Primary Cilia: Highly Sophisticated Biological Sensors
Abstract
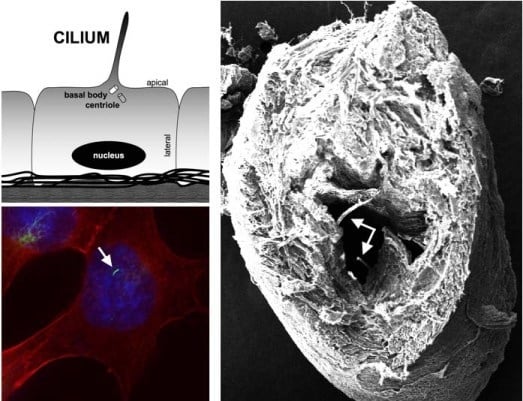
:1. Introduction
2. How Are Cilia Structured, and What Are Cilia Made of?
3. Primary Cilia and Sensing
4. Cilia and Cell Cycle Regulation
5. Cilia and Development
6. Cilia in Renal and Cardiovascular Disease
7. Cilia Involvement in Cell Signaling and Cancer
8. Ciliopathies
9. Future Prospectives
Acknowledgments
References and Notes
- Satir, P.; Christensen, S.T. Structure and function of mammalian cilia. Histochem. Cell. Biol 2008, 129, 687–693. [Google Scholar]
- Wheatley, D.N.; Wang, A.M.; Strugnell, G.E. Expression of primary cilia in mammalian cells. Cell Biol. Int 1996, 20, 73–81. [Google Scholar]
- Nauli, S.M.; Zhou, J. Polycystins and mechanosensation in renal and nodal cilia. Bioessays 2004, 26, 844–856. [Google Scholar]
- Rosenbaum, J.L.; Witman, G.B. Intraflagellar transport. Nat. Rev. Mol. Cell Biol 2002, 3, 813–825. [Google Scholar]
- Satir, P.; Christensen, S.T. Overview of structure and function of mammalian cilia. Annu. Rev. Physiol 2007, 69, 377–400. [Google Scholar]
- Gerdes, J.M.; Davis, E.E.; Katsanis, N. The vertebrate primary cilium in development, homeostasis, and disease. Cell 2009, 137, 32–45. [Google Scholar]
- Sharma, N.; Berbari, N.F.; Yoder, B.K. Ciliary dysfunction in developmental abnormalities and diseases. Curr. Top Dev. Biol 2008, 85, 371–427. [Google Scholar]
- Sorokin, S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J. Cell Biol 1962, 15, 363–377. [Google Scholar]
- Fliegauf, M.; Benzing, T.; Omran, H. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol 2007, 8, 880–893. [Google Scholar]
- Salathe, M. Regulation of mammalian ciliary beating. Annu. Rev. Physiol 2007, 69, 401–422. [Google Scholar]
- Wanner, A.; Salathe, M.; O'Riordan, T.G. Mucociliary clearance in the airways. Am. J. Respir. Crit. Care Med 1996, 154, 1868–1902. [Google Scholar]
- Kozminski, K.G.; Johnson, K.A.; Forscher, P.; Rosenbaum, J.L. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. USA 1993, 90, 5519–5523. [Google Scholar]
- Corbit, K.C.; Aanstad, P.; Singla, V.; Norman, A.R.; Stainier, D.Y.; Reiter, J.F. Vertebrate Smoothened functions at the primary cilium. Nature 2005, 437, 1018–1021. [Google Scholar]
- Dutcher, S.K. Flagellar assembly in two hundred and fifty easy-to-follow steps. Trends Genet 1995, 11, 398–404. [Google Scholar]
- Gherman, A.; Davis, E.E.; Katsanis, N. The ciliary proteome database: an integrated community resource for the genetic and functional dissection of cilia. Nat. Genet 2006, 38, 961–962. [Google Scholar]
- Liu, Q.; Tan, G.; Levenkova, N.; Li, T.; Pugh, E.N., Jr.; Rux, J.J.; Speicher, D.W.; Pierce, E.A. The proteome of the mouse photoreceptor sensory cilium complex. Mol. Cell Proteomics 2007, 6, 1299–1317. [Google Scholar]
- Pazour, G.J.; Agrin, N.; Leszyk, J.; Witman, G.B. Proteomic analysis of a eukaryotic cilium. J. Cell Biol 2005, 170, 103–113. [Google Scholar]
- Schwartz, E.A.; Leonard, M.L.; Bizios, R.; Bowser, S.S. Analysis and modeling of the primary cilium bending response to fluid shear. Am. J. Physiol 1997, 272, F132–F138. [Google Scholar]
- Praetorius, H.A.; Spring, K.R. Bending the MDCK cell primary cilium increases intracellular calcium. J. Membr. Biol 2001, 184, 71–79. [Google Scholar]
- Nauli, S.M.; Alenghat, F.J.; Luo, Y.; Williams, E.; Vassilev, P.; Li, X.; Elia, A.E.; Lu, W.; Brown, E.M.; Quinn, S.J.; Ingber, D.E.; Zhou, J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet 2003, 33, 129–137. [Google Scholar]
- Yoder, B.K.; Hou, X.; Guay-Woodford, L.M. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J. Am. Soc. Nephrol 2002, 13, 2508–2516. [Google Scholar]
- Nauli, S.M.; Rossetti, S.; Kolb, R.J.; Alenghat, F.J.; Consugar, M.B.; Harris, P.C.; Ingber, D.E.; Loghman-Adham, M.; Zhou, J. Loss of polycystin-1 in human cyst-lining epithelia leads to ciliary dysfunction. J. Am. Soc. Nephrol 2006, 17, 1015–1025. [Google Scholar]
- Xu, C.; Rossetti, S.; Jiang, L.; Harris, P.C.; Brown-Glaberman, U.; Wandinger-Ness, A.; Bacallao, R.; Alper, S.L. Human ADPKD primary cyst epithelial cells with a novel, single codon deletion in the PKD1 gene exhibit defective ciliary polycystin localization and loss of flow-induced Ca2+ signaling. Am. J. Physiol. Renal. Physiol 2007, 292, F930–F945. [Google Scholar]
- Xu, C.; Shmukler, B.E.; Nishimura, K.; Kaczmarek, E.; Rossetti, S.; Harris, P.C.; Wandinger-Ness, A.; Bacallao, R.L.; Alper, S.L. Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am. J. Physiol. Renal. Physiol 2009, 296, F1464–F1476. [Google Scholar]
- Hou, B.; Kolpakova-Hart, E.; Fukai, N.; Wu, K.; Olsen, B.R. The polycystic kidney disease 1 (Pkd1) gene is required for the responses of osteochondroprogenitor cells to midpalatal suture expansion in mice. Bone 2009, 44, 1121–1133. [Google Scholar]
- Xiao, Z.; Zhang, S.; Mahlios, J.; Zhou, G.; Magenheimer, B.S.; Guo, D.; Dallas, S.L.; Maser, R.; Calvet, J.P.; Bonewald, L.; Quarles, L.D. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. J. Biol. Chem 2006, 281, 30884–30895. [Google Scholar]
- Masyuk, A.I.; Masyuk, T.V.; Splinter, P.L.; Huang, B.Q.; Stroope, A.J.; LaRusso, N.F. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 2006, 131, 911–920. [Google Scholar]
- McGrath, J.; Somlo, S.; Makova, S.; Tian, X.; Brueckner, M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 2003, 114, 61–73. [Google Scholar]
- Nauli, S.M.; Kawanabe, Y.; Kaminski, J.J.; Pearce, W.J.; Ingber, D.E.; Zhou, J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 2008, 117, 1161–1171. [Google Scholar]
- AbouAlaiwi, W.A.; Takahashi, M.; Mell, B.R.; Jones, T.J.; Ratnam, S.; Kolb, R.J.; Nauli, S.M. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ. Res 2009, 104, 860–869. [Google Scholar]
- Boekhoff, I.; Tareilus, E.; Strotmann, J.; Breer, H. Rapid activation of alternative second messenger pathways in olfactory cilia from rats by different odorants. Embo J 1990, 9, 2453–2458. [Google Scholar]
- Nakamura, T.; Gold, G.H. A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature 1987, 325, 442–444. [Google Scholar]
- Elias, R.V.; Sezate, S.S.; Cao, W.; McGinnis, J.F. Temporal kinetics of the light/dark translocation and compartmentation of arrestin and alpha-transducin in mouse photoreceptor cells. Mol. Vis 2004, 10, 672–681. [Google Scholar]
- Li, T.; Snyder, W.K.; Olsson, J.E.; Dryja, T.P. Transgenic mice carrying the dominant rhodopsin mutation P347S: evidence for defective vectorial transport of rhodopsin to the outer segments. Proc. Natl. Acad. Sci. USA 1996, 93, 14176–14181. [Google Scholar]
- Zaghloul, N.A.; Katsanis, N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J. Clin. Invest 2009, 119, 428–437. [Google Scholar]
- Kolb, R.J.; Nauli, S.M. Ciliary dysfunction in polycystic kidney disease: an emerging model with polarizing potential. Front. Biosci 2008, 13, 4451–4466. [Google Scholar]
- Snell, W.J.; Pan, J.; Wang, Q. Cilia and flagella revealed: from flagellar assembly in Chlamydomonas to human obesity disorders. Cell 2004, 117, 693–697. [Google Scholar]
- Tobin, J.L.; Beales, P.L. Bardet-Biedl syndrome: beyond the cilium. Pediatr. Nephrol 2007, 22, 926–936. [Google Scholar]
- Gong, Z.; Son, W.; Chung, Y.D.; Kim, J.; Shin, D.W.; McClung, C.A.; Lee, Y.; Lee, H.W.; Chang, D.J.; Kaang, B.K.; Cho, H.; Oh, U.; Hirsh, J.; Kernan, M.J.; Kim, C. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J. Neurosci 2004, 24, 9059–9066. [Google Scholar]
- Tobin, D.; Madsen, D.; Kahn-Kirby, A.; Peckol, E.; Moulder, G.; Barstead, R.; Maricq, A.; Bargmann, C. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron 2002, 35, 307–318. [Google Scholar]
- Alenghat, F.J.; Nauli, S.M.; Kolb, R.; Zhou, J.; Ingber, D.E. Global cytoskeletal control of mechanotransduction in kidney epithelial cells. Exp. Cell Res 2004, 301, 23–30. [Google Scholar]
- Anderson, C.T.; Castillo, A.B.; Brugmann, S.A.; Helms, J.A.; Jacobs, C.R.; Stearns, T. Primary cilia: cellular sensors for the skeleton. Anat. Rec. (Hoboken) 2008, 291, 1074–1078. [Google Scholar]
- Haycraft, C.J.; Serra, R. Cilia involvement in patterning and maintenance of the skeleton. Curr. Top Dev. Biol 2008, 85, 303–332. [Google Scholar]
- Whitfield, J.F. The solitary (primary) cilium--a mechanosensory toggle switch in bone and cartilage cells. Cell Signal 2008, 20, 1019–1024. [Google Scholar]
- Rohatgi, R.; Milenkovic, L.; Scott, M.P. Patched1 regulates hedgehog signaling at the primary cilium. Science 2007, 317, 372–376. [Google Scholar]
- Haycraft, C.J.; Banizs, B.; Aydin-Son, Y.; Zhang, Q.; Michaud, E.J.; Yoder, B.K. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 2005, 1, e53. [Google Scholar]
- Huangfu, D.; Liu, A.; Rakeman, A.S.; Murcia, N.S.; Niswander, L.; Anderson, K.V. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 2003, 426, 83–87. [Google Scholar]
- Moorman, S.J.; Shorr, A.Z. The primary cilium as a gravitational force transducer and a regulator of transcriptional noise. Dev. Dyn 2008, 237, 1955–1959. [Google Scholar]
- Badano, J.L.; Teslovich, T.M.; Katsanis, N. The centrosome in human genetic disease. Nat. Rev. Genet 2005, 6, 194–205. [Google Scholar]
- Pan, J.; Wang, Q.; Snell, W.J. Cilium-generated signaling and cilia-related disorders. Lab. Invest 2005, 85, 452–463. [Google Scholar]
- Hovater, M.B.; Olteanu, D.; Hanson, E.L.; Cheng, N.L.; Siroky, B.; Fintha, A.; Komlosi, P.; Liu, W.; Satlin, L.M.; Bell, P.D.; Yoder, B.K.; Schwiebert, E.M. Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinerg. Signal 2008, 4, 155–170. [Google Scholar]
- Siroky, B.J.; Ferguson, W.B.; Fuson, A.L.; Xie, Y.; Fintha, A.; Komlosi, P.; Yoder, B.K.; Schwiebert, E.M.; Guay-Woodford, L.M.; Bell, P.D. Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. Am. J. Physiol. Renal. Physiol 2006, 290, F1320–F1328. [Google Scholar]
- Rohatgi, R.; Battini, L.; Kim, P.; Israeli, S.; Wilson, P.D.; Gusella, G.L.; Satlin, L.M. Mechanoregulation of intracellular Ca2+ in human autosomal recessive polycystic kidney disease cyst-lining renal epithelial cells. Am. J. Physiol. Renal. Physiol 2008, 294, F890–F899. [Google Scholar]
- Wang, S.; Zhang, J.; Nauli, S.M.; Li, X.; Starremans, P.G.; Luo, Y.; Roberts, K.A.; Zhou, J. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol. Cell Biol 2007, 27, 3241–3252. [Google Scholar]
- Bhunia, A.K.; Piontek, K.; Boletta, A.; Liu, L.; Qian, F.; Xu, P.N.; Germino, F.J.; Germino, G.G. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell 2002, 109, 157–168. [Google Scholar]
- Yamaguchi, T.; Wallace, D.P.; Magenheimer, B.S.; Hempson, S.J.; Grantham, J.J.; Calvet, J.P. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J. Biol. Chem 2004, 279, 40419–40430. [Google Scholar]
- Aguiari, G.; Trimi, V.; Bogo, M.; Mangolini, A.; Szabadkai, G.; Pinton, P.; Witzgall, R.; Harris, P.C.; Borea, P.A.; Rizzuto, R.; del Senno, L. Novel role for polycystin-1 in modulating cell proliferation through calcium oscillations in kidney cells. Cell Prolif 2008, 41, 554–573. [Google Scholar]
- Battini, L.; Macip, S.; Fedorova, E.; Dikman, S.; Somlo, S.; Montagna, C.; Gusella, G.L. Loss of polycystin-1 causes centrosome amplification and genomic instability. Hum. Mol. Genet 2008, 17, 2819–2833. [Google Scholar]
- Burtey, S.; Riera, M.; Ribe, E.; Pennenkamp, P.; Rance, R.; Luciani, J.; Dworniczak, B.; Mattei, M.G.; Fontes, M. Centrosome overduplication and mitotic instability in PKD2 transgenic lines. Cell Biol. Int 2008, 32, 1193–1198. [Google Scholar]
- Mahjoub, M.R.; Montpetit, B.; Zhao, L.; Finst, R.J.; Goh, B.; Kim, A.C.; Quarmby, L.M. The FA2 gene of Chlamydomonas encodes a NIMA family kinase with roles in cell cycle progression and microtubule severing during deflagellation. J. Cell Sci 2002, 115, 1759–1768. [Google Scholar]
- Bradley, B.A.; Quarmby, L.M. A NIMA-related kinase, Cnk2p, regulates both flagellar length and cell size in Chlamydomonas. J. Cell Sci 2005, 118, 3317–3326. [Google Scholar]
- White, M.C.; Quarmby, L.M. The NIMA-family kinase, Nek1 affects the stability of centrosomes and ciliogenesis. BMC Cell Biol 2008. [Google Scholar] [CrossRef]
- Qin, H.; Wang, Z.; Diener, D.; Rosenbaum, J. Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control. Curr. Biol 2007, 17, 193–202. [Google Scholar]
- Robert, A.; Margall-Ducos, G.; Guidotti, J.E.; Bregerie, O.; Celati, C.; Brechot, C.; Desdouets, C. The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. J. Cell Sci 2007, 120, 628–637. [Google Scholar]
- McMahon, A.P.; Ingham, P.W.; Tabin, C.J. Developmental roles and clinical significance of hedgehog signaling. Curr. Top Dev. Biol 2003, 53, 1–114. [Google Scholar]
- May, S.R.; Ashique, A.M.; Karlen, M.; Wang, B.; Shen, Y.; Zarbalis, K.; Reiter, J.; Ericson, J.; Peterson, A.S. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev. Biol 2005, 287, 378–389. [Google Scholar]
- Rana, A.A.; Barbera, J.P.; Rodriguez, T.A.; Lynch, D.; Hirst, E.; Smith, J.C.; Beddington, R.S. Targeted deletion of the novel cytoplasmic dynein mD2LIC disrupts the embryonic organiser, formation of the body axes and specification of ventral cell fates. Development 2004, 131, 4999–5007. [Google Scholar]
- Zhang, Q.; Murcia, N.S.; Chittenden, L.R.; Richards, W.G.; Michaud, E.J.; Woychik, R.P.; Yoder, B.K. Loss of the Tg737 protein results in skeletal patterning defects. Dev. Dyn 2003, 227, 78–90. [Google Scholar]
- Das, G.; Jenny, A.; Klein, T.J.; Eaton, S.; Mlodzik, M. Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development 2004, 131, 4467–4476. [Google Scholar]
- Theisen, H.; Purcell, J.; Bennett, M.; Kansagara, D.; Syed, A.; Marsh, J.L. dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development 1994, 120, 347–360. [Google Scholar]
- Tree, D.R.; Shulman, J.M.; Rousset, R.; Scott, M.P.; Gubb, D.; Axelrod, J.D. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell 2002, 109, 371–381. [Google Scholar]
- Vinson, C.R.; Adler, P.N. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature 1987, 329, 549–551. [Google Scholar]
- Fischer, E.; Legue, E.; Doyen, A.; Nato, F.; Nicolas, J.F.; Torres, V.; Yaniv, M.; Pontoglio, M. Defective planar cell polarity in polycystic kidney disease. Nat. Genet 2006, 38, 21–23. [Google Scholar]
- Jonassen, J.A.; San Agustin, J.; Follit, J.A.; Pazour, G.J. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J. Cell Biol 2008, 183, 377–384. [Google Scholar]
- Patel, V.; Li, L.; Cobo-Stark, P.; Shao, X.; Somlo, S.; Lin, F.; Igarashi, P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum. Mol. Genet 2008, 17, 1578–1590. [Google Scholar]
- Saburi, S.; Hester, I.; Fischer, E.; Pontoglio, M.; Eremina, V.; Gessler, M.; Quaggin, S.E.; Harrison, R.; Mount, R.; McNeill, H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat. Genet 2008, 40, 1010–1015. [Google Scholar]
- Simons, M.; Gloy, J.; Ganner, A.; Bullerkotte, A.; Bashkurov, M.; Kronig, C.; Schermer, B.; Benzing, T.; Cabello, O.A.; Jenny, A.; Mlodzik, M.; Polok, B.; Driever, W.; Obara, T.; Walz, G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat. Genet 2005, 37, 537–543. [Google Scholar]
- Yoder, B.K. Role of primary cilia in the pathogenesis of polycystic kidney disease. J. Am. Soc. Nephrol 2007, 18, 1381–1388. [Google Scholar]
- Moyer, J.H.; Lee-Tischler, M.J.; Kwon, H.Y.; Schrick, J.J.; Avner, E.D.; Sweeney, W.E.; Godfrey, V.L.; Cacheiro, N.L.; Wilkinson, J.E.; Woychik, R.P. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science 1994, 264, 1329–1333. [Google Scholar]
- Hateboer, N.; Veldhuisen, B.; Peters, D.; Breuning, M.H.; San-Millan, J.L.; Bogdanova, N.; Coto, E.; van Dijk, M.A.; Afzal, A.R.; Jeffery, S.; Saggar-Malik, A.K.; Torra, R.; Dimitrakov, D.; Martinez, I.; de Castro, S.S.; Krawczak, M.; Ravine, D. Location of mutations within the PKD2 gene influences clinical outcome. Kidney Int 2000, 57, 1444–1451. [Google Scholar]
- Hierck, B.P.; van der Heiden, K.; Alkemade, F.E.; van de Pas, S.; van Thienen, J.V.; Groenendijk, B.C.; Bax, W.H.; van der Laarse, A.; Deruiter, M.C.; Horrevoets, A.J.; Poelmann, R.E. Primary cilia sensitize endothelial cells for fluid shear stress. Dev. Dyn 2008, 237, 725–735. [Google Scholar]
- Poelmann, R.E.; van der Heiden, K.; Gittenberger-de Groot, A.C.; Hierck, B.P. Deciphering the endothelial shear stress sensor. Circulation 2008, 117, 1124–1126. [Google Scholar]
- van der Heiden, K.; Groenendijk, B.C.; Hierck, B.P.; Hogers, B.; Koerten, H.K.; Mommaas, A.M.; Gittenberger-de Groot, A.C.; Poelmann, R.E. Monocilia on chicken embryonic endocardium in low shear stress areas. Dev. Dyn 2006, 235, 19–28. [Google Scholar]
- Van der Heiden, K.; Hierck, B.P.; Krams, R.; de Crom, R.; Cheng, C.; Baiker, M.; Pourquie, M.J.; Alkemade, F.E.; DeRuiter, M.C.; Gittenberger-de Groot, A.C.; Poelmann, R.E. Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis 2008, 196, 542–550. [Google Scholar]
- Schneider, L.; Clement, C.A.; Teilmann, S.C.; Pazour, G.J.; Hoffmann, E.K.; Satir, P.; Christensen, S.T. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr. Biol 2005, 15, 1861–1866. [Google Scholar]
- Alvarez, R.H.; Kantarjian, H.M.; Cortes, J.E. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin. Proc 2006, 81, 1241–1257. [Google Scholar]
- Yu, J.; Ustach, C.; Kim, H.R. Platelet-derived growth factor signaling and human cancer. J. Biochem. Mol. Biol 2003, 36, 49–59. [Google Scholar]
- Mao, J.H.; Wu, D.; Perez-Losada, J.; Jiang, T.; Li, Q.; Neve, R.M.; Gray, J.W.; Cai, W.W.; Balmain, A. Crosstalk between Aurora-A and p53: frequent deletion or downregulation of Aurora-A in tumors from p53 null mice. Cancer Cell 2007, 11, 161–173. [Google Scholar]
- Portier, N.; Audhya, A.; Maddox, P.S.; Green, R.A.; Dammermann, A.; Desai, A.; Oegema, K. A microtubule-independent role for centrosomes and aurora a in nuclear envelope breakdown. Dev. Cell 2007, 12, 515–529. [Google Scholar]
- Pugacheva, E.N.; Golemis, E.A. The focal adhesion scaffolding protein HEF1 regulates activation of the Aurora-A and Nek2 kinases at the centrosome. Nat. Cell Biol 2005, 7, 937–946. [Google Scholar]
- Pugacheva, E.N.; Jablonski, S.A.; Hartman, T.R.; Henske, E.P.; Golemis, E.A. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 2007, 129, 1351–1363. [Google Scholar]
- Thoma, C.R.; Frew, I.J.; Hoerner, C.R.; Montani, M.; Moch, H.; Krek, W. pVHL and GSK3beta are components of a primary cilium-maintenance signalling network. Nat. Cell Biol 2007, 9, 588–595. [Google Scholar]
- Badano, J.L.; Mitsuma, N.; Beales, P.L.; Katsanis, N. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genom. Hum. Genet 2006, 7, 125–148. [Google Scholar]
- Hildebrandt, F.; Otto, E. Cilia and centrosomes: A unifying pathogenic concept for cystic kidney disease? Nat. Rev. Genet 2005, 6, 928–940. [Google Scholar]
- Praetorius, H.A.; Spring, K.R. A physiological view of the primary cilium. Annu. Rev. Physiol 2005, 67, 515–529. [Google Scholar]
- Masyuk, A.I.; Masyuk, T.V.; LaRusso, N.F. Cholangiocyte primary cilia in liver health and disease. Dev. Dyn 2008, 237, 2007–2012. [Google Scholar]
- Hong, D.H.; Pawlyk, B.; Sokolov, M.; Strissel, K.J.; Yang, J.; Tulloch, B.; Wright, A.F.; Arshavsky, V.Y.; Li, T. RPGR isoforms in photoreceptor connecting cilia and the transitional zone of motile cilia. Invest. Ophthal. Mol. Vis. Sci 2003, 44, 2413–2421. [Google Scholar]
- Beales, P.L. Lifting the lid on Pandora's box: the Bardet-Biedl syndrome. Curr. Opin. Genet. Dev 2005, 15, 315–323. [Google Scholar]
- Katsanis, N.; Beales, P.L.; Woods, M.O.; Lewis, R.A.; Green, J.S.; Parfrey, P.S.; Ansley, S.J.; Davidson, W.S.; Lupski, J.R. Mutations in MKKS cause obesity, retinal dystrophy and renal malformations associated with Bardet-Biedl syndrome. Nat. Genet 2000, 26, 67–70. [Google Scholar]
- Maffei, P.; Munno, V.; Marshall, J.D.; Scandellari, C.; Sicolo, N. The Alstrom syndrome: Is it a rare or unknown disease? Ann. Ital. Med. Int 2002, 17, 221–228. [Google Scholar]
- Kulaga, H.M.; Leitch, C.C.; Eichers, E.R.; Badano, J.L.; Lesemann, A.; Hoskins, B.E.; Lupski, J.R.; Beales, P.L.; Reed, R.R.; Katsanis, N. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat. Genet 2004, 36, 994–998. [Google Scholar]
- Adato, A.; Lefevre, G.; Delprat, B.; Michel, V.; Michalski, N.; Chardenoux, S.; Weil, D.; El-Amraoui, A.; Petit, C. Usherin, the defective protein in Usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Hum. Mol. Genet 2005, 14, 3921–3932. [Google Scholar]



© 2009 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abou Alaiwi, W.A.; Lo, S.T.; Nauli, S.M. Primary Cilia: Highly Sophisticated Biological Sensors. Sensors 2009, 9, 7003-7020. https://doi.org/10.3390/s90907003
Abou Alaiwi WA, Lo ST, Nauli SM. Primary Cilia: Highly Sophisticated Biological Sensors. Sensors. 2009; 9(9):7003-7020. https://doi.org/10.3390/s90907003
Chicago/Turabian StyleAbou Alaiwi, Wissam A., Shao T. Lo, and Surya M. Nauli. 2009. "Primary Cilia: Highly Sophisticated Biological Sensors" Sensors 9, no. 9: 7003-7020. https://doi.org/10.3390/s90907003
APA StyleAbou Alaiwi, W. A., Lo, S. T., & Nauli, S. M. (2009). Primary Cilia: Highly Sophisticated Biological Sensors. Sensors, 9(9), 7003-7020. https://doi.org/10.3390/s90907003