivation CB1 Receptor Ligands as a Novel Strategy to Lower Intraocular Pressure. Pharmaceuticals, 11(2), 50. https://doi.org/10.3390/ph11020050





