Sodium Gill Potential as a Tool to Monitor Valve Closure Behavior in Freshwater Clam Corbicula fluminea in Response to Copper
Abstract
:1. Introduction
2. Results and Discussion
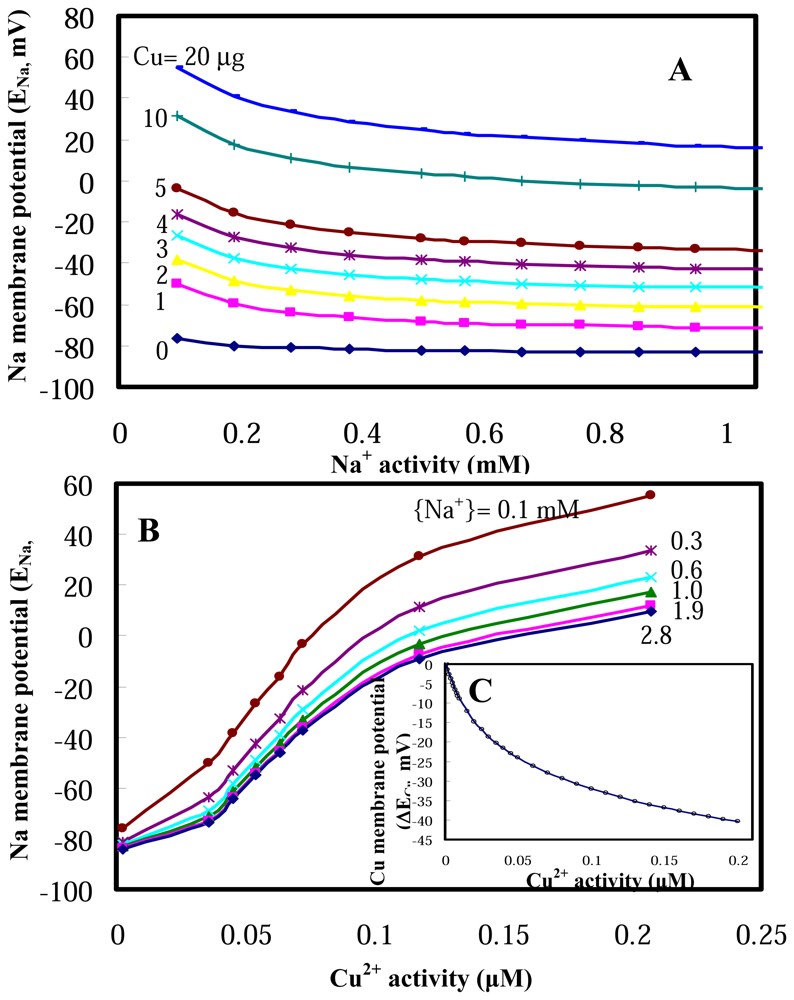
2.1 Model performances
2.2 Model applications
2.3 Clam gill potential as a determinant in environmental risk assessment
2.4 Implications for biomonitoring systems
3. Materials and Methods
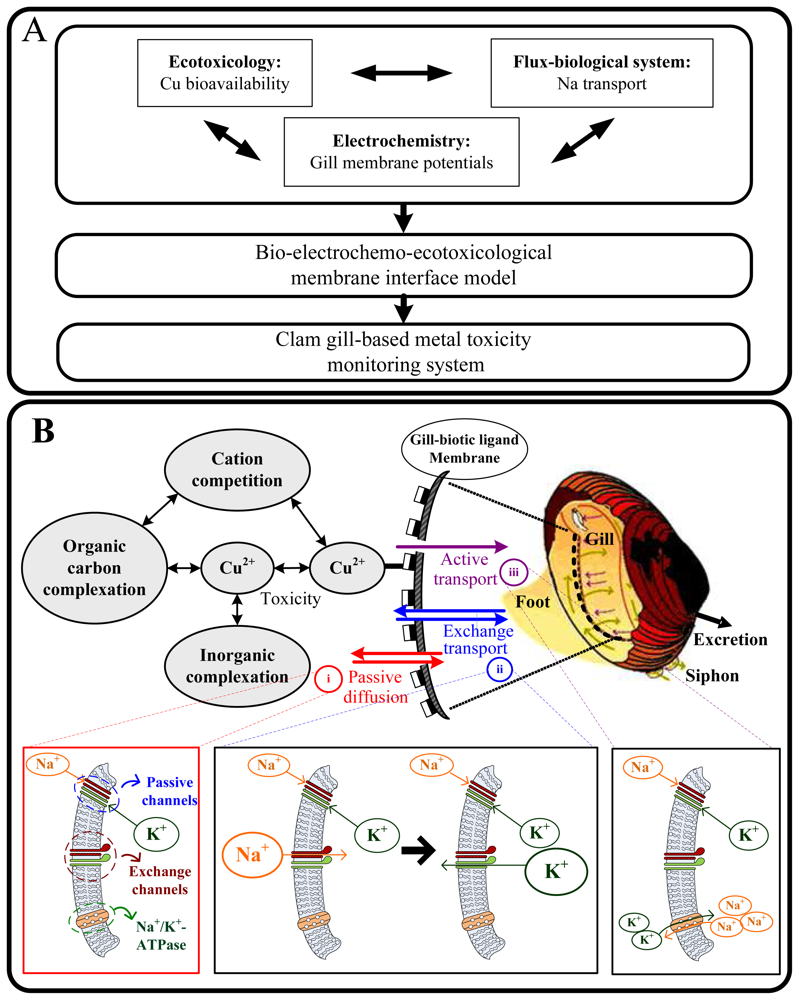
3.1 Integration model
3.2 Clam gill-based electrophysiological response model
5. Conclusions
References and Notes
- Doherty, F.G.; Cherry, D.S.; Cairns, J. Valve closure responses of the Asiatic clam Corbicula fluminea exposed to cadmium and zinc. Hydrobiologia 1987, 153, 159–167. [Google Scholar]
- Sluyts, H.; VanHoof, F.; Cornet, A.; Paulussen, J. A dynamic new alarm system for use in biological early warning systems. Environ. Toxicol. Chem. 1996, 15, 1317–1323. [Google Scholar]
- Borcherding, J.; Jantz, B. Valve movement response of the mussel Dreissena polymorpha–the influence of pH and turbidity on the acute toxicity of pentachlorophenol under laboratory and field conditions. Ecotoxicology 1997, 6, 153–165. [Google Scholar]
- Curtis, T.M.; Williamson, R.; Depledge, M.H. Simultaneous, long-term monitoring of valve and cardiac activity in the blue mussel Mytilus edulis exposed to copper. Mar. Biol. 2000, 136, 837–846. [Google Scholar]
- Weis, J. S.; Samson, J.; Zhou, T.; Skurnick, J.; Weis, P. Prey capture ability of mummichogs (Fundulus heteroclitus) as a behavioral biomarker for contaminants in estuarine systems. Can. J. Fish. Aquat. Sci. 2001, 58, 1442–1452. [Google Scholar]
- Tran, D.; Ciret, P.; Ciutat, A.; Durrieu, G.; Massabuau, J.C. Estimation of potential and limits of bivalve closure response to detect contaminants: Application to cadmium. Environ. Toxicol. Chem. 2003, 22, 914–920. [Google Scholar]
- Tran, D.; Fournier, E.; Durrieu, G.; Massabuau, J.C. Copper detection in the Asiatic clam Corbicula fluminea: optimum valve closure response. Aquat. Toxicol. 2004, 66, 333–343. [Google Scholar]
- El-Shenawy, N.S. Heavy-metal and microbial depuration of the clam Ruditapes decussatus and its effect on bivalve behavior and physiology. Environ. Toxicol. 2004, 19, 143–153. [Google Scholar]
- Perez, M.H.; Wallace, W.G. Differences in prey capture in grass shrimp, Palaemonetes pugio, collected along an environmental impact gradient. Arch. Environ.l Contam. Toxicol. 2004, 46, 81–89. [Google Scholar]
- Booij, K.; Smedes, F.; van Weerlee, E.M.; Honkoop, P.J.C. Environmental monitoring of hydrophobic organic contaminants: The case of mussels versus semipermeable membrane devices. Environ. Sci. Technol. 2006, 40, 3893–3900. [Google Scholar]
- van Leeuwen, H.P.; Köster, W. Physicochemical kinetics and transport at biointerface. In Physicochemical Kinetics and Transport at Biointerfaces; van Leeuwen, H. P., Köster, W., Eds.; Wiley: Chichester, 2004; pp. 1–14. [Google Scholar]
- van Leeuwen, H.P.; Town, R.M.; Buffle, J.; Cleven, R.; Davison, W.; Puy, J.; van Riemsdijk, W. H.; Sigg, L. Dynamic speciation analysis and bioavailability of metals in aquatic systems. Environ. Sci. Technol. 2005, 39, 8545–8556. [Google Scholar]
- Tercier-Waeber, M.L.; Confalonieri, F.; Riccardi, G.; Sina, A.; Noel, S.; Buffle, J.; Graziottin, F. Multi Physical-Chemical profiler for real-time in situ monitoring of trace metal speciation and master variables: Development, validation and field applications. Mar. Chem. 2005, 97, 216–235. [Google Scholar]
- Morgan, T.P.; Wood, C.M. A relationship between gill silver accumulation and acute silver toxicity in the freshwater rainbow trout: Support for the acute silver biotic ligand model. Environ. Toxicol. Chem. 2004, 23, 1261–1267. [Google Scholar]
- Zhou, B.S.; Nichols, J.; Playle, R.C.; Wood, C.M. An in vitro biotic ligand model (BLM) for silver binding to cultured gill epithelia of freshwater rainbow trout (Oncorhynchus mykiss). Toxicol. Appl. Pharmacol. 2005, 202, 25–37. [Google Scholar]
- Hedgepeth, M.E. Salinity related changes in ATPase levels in the Virginia oyster, Crassostrea virginiaand the hard clam, Mercenaria mercenaria. Va. J. Sci. 1974, 25, 64, (abstract). [Google Scholar]
- Dietz, T.H.; Findley, A.M. Ion-stimulated ATPase activity and NaCl uptake in the gills of fresh-water mussels. Can. J. Zool. 1980, 58, 917–923. [Google Scholar]
- Handy, R.D.; Eddy, F.B. Transport of solutes across biological membranes in eukaryotes. In Physicochemical Kinetics and Transport at Biointerfaces; van Leeuwen, H. P., Köster, W., Eds.; Wiley: Chichester, 2004; pp. 337–356. [Google Scholar]
- Reyes, N.; Gadsby, D.C. Ion permeation through the Na+, K+-ATPase. Nature 2006, 443, 470–474. [Google Scholar]
- Wilkinson, K.J.; Buffle, J. Critical evaluation of the physicochemical parameters and processes for modeling the biological uptake of trace metals in environmental (aquatic) system. In Physicochemical Kinetics and Transport at Biointerfaces; van Leeuwen, H. P., Köster, W., Eds.; Wiley: Chichester, 2004; pp. 445–533. [Google Scholar]
- Potts, W.T.W.; Eddy, F.B. Gill potentials and sodium fluxes in flounder Platichthys flesus. J. Comp. Physiol. 1973, 87, 29–48. [Google Scholar]
- Ussing, H.H.; Erlij, D.; Lassen, U. Transport pathways in biological membranes. Annu. Rev. Physiol. 1974, 36, 17–49. [Google Scholar]
- Fletcher, C.R. Potential dependence of sodium fluxes across gills of marine teleosts. J. Cel. Comp. Physiol. 1977, 117, 277–289. [Google Scholar]
- McCorkle, S.; Dietz, T.H. Sodium transport in the freshwater Asiatic clam Corbicula fluminea. Biol. Bull. 1980, 159, 325–336. [Google Scholar]
- Potts, W.T.W.; Hedges, A.J. Gill potentials in marine teleosts. J. Comp. Physiol. B, Biochem. Syst. Environ. Physiol. 1991, 161, 401–405. [Google Scholar]
- Dietz, T.H.; Byrne, R.A. Potassium and rubidium uptake in freshwater bivalves. J. Exp. Biol. 1990, 150, 395–405. [Google Scholar]
- Dietz, T.H.; Hagar, A.F. Chloride uptake in isolated gills of the freshwater mussel Ligumia subrostrata. Can. J. Zool. 1990, 68, 6–9. [Google Scholar]
- Wilcox, S.J.; Dietz, T.H. Potassium transport in the freshwater bivalve Dreissena polymorpha. J. Exp. Biol. 1995, 198, 861–868. [Google Scholar]
- Zheng, H.Y.; Dietz, T.H. Ion transport in the freshwater bivalve Corbicula fluminea. Biol. Bull. 1998, 194, 161–169. [Google Scholar]
- Haynie, D.T. Biological Thermodynamics.; Cambridge University Press: New York, 2001. [Google Scholar]
- Liao, C.M.; Jou, L.J.; Lin, C.M.; Chiang, K.C.; Yeh, C.H.; Chou, B.Y.H. Predicting acute copper toxicity to valve closure behavior in the freshwater clam Corbicula fluminea supports the biotic ligand model. Environ. Toxicol. 2007, 22, 295–307. [Google Scholar]
- Børseth, J.F.; Aunaas, T.; Einarson, S.; Nordtug, T.; Olsen, A.J.; Zachariassen, K.E. Pollutant-induced depression of the transmembrane sodium-gradient in muscles of mussels. J. Exp. Biol. 1992, 169, 1–18. [Google Scholar]
- Kinraide, T.B. Plasma membrane surface potential (ΨPM) as a determinant of ion bioavailability: A critical analysis of new and published toxicological studies and a simplified method for the computation of plant ΨPM. Environ.l Toxicol. Chem. 2006, 25, 3188–3198. [Google Scholar]
- Cereijido, M.; Shoshani, L.; Contreras, R.G. The polarized distribution of Na+, K+-ATPase and active transport across epithelia. J. Membr. Biol. 2001, 184, 299–304. [Google Scholar]
- Ussing, H.H.; Zerahn, K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol. Scand. 1951, 23, 27–110. [Google Scholar]
- Kelly, S.P.; Wood, C.M. Cultured gill epithelia from freshwater tilapia (Oreochromis niloticus): Effect of cortisol and homologous serum supplements from stressed and unstressed fish. J. Membr. Biol. 2002, 190, 29–42. [Google Scholar]
- Bricelj, V.M.; Connell, L.; Konoki, K.; MacQuarrie, S.P.; Scheuer, T.; Catterall, W.A.; Trainer, V.L. Sodium channel mutation leading to saxitoxin resistance in clams increases risk of PSP. Nature 2005, 434, 763–767. [Google Scholar]
- Kirschner, L.B. The study of NaCl transport in aquatic animals. Am. Zool. 1970, 10, 365–376. [Google Scholar]
- Palmer, L.G. Na+ transport and flux ratio through apical Na+ channels in toad bladder. Nature 1982, 297, 688–690. [Google Scholar]
- Geffeney, S.L.; Fujimoto, E.; Brodie, E.D.; Ruben, P.C. Evolutionary diversification of TTX-resistant sodium channels in a predator-prey interaction. Nature 2005, 434, 759–763. [Google Scholar]
- Luoma, S.N.; Rainbow, P.S. Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environ. Sci. Technol. 2005, 39, 1921–1931. [Google Scholar]
- Tsai, J.W.; Liao, C.M. A dose-based modeling approach for accumulation and toxicity of arsenic in tilapia Oreochromis mossambicus. Environ. Toxicol. 2006, 21, 8–21. [Google Scholar]
- De Schamphelaere, K.A.C.; Janssen, C.R. A biotic ligand model predicting acute copper toxicity for Daphnia magna: The effect of calcium, magnesium, sodium, potassium, and pH. Environ. Sci. Technol. 2002, 36, 48–54. [Google Scholar]
- Rainbow, P.S.; Poirier, L.; Smith, B.D.; Brix, K.V.; Luoma, S.N. Trophic transfer of trace metals: Subcellular compartmentalization in a polychaete and assimilation by a decapod crustacean. Mar Ecol. Prog. Ser. 2006, 308, 91–100. [Google Scholar]
- Jou, L.J.; Liao, C.M. A dynamic artificial clam (Corbicula fluminea) allows parsimony on-line measurement of waterborne metals. Environ. Pollut. 2006, 144, 172–183. [Google Scholar]
- Wu, R.S.S.; Lau, T.C.; Fung, W.K; Ko, P. H.; Leung, K. M. Y. An ‘artificial mussel’ for monitoring heavy metals in marine environments. Environ. Pollut. 2007, 145, 104–110. [Google Scholar]
- Bayen, S.; Worms, I.; Parthasarathy, N.; Wilkinson, K.; Buffle, J. Cadmium bioavailability and speciation using the permeation liquid membrane. Anal. Chim. Acta 2006, 575, 267–273. [Google Scholar]
- Kwon, J.H.; Katz, L.E.; Liljestrand, H.M. Use of a parallel artificial membrane system to evaluate passive absorption and elimination in small fish. Environ. Toxicol. Chem. 2006, 25, 3083–3092. [Google Scholar]
- LaBarbera, M. Principles of fluid transport-system in zoology. Science 1990, 249, 992–1000. [Google Scholar]
- Medler, S.; Silverman, H. Muscular alteration of gill geometry in vitro: Implications for bivalve pumping processes. Biol. Bull. 2001, 200, 77–86. [Google Scholar]
- Bauer, W.R.; Nadler, W. Molecular transport through channels and pores: Effects of in-channel interactions and blocking. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 11446–11451. [Google Scholar]
- Liao, C.M.; Lin, C.M.; Jou, L.J.; Chiang, K.C. Linking valve closure behavior and sodium transport mechanism in freshwater clam Corbicula fluminea in response to copper. Environ. Pollut. 2007, 147, 656–667. [Google Scholar]
- Ussing, H.H. The distinction by means of tracers between active transport and diffusion. Acta Physiol. Scand. 1949, 19, 43–56. [Google Scholar]




| Gill (transepithelial) potential (mV) | ||
|---|---|---|
| Equilibrium | Nonequilibrium | |
| McCorkle and Dietz [24] | −74 (estimated) | –7 (measured) |
| This study a | −84.2 (−93.9 − −67.9) b | −8.2 c |
| Ion activities (mM) | |||||||
|---|---|---|---|---|---|---|---|
| pH | Temp. (°C) | Ca2+ | Mg2+ | Na+ | Cl− | ||
| Changhua a | 8.01±0.19 | 29.3±0.9 | 0.41±0.14 | 0.34±0.08 | 0.43±0.23 | 0.40±0.25 | 0.098±0.15 |
| Hualien a | 7.80 | 30.5 | 0.36 | 1.17 | 12.28 | 55.57 | 1.42 |
|
|
|
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. Thisarticle is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liao, C.-M.; Lin, C.-M.; Jou, L.-J.; Chen, W.-Y. Sodium Gill Potential as a Tool to Monitor Valve Closure Behavior in Freshwater Clam Corbicula fluminea in Response to Copper. Sensors 2008, 8, 5250-5269. https://doi.org/10.3390/s8095250
Liao C-M, Lin C-M, Jou L-J, Chen W-Y. Sodium Gill Potential as a Tool to Monitor Valve Closure Behavior in Freshwater Clam Corbicula fluminea in Response to Copper. Sensors. 2008; 8(9):5250-5269. https://doi.org/10.3390/s8095250
Chicago/Turabian StyleLiao, Chung-Min, Chieh-Ming Lin, Li-John Jou, and Wei-Yu Chen. 2008. "Sodium Gill Potential as a Tool to Monitor Valve Closure Behavior in Freshwater Clam Corbicula fluminea in Response to Copper" Sensors 8, no. 9: 5250-5269. https://doi.org/10.3390/s8095250
APA StyleLiao, C.-M., Lin, C.-M., Jou, L.-J., & Chen, W.-Y. (2008). Sodium Gill Potential as a Tool to Monitor Valve Closure Behavior in Freshwater Clam Corbicula fluminea in Response to Copper. Sensors, 8(9), 5250-5269. https://doi.org/10.3390/s8095250




