Thiopental and Phenytoin as Novel Ionophores for Potentiometric Determination of Lead (II) Ions
Abstract
:1. Introduction
2. Experimental
2.1. Equipment
2.2. Reagents
2.3. Sensor Construction
2.4. Calibration of Lead Membrane Sensors
2.5. Sensor Selectivity
2.6. Analytical Applications
2.6.1. Determination of Lead in Solder Alloys
2.6.2. Determination of Lead in Rocks
3. Results and Discussion
3.1. Characteristics of the Sensors
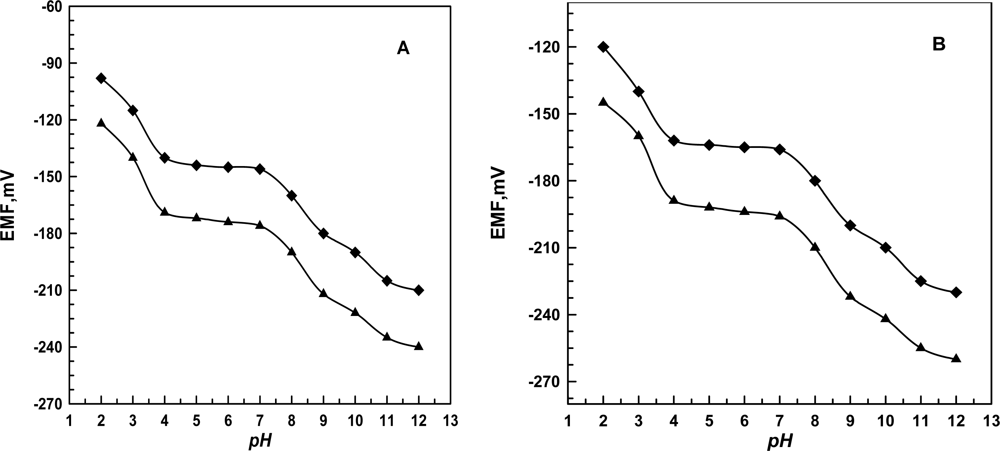
3.2. Effect of pH and Foreign Ions
3.3. Comparison with Other Reported Lead Sensors
3.4. Direct Determination of Pb2+ Ions
3.5. Analytical Applications
3.5.1 Determination of Lead in Solder Alloys
3.5.2 Determination of Pb2+ in Galena Rocks
3.6. Conclusions
References and Notes
- Al-Saleh, I.A.S. The biochemical and clinical consequences of lead poisoning. Med. Res. Rev 1994, 14, 415–486. [Google Scholar]
- Bressler, J.; Kim, K.; Chakraborti, T.; Goldstein, G. Molecular mechanisms of lead neurotoxicity. Neurochem. Res 1999, 24, 595–600. [Google Scholar]
- Xian, Y.; Li, X.; Lian, T.; Zhu, X.; Wang, X. Standardization of urinary lead determination by differential potentiometric-stripping analysis. Gongye Weisheng Yu Zhiyebing 1989, 4, 226–229. (In Chinese) [Google Scholar]
- Wang, J.; Tian, B. Screen-printed stripping voltammetric-potentiometric electrodes for decentralized testing of trace lead. Anal. Chem 1992, 64, 1706–1709. [Google Scholar]
- Marrero, J.; Arisnabarreta, S.P.; Smichowski, P. Comparison of effects of four acid oxidant mixtures in the determination of lead in foods and beverages by hydride generation-ICP OES. At. Spectrosc 2003, 24, 133–142. [Google Scholar]
- Packer, A.P.; Gervasio, A.P.G.; Miranda, C.E.S.; Reis, B.F.; Menegario, A.A.; Giné, M.F. Online electrolytic dissolution for lead determination in high-purity copper by isotope dilution inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2003, 485, 145–153. [Google Scholar]
- Le-Bihan, A.; Lijour, Y.; Giamarchi, P.; Burel-Deschamps, L.; Stephan, L. Laser-induced fluorescence with an OPO system. Part II: direct determination of lead content in seawater by electrothermal atomization laser excited atomic fluorescence (ETA-LEAF). Anal. Bioanal. Chem 2003, 375, 815–819. [Google Scholar]
- Akman, S.; Tokman, N. Determination of lead and nickel in apple-leaves and sea-water by electrothermal atomic absorption spectrometry after solid-phase extraction using Chromo- sorb-107 filled in a syringe. Talanta 2003, 60, 199–204. [Google Scholar]
- Ensafi, A.A.; Khayamian, T.; Karbasi, M.H. On-line preconcentration system for lead(II) determination in waste water by atomic absorption spectrometry using active carbon loaded with Pyrogallol Red. Anal. Sci 2003, 19, 953–956. [Google Scholar]
- Nakajima, J.; Hirano, Y.; Oguma, K. Determination of lead in sea water by flow- injection online preconcentration-electrothermal atomic absorption spectrometry after coprecipitation with iron (III) hydroxide. Anal. Sci 2003, 19, 585–588. [Google Scholar]
- Ensafi, A.A.; Khayamian, T.; Atabati, M. Differential pulse cathodic stripping adsorption voltammetric determination of trace amounts of lead using factorial design for optimization. Talanta 2003, 59, 727–733. [Google Scholar]
- Hu, C.G.; Wu, K.B.; Dai, X.A.; Hu, S.S. Simultaneous determination of lead(II) and cadmium(II) at a diacetyldioxime modified carbon paste electrode by differential pulse stripping voltammetry. Talanta 2003, 60, 17–24. [Google Scholar]
- Li, Z.J.; Chen, Y.P.; Pan, J.M.; Tang, J. The determination of lead in industrial samples by spectrophotometry with 2-(2-sulfophenylazo)-7-(2,6-dibromo-4-methylphenylazo)-1,8-dihydroxy- naphthalene-3,6-disulfonic acid. Anal. Lett 2002, 35, 2157–2171. [Google Scholar]
- Demkin, A.M. Coulometric determination of lead (II) and lead (IV) in high Tc superconductors containing copper and bismuth. J. Anal. Chem 2000, 55, 175–181. [Google Scholar]
- Karami, H.; Mousavi, M.F.; Shamsipur, M. Flow injection potentiometry by a new coated graphite ion-selective electrode for the determination of lead ion. Talanta 2003, 60, 775–786. [Google Scholar]
- Hassan, S.S.M.; Ghalia, M.H.A.; Amr, G.E.; Mohamed, A.H.K. New lead (II) selective membrane potentiometric sensors based on chiral 2,6-bis- pyridinecarboximide derivatives. Talanta 2003, 1, 81–91. [Google Scholar]
- Yaftian, M.F.; Rayati, S.; Emadi, D.; Matt, D. A coated wire-type lead (II) ion selective electrode based on a phosphorylated calix[4]arene derivative. Anal. Sci 2006, 22, 1075–1078. [Google Scholar]
- Kim, H.; Kyoung-Lee, H.; Young-Choi, A.; Jeon, S. Polymeric lead (II)- selective electrode based on N,N′-bis-thiophen-2-ylmethylene-pyridine-2,6- diamine as an ion carrier. Bull. Korean Chem. Soc 2007, 28, 538–548. [Google Scholar]
- Isvoranu, M.; Luca, C.; Pleniceanu, M.; Spinu, C. Stuides on a Pb2+-selective electrode with a macrocyclic liquid membrane. Potentiometric determination of Pb2+. J. Serb. Chem. Soc 2006, 71, 1345–1352. [Google Scholar]
- Ardakani, M.M.; Pourhakak, P.; Niasari, M.S. Bis(2 hydroxyacetophenone) ethylenediimine as a neutral carrier in a coated wire membrane electrode for lead(II). Anal. Sci 2006, 22, 865–870. [Google Scholar]
- Gupta, V.K.; Jain, A.K.; Maheshwari, G. Pb(II) selective sensor based on of N, N′- bis(2-hydroxy-1-napthalene)-2,6-pyridiamine in (Polyvinyl) chloride matrix. Int. J. Electrochem. Sci 2007, 2, 102–112. [Google Scholar]
- Gupta, V.K.; Jain, A.K.; Kumar, P. PVC-based membranes of N, N’-dibenzyl-1, 4,10,13-tetraoxa-7, 16-diazacyclooctadecane as Pb(II)-selective sensor. Sens. Actuat. B 2006, 120, 259–265. [Google Scholar]
- Sadeghi, S.; Dashti, G.R.; Shamsipur, M. Lead-selective poly(vinyl chloride) membrane electrode based on piroxicam as a neutral carrier. Sens. Actuat. B 2002, 81, 223–228. [Google Scholar]
- Borraccino, A.; Campanella, L.; Sammartino, M.P.; Tomassetti, M.; Battilotti, M. Suitable ion selective sensors for lead and cadmium analysis. Sens.. Actuat. B 1992, 7, 535–539. [Google Scholar]
- Lerchi, M.; Bakker, E.; Rusterholz, B.; Simon, W. Lead selective bulk optodes based on neutral ionophores with subnanomolar detection limits. Anal. Chem 1992, 64, 1534–1540. [Google Scholar]
- Barzegar, M.; Mousavi, M.F.; Khajehsharifi, H.; Shamsipur, M.; Sharghi, H. Application of some recently synthesized 9, 10-anthraquinone derivatives as new class of ionophores responsive to lead (II) ion. IEEE Sens. J 2005, 5, 392–397. [Google Scholar]
- Jain, A.K.; Gupta, V.K.; Singh, L.P.; Raisoni, J.R. A comparative study of Pb2+ sensors based on derivatized tetrapyrazole and calix[4]arene receptors. Electrochim. Acta 2006, 51, 2547–2553. [Google Scholar]
- Wroblewski, W.; Brzozka, Z. Switching of ion selectivity of membranes by lipophilic ionic sites. Anal. Chim. Acta 1996, 326, 163–168. [Google Scholar]
- Buhlmann, P.; Pretsch, E.; Bakker, E. Carrier based ion selective electrodes and bulk optodes. 2. ionophores for potentiometric and optical sensors. Chem. Rev 1998, 98, 1593–1687. [Google Scholar]
- Cadogan, F.; Kan, P.; McKervey, M.A.; Diamond, D. Lead-selective electrodes based on calixarene phosphine oxide derivatives. Anal. Chem 1999, 71, 5544–5550. [Google Scholar]
- Gupta, V.K.; Mangla, R.; Agarwal, S. Pb (II) selective potentiometric sensor based on 4-tert-Butylcalix[4]arene in PVC matrix. Electroanalysis 2002, 14, 1127–1132. [Google Scholar]
- Chen, L.; Zhang, J.; Zhao, W.; He, X.; Liu, Y. Double-armed calix[4]arene amide derivatives as ionophores for lead ion-selective electrodes. J. Electroanal. Chem 2006, 589, 106–111. [Google Scholar]
- Mousavi, M.F.; Barzegar, M.B.; Sahari, S. A PVC-based capric acid membrane potentiometric sensor for lead (II) ions. Sens. Actuat. B 2001, 73, 199–204. [Google Scholar]
- Bakker, E.; Willer, M.; Pretsch, E. Detection limit of ion-selective bulk optodes and corresponding electrodes. Anal. Chim. Acta 1993, 282, 265–271. [Google Scholar]
- Srivastava, S.K.; Gupta, V.K.; Jain, S. Determination of lead using poly (vinyl chloride) based crown ether membrane. Analyst 1995, 120, 495–498. [Google Scholar]
- Shamsipur, M.; Ganjali, M.R.; Rouhollahi, A. Lead selective membrane potentiometric sensor based on an 18-membered thiacrown derivative. Anal. Sci 2001, 17, 935–938. [Google Scholar]
- Mousavi, M.F.; Sahari, S.; Alizadeh, N.; Shamsipur, M. Lead ion-selective membrane electrode based on 1,10-dibenzyl-1,10-diaza-18-crown-6. Anal. Chim. Acta 2000, 414, 189–194. [Google Scholar]
- Xu, D.; Katsu, T. Tetrabenzyl pyrophosphate as a new class of neutral carrier responsive to lead ion. Talanta 2000, 51, 365–371. [Google Scholar]
- Jeong, T.; Lee, H.K.; Jeong, D.C.; Jeon, S. A lead(II)-selective PVC membrane based on a Schiff base complex of N,N’-bis(salicylidene)-2,6-pyridinediamine. Talanta 2005, 65, 543–548. [Google Scholar]
- Zimkus, A.; Cretscu, I.; Grzybowska, I.; Radecka, H.; Geise, H.J.; Dieltiens, P.; Aleksandrzak, K. Potentiometric lead (II) ions recognition by liquid membrane electrodes incorporating methoxy substituted arylenevinylene derivatives. Pol. J. Environ. Stud 2003, 12, 773–778. [Google Scholar]
- Arain, S.R.; Navani, A.; Ebert, T.J. The effects of thiopental and generic and nongeneric propofol on respiratory resistance during anesthetic induction in patients with reactive airways. J. Clin. Anesth 2002, 14, 257–261. [Google Scholar]
- Moffat, A.C.; Jackson, J.V.; Moss, M.S.; Widdop, B.; Greenfield, E.S. Clarke's Isolation and Identification of Drugs, 2nd Ed ed; The Pharmaceutical Press: London, U.K., 1986. [Google Scholar]
- Saleh, G.A. Charge-transfer complexes of barbiturates and phenytoin. Talanta 1998, 46, 111–121. [Google Scholar]
- Hassan, S.S.M. Organic Analysis Using Atomic Absorption Spectrometry; Ellis Horwood: Chichester, U.K., 1984. [Google Scholar]
- Hassan, S.S.M.; Elnemma, E.M.; Mohamed, A.H.K. Novel potentiometric copper (II) selective membrane sensors based on tetrapeptide derivatives as neutral ionophores. Talanta 2005, 66, 1034–1041. [Google Scholar]
- Umezawa, Y.; Buhlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric selectivity coefficients of ion-selective electrodes, part I. inorganic cations. Pure Appl. Chem 2000, 72, 1851–2082. [Google Scholar]
- Vanloon, J.C.; Barefoot, R.R. Analytical Methods for Geochemical Exploration Textbook; University of Toronto: Toronto, ON, Canada, 1989. [Google Scholar]
- Buck, P.R.; Coşofret, V.V. Recommended procedures for calibration of ion-selective electrodes. Pure Appl. Chem 1993, 65, 1849–1858. [Google Scholar]
- Ammann, D.; Pretsch, E.; Simon, W.; Lindler, E.; Bezegh, A.; Pungor, E. Lipophilic salts as membrane additives and their influence on the properties of macro- and micro-electrodes based on neutral carriers. Anal. Chim. Acta 1985, 171, 119–129. [Google Scholar]
- The United States Phamacopeia/National Formulary 2007, 25th Ed ed; United States Pharmacopeial Convention: Rockville, MD, USA, 2007.
- Rose, J. Advanced Physico Chemical Experiments; Sir Issac Pitman and Sons Ltd: London, U.K., 1964. [Google Scholar]
- Francisco, J.O.; Janick, F.A.; Raina, M.M. Stability constants for the complexation of various metals with a rhamnolipid biosurfactant. J. Environ. Qual 2001, 30, 479–485. [Google Scholar]
- Taylor, J. K. Quality Assurance of Chemical Measurements; Lewis Publishers CRC Press: Chelsea, MI, USA, 1987. [Google Scholar]



| Sensor No. | Composition (mg) | Slope (mV decade−1) | Linear range (M) | Lower detection limit (M) | |||
|---|---|---|---|---|---|---|---|
| Ionophore | PVC | Additive | plasticizer | ||||
| 1 | 2.0 (TP) | 66.0 | - | 132.0 (o-NPOE) | 28.5 | 1×10−2–9×10−6 | 7.0×10−6 |
| 2 | 2.1 (PT) | 66.1 | - | 131.7 (o-NPOE) | 27.3 | 1×10−2–1×10−5 | 6.5×10−6 |
| 3 | 1.9 (TP) | 65.9 | 0.1 (KTpClPB) | 132.1 (o-NPOE) | 31.5 | 1×10−2–7×10−6 | 5.0×10−6 |
| 4 | 2.0 (PT) | 65.7 | 0.1 (KTpClPB) | 132.0 (o-NPOE) | 30.5 | 1×10−2–8×10−6 | 4.5×10−6 |
| 5 | 1.8 (TP) | 66.2 | - | 131.8 (DOP) | 25.0 | 5×10−3–1×10−5 | 8.0×10−6 |
| 6 | 2.1 (PT) | 66.0 | - | 132.2 (DOP) | 23.6 | 5×10−3–6×10−5 | 1.0×10−5 |
| 7 | 2.0 (TP) | 66.4 | - | 131.6 (DBS) | 22.5 | 5×10−3–8.5×10−5 | 4.0×10−5 |
| 8 | 1.9 (PT) | 66.1 | - | 132.4 (DBS) | 21.6 | 5×10−3–1×10−4 | 8.0×10−5 |
| Parametera | Sensor 3 | Sensor 4 |
|---|---|---|
| Slope (mV decade−1) | 31.5 | 30.5 |
| Intercept (mV) | −330 | −270 |
| Correlation coefficient (r) (n=6) | 0.998 | 0.999 |
| Linear range (M) | 1×10−2–7×10−6 | 1×10−2–8×10−6 |
| Lower limit of detection (M) | 5.0×10−6 | 4.5×10−6 |
| Response time for 10−3 M (s) | ∼20 | ∼20 |
| Working pH range | 4–7 | 4–7 |
| Life span (week) | >9 | >9 |
| Accuracy (%) | 99.3 | 99.0 |
| Standard deviation (%) | 0.7 | 0.6 |
| Repeatability (CVW%) | 0.8 | 0.7 |
| between day variability (CVb%) | 0.9 | 1.0 |
| Robustnessb | 101.4 ± 1.7 | 102.0 ± 1.2 |
| Ruggednessc | 100.6 ± 1.5 | 101.7 ± 1.6 |
| Interfering iona | log
| |||
|---|---|---|---|---|
| TP without additives | TP with additives | PT without additives | PT with additives | |
| Co2+ | −2.06 | −3.17 | −2.01 | −2.16 |
| Hg2+ | −1.17 | −1.5 | −1.07 | −1.37 |
| Ca2+ | −2.77 | −5.0 | −2.12 | −4.19 |
| Sr2+ | −2.17 | −3.96 | −2.4 | −3.12 |
| Zn2+ | −2.31 | −2.38 | −2.27 | −2.83 |
| Cu2+ | −1.06 | −1.58 | −1.01 | −1.53 |
| Mn2+ | −2.17 | −3.34 | −3.14 | −3.52 |
| Ag+ | −2.0 | −2.52 | −2.0 | −2.69 |
| Na+ | −3.86 | −5.36 | −3.51 | −3.67 |
| K+ | −3.1 | −4.66 | −3.3 | −3.9 |
| Fe2+ | −2.52 | −3.62 | −2.67 | −3.34 |
| Al3+ | −2.67 | −3.0 | −3.35 | −4.33 |
| Ni2+ | −1.68 | −2.34 | −1.85 | −2.06 |
| Cd2+ | −2.33 | −2.37 | −1.34 | −2.03 |
| Ionophore | Linear Range (M) | Lower limit of detection (M) | Slope (mV/decade) | Interferent (M, Selectivity) | Ref. |
|---|---|---|---|---|---|
| 9,10-Anthraquinone derivatives | 1×10−6 – 1×10−2 | 6.7×10−7 | 28.9 | Zn2+,Cd2+ | [22] |
| Methoxy substituted arylenevinylene derivatives | 4.2×10−4 – 2.0×10 −2 | NR | 33–36 | Na+ −1.33, K+ −1.66, Mg2+ −1.3, Zn2+ −1.3, Cd2+ −1.28, Ca2+ −1.39, Cu2+−0.17, Ni2+−1.11 | [40] |
| Dioxamide | 1×10−6 – 8.4×10 −3 | NR | 31.9 | Hg2+ −1.6, Fe2+ −1.67, Cd2+−2.1 | [24] |
| Thia crowm derivatives | 1×10−6 – 8×10−3 | 8×10−7 | 29 | Hg2+ −2.1 | [36] |
| Piroxicam | 1×10−5 – 1×10−2 | 4×10−6 | 30 | UO2+ −0.43, Ag+ −1.2.K+ −1.29, Zn2+ −1.08, Mg2+−1.24 | [23] |
| N,N’-bis(salicylidene)-2,6-pyridinediamine | NR | 9.12×10−7 | 29.4 | Na+ −2.5, K+ −2.2, Ag+ −2.2, Zn2+ −4.1, Co2+ −4.2, Mg2+ −4.9, Cu2+−2.7 | [39] |
| Chiral 2,6-bis-pyridine-carboximide derivatives | 9×10−6– 1×10−2 | 4.4×10−6 | 21.6 | Li+ −3.4, Na+ −3.41, K+ −3.5, Ca2+ −1.45, Cu2+−1.06, Cd2+ −1.61, Ag+ −2.89, Hg2+ −1.00. | [16] |
| 5.8×10−5 – 1×10−2 | 1.8×10−5 | 33.1 | Li+−3.83, Na+ −4.24, K+ −3.83, Ca2+ −2.14, Cu2+−2.03, Cd2+ −2.17, Ag+ −2.25, Hg2+ −2.10. | [16] | |
| 4×10−6–1×10−2 | 2.1×10−6 | 25.0 | Li+ −4.12, Na+ −3.70, K+ −4.11, Ca2+ −1.91, Cu2+−1.99, Cd2+ −1.94, Ag+ −2.89, Hg2+ −1.5. | [16] | |
| Fatty acids | 1×10−6–1×10−2 | NR | 29 | Ag+ −0.9. K+−0.89, Na+−0.80 | [33] |
| Thiopental (Sensor 3) | 4.5×10−6 − 1×10−2 | 1×10−6 | 30.5 | Na+−3.86, Zn2+ −2.38, Mn2+−2.17, Cd2+ −1.37, Ag+ −3.52, K+−4.66, Ca2+ −3.0, Cu2+−2.08 | This work |
| Phenytoin (Sensor 4) | 6.4×10−6– 1×10−2 | 1×10−6 | 31.5 | Na+−3.67, Zn2+ −2.83, Mn2+−3.52, Cd2+ −1.34, Ag+ −3.0. K+ −4.19, Ca2+ −1.19, Cu2+−2.03 | This work |
| Sample | Lead contenta (mg/g) | ||
|---|---|---|---|
| Sensor 3 | Sensor 4 | AAS [8] | |
| 1 | 70.2 mg ± 0.5 mg | 69.7 mg ± 0.4 mg | 66.7 mg ± 0.9 mg |
| 2 | 70.0 mg ± 0.6 mg | 69.9 mg ± 0.5 mg | 66.9 mg ± 0.9 mg |
| 3 | 70.5 mg ± 0.5 mg F= 1.49 t = 17.39 | 69.9 mg ± 0.3 mg F = 3.31 (19*) t = 21.1 (2.77*) | 67.2 mg ± 0.8 mg |
| Sample | Lead contenta (mg/g) | ||
|---|---|---|---|
| Sensor 3 | Sensor 4 | AAS [8] | |
| 1 | 12.2 mg ± 0.8 mg | 12.0 mg ± 0.5 mg | 12.5 mg ± 0.9 mg |
| 2 | 12.7 mg ± 0.4 mg | 12.9 mg ± 0.2 mg | 12.7 mg ± 0.8 mg |
| 3 | 12.7 mg ± 0.8 mg F= 6.46 t = 0.222 | 12.1 mg ± 0.5 mg F = 18.69 (19*) t = 0.822 (2.77*) | 12.4 mg ± 0.7 mg |
© 2009 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rizk, N.M.H.; Abbas, S.S.; Hamza, S.M.; Hamza, S.M.; Abd EL-Karem, Y.M. Thiopental and Phenytoin as Novel Ionophores for Potentiometric Determination of Lead (II) Ions. Sensors 2009, 9, 1860-1875. https://doi.org/10.3390/s90301860
Rizk NMH, Abbas SS, Hamza SM, Hamza SM, Abd EL-Karem YM. Thiopental and Phenytoin as Novel Ionophores for Potentiometric Determination of Lead (II) Ions. Sensors. 2009; 9(3):1860-1875. https://doi.org/10.3390/s90301860
Chicago/Turabian StyleRizk, Nashwa M.H., Samah S. Abbas, Salem M. Hamza, Salem M. Hamza, and Yasser M. Abd EL-Karem. 2009. "Thiopental and Phenytoin as Novel Ionophores for Potentiometric Determination of Lead (II) Ions" Sensors 9, no. 3: 1860-1875. https://doi.org/10.3390/s90301860
APA StyleRizk, N. M. H., Abbas, S. S., Hamza, S. M., Hamza, S. M., & Abd EL-Karem, Y. M. (2009). Thiopental and Phenytoin as Novel Ionophores for Potentiometric Determination of Lead (II) Ions. Sensors, 9(3), 1860-1875. https://doi.org/10.3390/s90301860




