The Nature of Aqueous Solutions of a Cationic Calix[4]arene: A Comparative Study of Dye–Calixarene and Dye–Surfactant Interactions
Abstract
:1. Introduction
2. Experimental
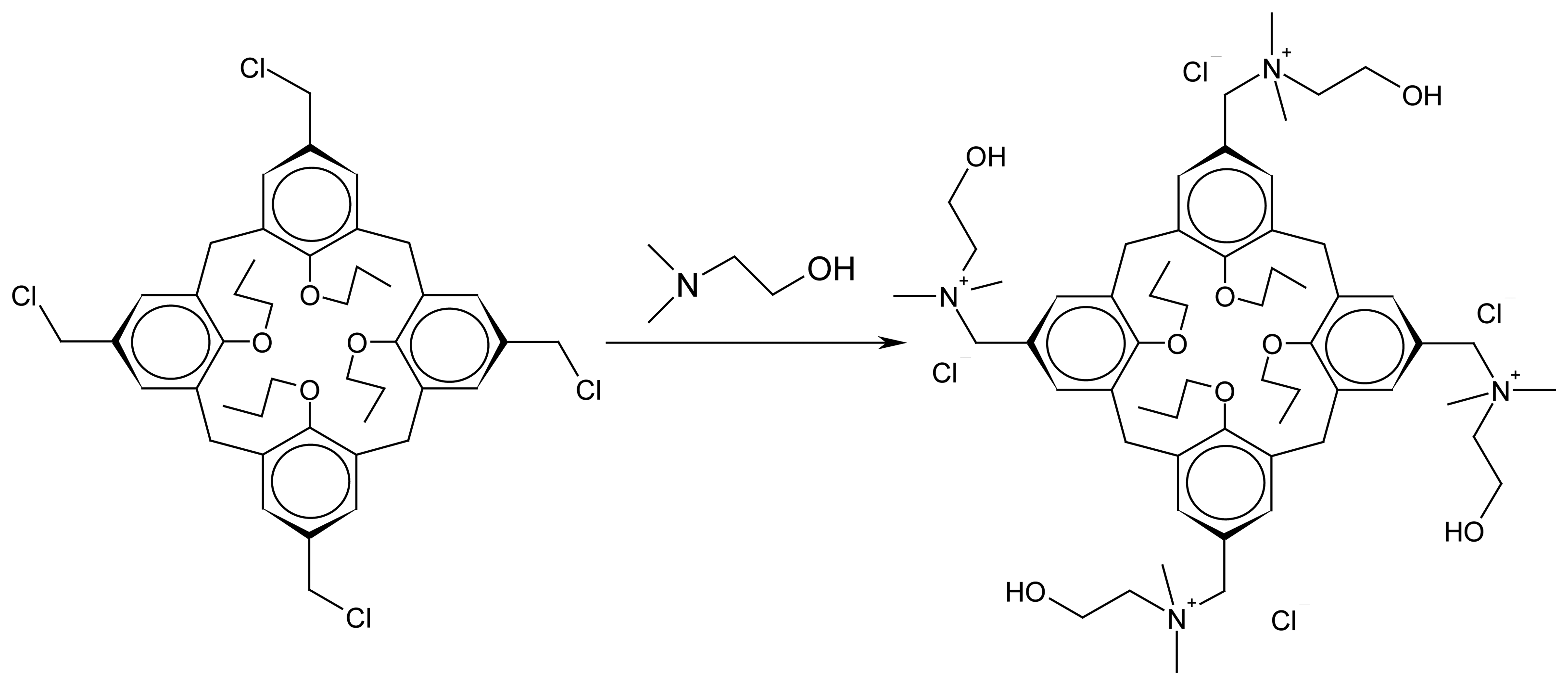
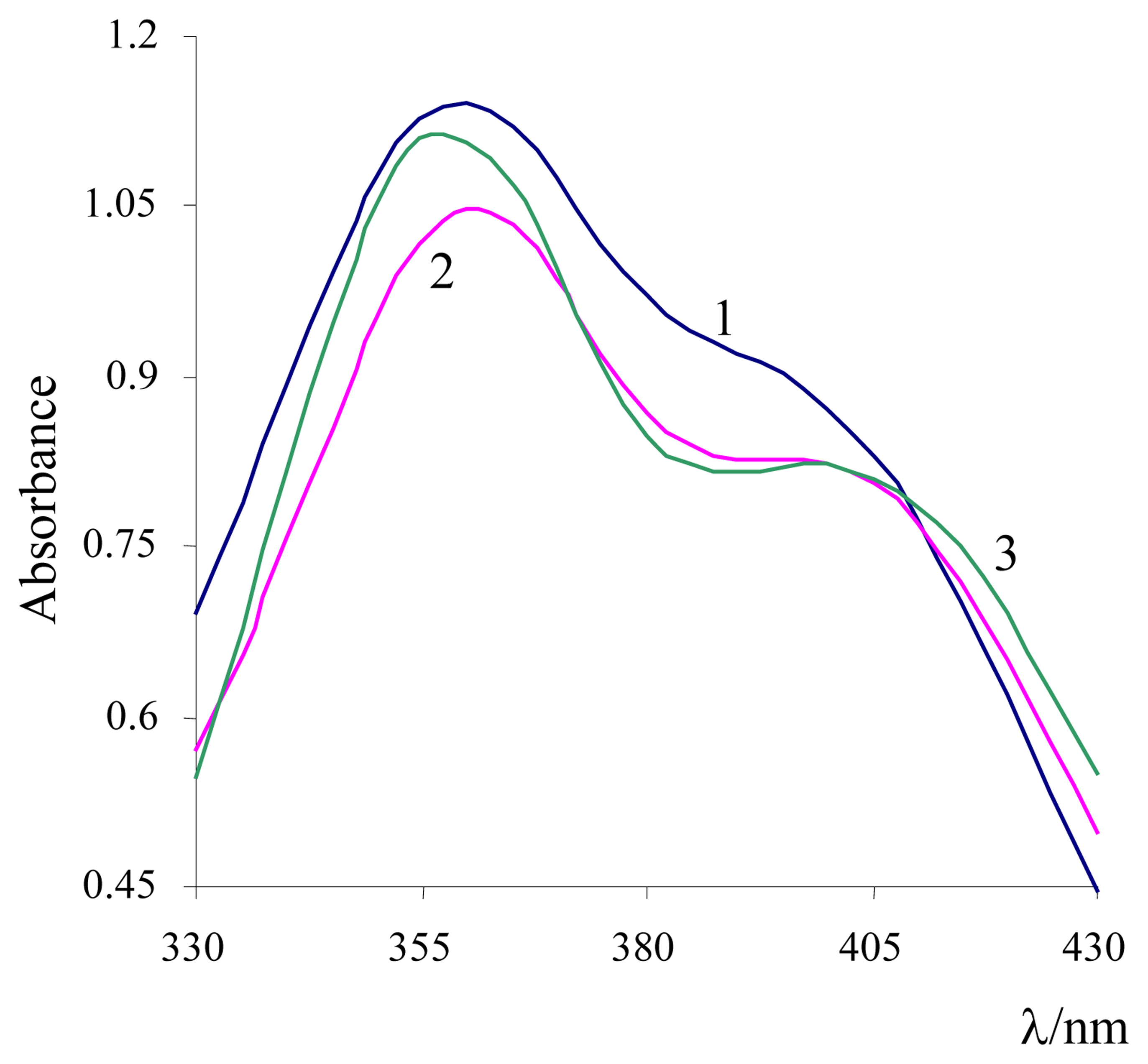
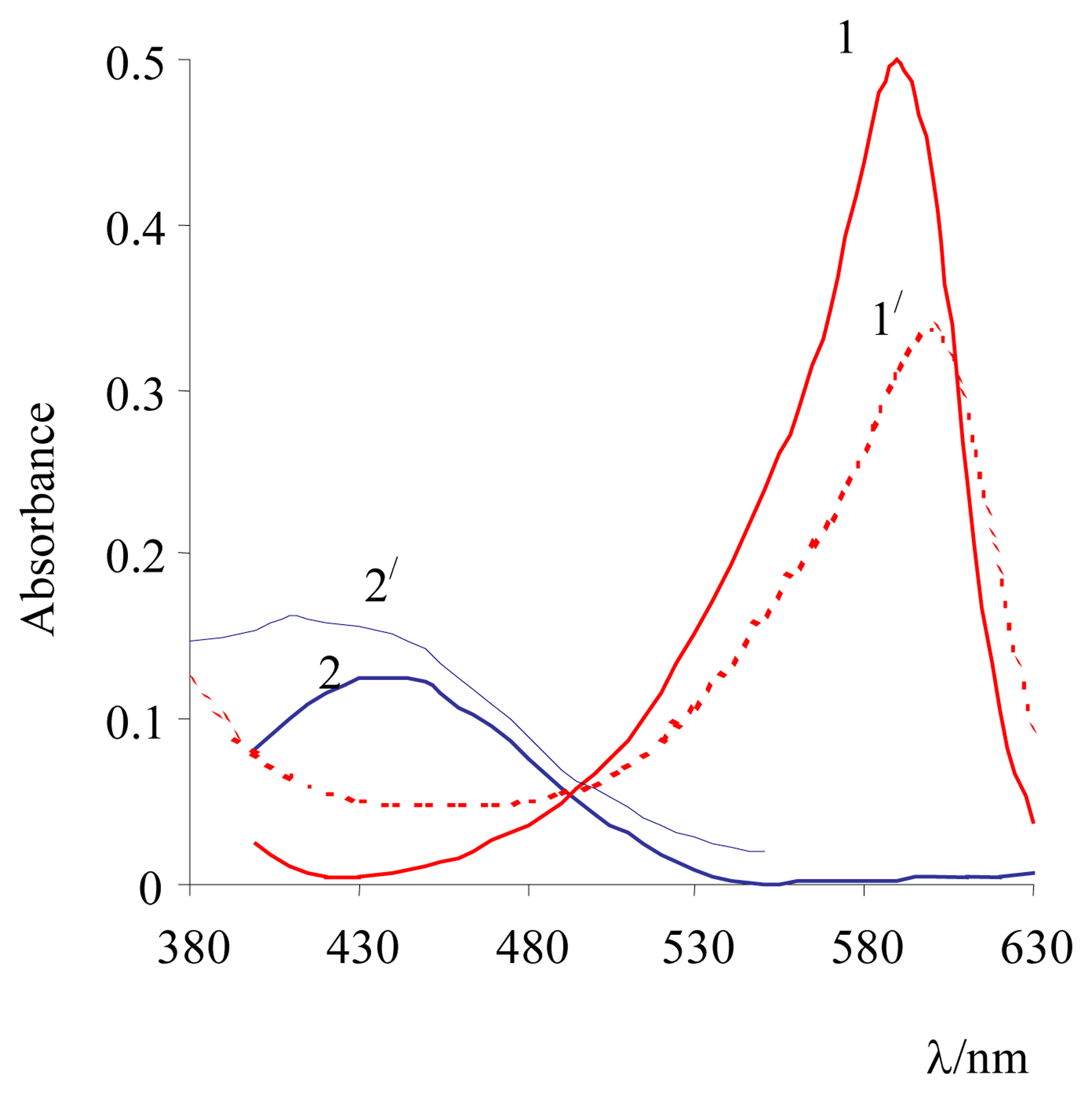
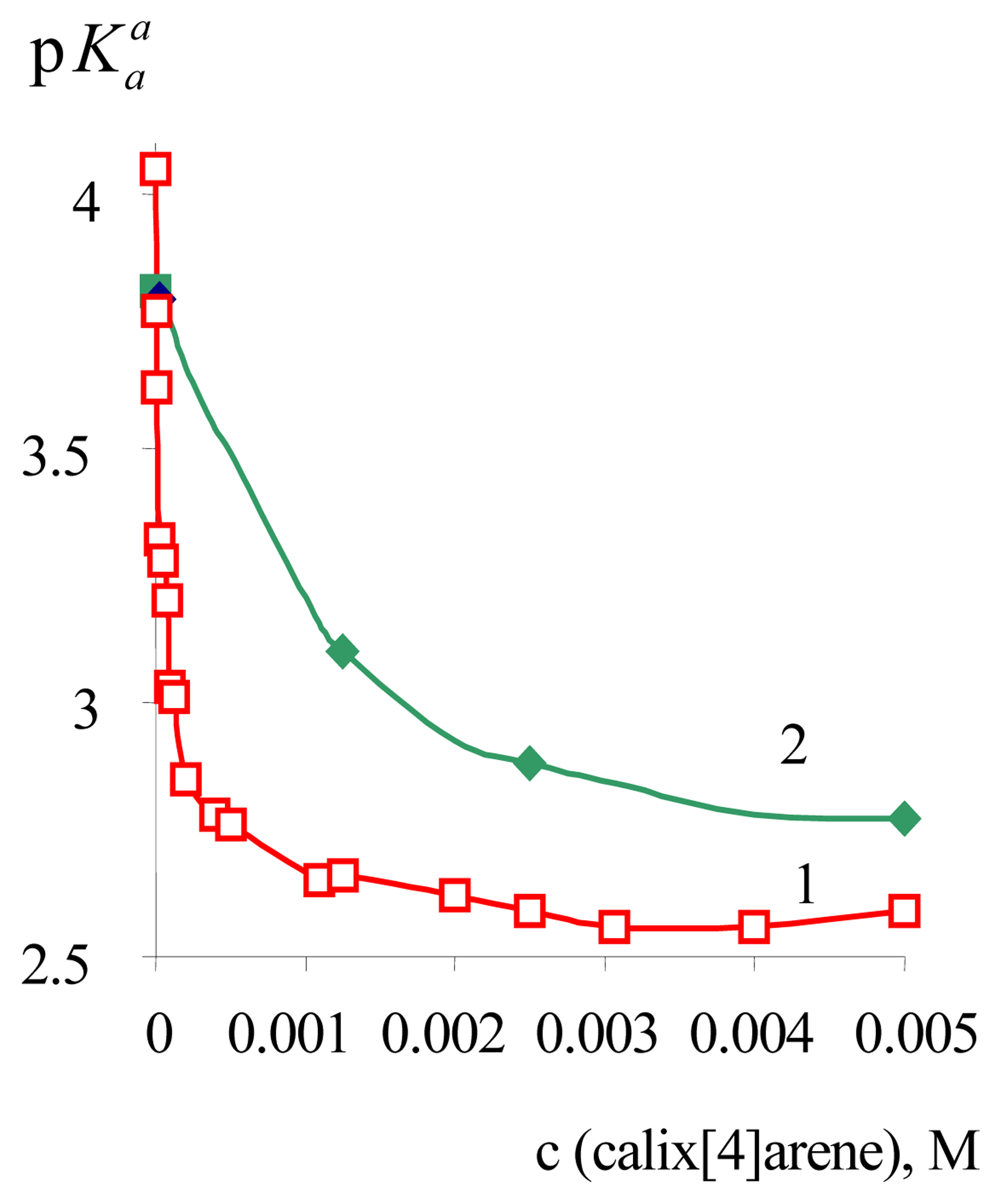
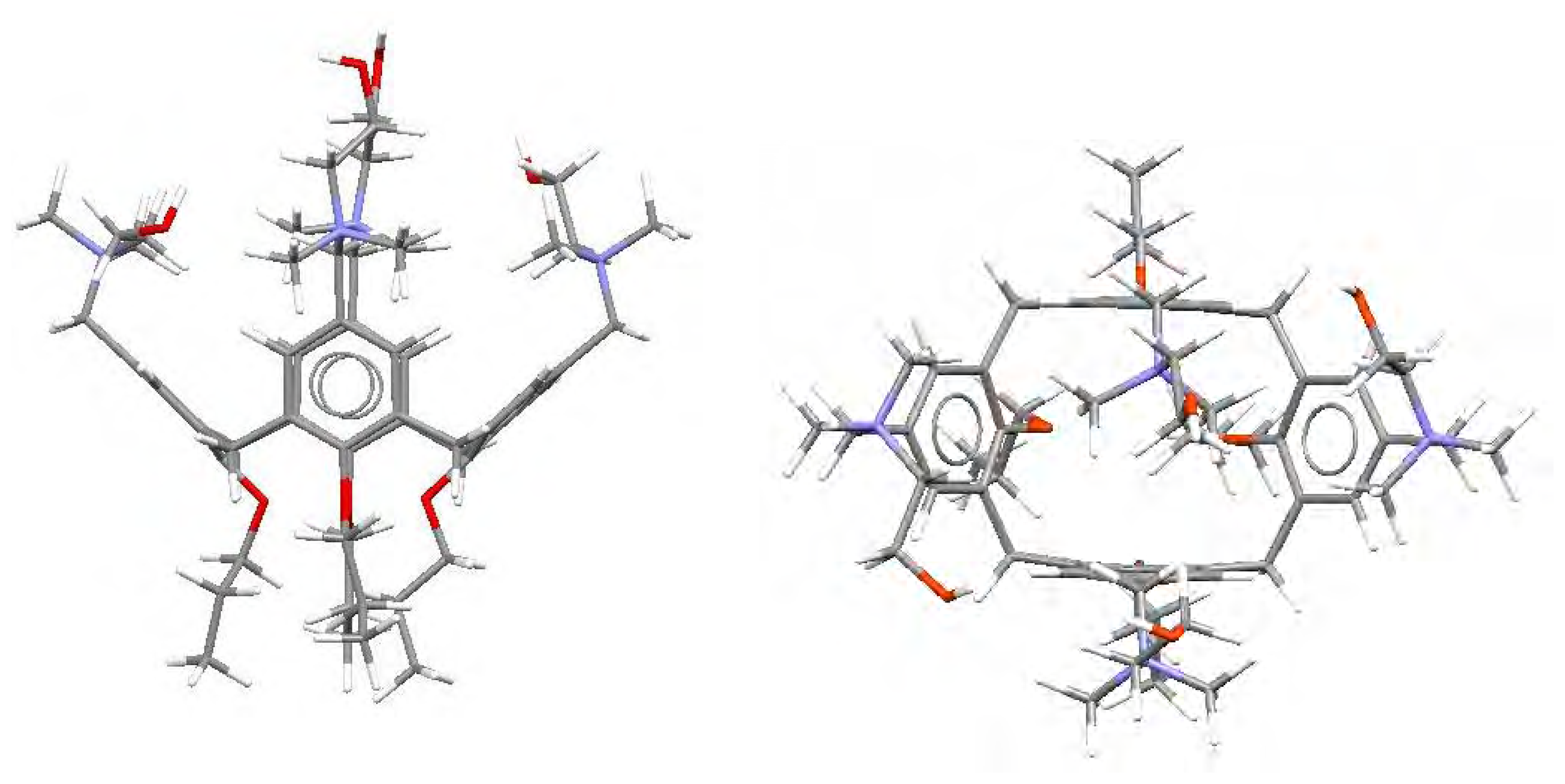
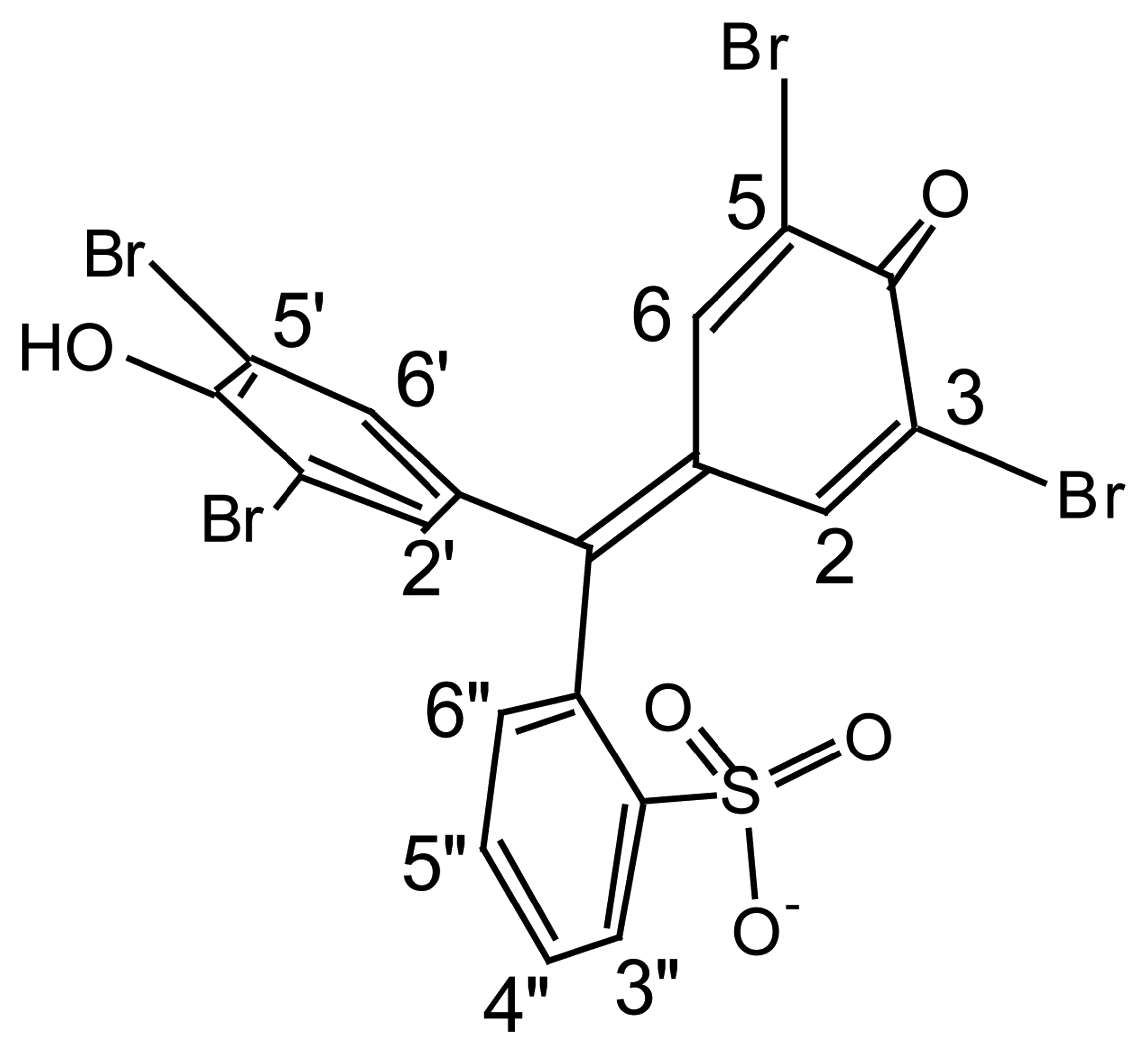
3. Results and Discussion
Acknowledgments
References
- Arimura, T.; Kawabata, H.; Matsuda, T.; Muramatsu, T.; Satoh, H.; Fujo, K.; Manabe, O.; Shinkai, S. New Water-Soluble Host Calixarenes Bearing Chiral Substituents. J. Org. Chem. 1991, 56, 301–306. [Google Scholar]
- Douteau-Guével, N.; Coleman, A. W.; Morel, J.-P.; Morel-Desrosiers, N. Complexation of Basic Amino Acids by Water-Soluble Calixarene Sulphonates as a Study of the Possible Mechanism of Recognition of Calixarene Sulphonates by Proteins. J. Phys. Org. Chem. 1998, 11, 693–696. [Google Scholar]
- Zhang, Y.; Pham, T. H.; Sánchez Pena, M.; Agbaria, R. A.; Warner, I. M. Spectroscopic Studies of Brilliant Cresyl Blue / Water-Soluble Sulfonated Calix[4]arene Complex. Applied Spectrosc. 1998, 52, 952–957. [Google Scholar]
- Hioki, H.; Yamada, T.; Fujioka, C.; Kodama, M. Peptide Library Based on Calix[4]arene. Tetrahedron Lett. 1999, 40, 6821–6825. [Google Scholar]
- Shi, Y.; Schneider, H.-J. Interaction Between Aminocalixarenes and Nucleotides or Nucleic Acids. J. Chem. Soc., Perkin Trans. 2 1999, 1797–1803. [Google Scholar]
- Jin, T. A New Fluorometric Method for the Detection of the Neurotransmitter Acetylcholine in Water Using a Dansylcholine Complex with p-Sulfonated Calix[8]arene. J. Incl. Phenom. Macro. 2003, 45, 195–201. [Google Scholar]
- Tao, W.; Barra, M. Inhibition of Quinone–Imine Dye Deamination by Complexation with Para-Sulfonated Calixarenes. J. Org. Chem. 2001, 66, 2158–2160. [Google Scholar]
- Iki, N.; Fujimoto, T.; Shindo, T.; Koyama, K.; Miyano, S. Almost Complete Removal of Trace Amount of Halogenated Organic Compounds in Water: An Approach by Use of a Combination of Water-Soluble Thiacalixarene and Ion-Exchange Resins. Chem. Lett. 1999, 777–778. [Google Scholar]
- Bügler, J.; Sommerdijk, N. A. J. M.; Visser, A. J. W. G.; van Hoek, A.; Nolte, R. J. M.; Engbersen, J. F. J.; Reinhoudt, D. N. Interconnective Host-Guest Complexation of β-Cyclodextrin – Calix[4]arene Couples. J. Am. Chem. Soc. 1999, 121, 28–33. [Google Scholar]
- Liu, Y.; Chen, Y.; Li, L.; Huang, G.; You, C.-C.; Zhang, H.-Y.; Wada, T.; Inoue, Y. Cooperative Multiple Recognition by Novel Calix[4]arene-Tethered β-Cyclodextrin and Calix[4]arene-Bridged Bis(β-cyclodextrin). J. Org. Chem. 2001, 66, 7209–7215. [Google Scholar]
- Zhang, Y.; Cao, W. Self-Assembly of Small Molecules: An Approach Combining Electrostatic Self-Assembly Technology with Host-Guest Chemistry. New J. Chem. 2001, 25, 483–486. [Google Scholar]
- Liu, Y.; Han, B.-H.; Chen, Y.-T. Inclusion Complexation of Acridine Red Dye by Calixarenesulfonates and Cyclodextrines: Opposite Fluorescent Behavior. J. Org. Chem. 2000, 65, 6227–6230. [Google Scholar]
- Liu, Y.; Han, B.-H.; Chen, Y.-T. Molecular Recognition and Complexation Thermodynamics of Dye Guest Molecules by Modified Cyclodextrins and Calixarenesulfonates. J. Phys. Chem. B 2002, 106, 4678–4687. [Google Scholar]
- Kunsági-Máte, S.; Szabo, K.; Lemli, B.; Bitter, I.; Nagy, G.; Kollár, L. Host-Guest Interaction Between Water-Soluble Calix[6]arene Hexasulfonate and p-Nitrophenol. Thermochim. Acta. 2005, 425, 121–126. [Google Scholar]
- Grieser, F.; Drummond, C. J. The Physicochemical Properties of Self-Assembled Surfactant Aggregates as Determined by Some Molecular Spectroscopic Probe Techniques. J. Phys. Chem. 1988, 92, 2580–2593. [Google Scholar]
- Bhattacharyya, K. Solvation Dynamics and Proton Transfer in Supramolecular Assemblies. Acc. Chem. Res. 2003, 36, 95–101. [Google Scholar]
- Ryzhkina, I. S.; Kudryavtseva, L. A.; Konovalov, A. I. The Reactivity of Associates of Aminomethylated Calix[4]resorcinolarenes Towards p-Nitrophenyl Esters of Phosphorous Acids. XIVth Internat. Conf. of Phosphorus Chemistry: Abstracts, Cincinnati, USA, Cincinnati, July 12-17, 1998; 1998; p. p. 114. [Google Scholar]
- Almi, M.; Arduini, A.; Casnati, A.; Pochini, A.; Ungaro, R. Chloromethylation of Calixarenes and Synthesis of New Water Soluble Macrocyclic Host. Tetrahedron 1989, 45, 2177–2182. [Google Scholar]
- Mchedlov-Petrossyan, N. O.; Kholin, Yu. V. Aggregation of Rhodamine B in Water. Russ. J. Appl. Chem. (English transl.) 2004, 27, 414–422, and references cited therein. [Google Scholar]
- Mchedlov-Petrossyan, N. O.; Vodolazkaya, N. A.; Reichardt, C. Unusual Findings on Studying Surfactant Solutions: Displacing Solvatochromic Pyridinium N-phenolate Towards Outlying Areas of Rod–Like Micelles? Colloids and Surfaces A 2002, 205, 215–229. [Google Scholar]
- Moller, J. V.; Kragh-Hanssen, U. Indicator Dyes and Probes of Electrostatic Potential Changes on Macromolecular Surfaces. Biochemistry 1975, 14, 2317–2322. [Google Scholar]
- Rozendorfova, J.; Cermakova, L. Spectrophotometric Study of the Interaction of Some Triphenylmethane Dyes and 1-Carbethoxypentadecyltrimethylammonium Bromide. Talanta 1980, 27, 705–708. [Google Scholar]
- Drummond, C.; Grieser, F.; Healy, T. W. Acid-Base Equilibria in Aqueous Micellar Solutions. J. Chem. Soc., Faraday Trans 1 1989, 85, 537–550. [Google Scholar]
- Funasaki, N. The Effect of the Solvent Property of the Surfactant Micelle on the Dissociation of Weak Electrolytes. Nippon Kagaku Kaishi 1976, 722–726. [Google Scholar]
- Fernandes, M.S.; Fromherz, P. Lipoid pH Indicators as Probes of Electrical Potential and Polarity in Micelles. J. Phys. Chem. 1977, 81, 1755–1761. [Google Scholar]
- Funasaki, N. Micellar Effects on the Kinetics and Equilibrium of Chemical Reactions in Salt Solutions. J. Phys. Chem. 1979, 83, 1998–2003. [Google Scholar]
- Mchedlov-Petrossyan, N. O.; Timiy, A. V.; Vodolazkaya, N. A. Binding of Sulfonephthalein Anions to the Micelles of an Anionic Surfactant. J. Mol. Liq. 2000, 87, 75–84. [Google Scholar]
- Mchedlov-Petrossyan, N. O.; Vodolazkaya, N. A.; Doroshenko, A. O. Ionic Equilibria of Fluorophores in Organized Solutions. The Influence of Micellar Microenvironment on Protolytic and Photophysical Properties of Rhodamine B. J. Fluoresc. 2003, 13, 235–248. [Google Scholar]
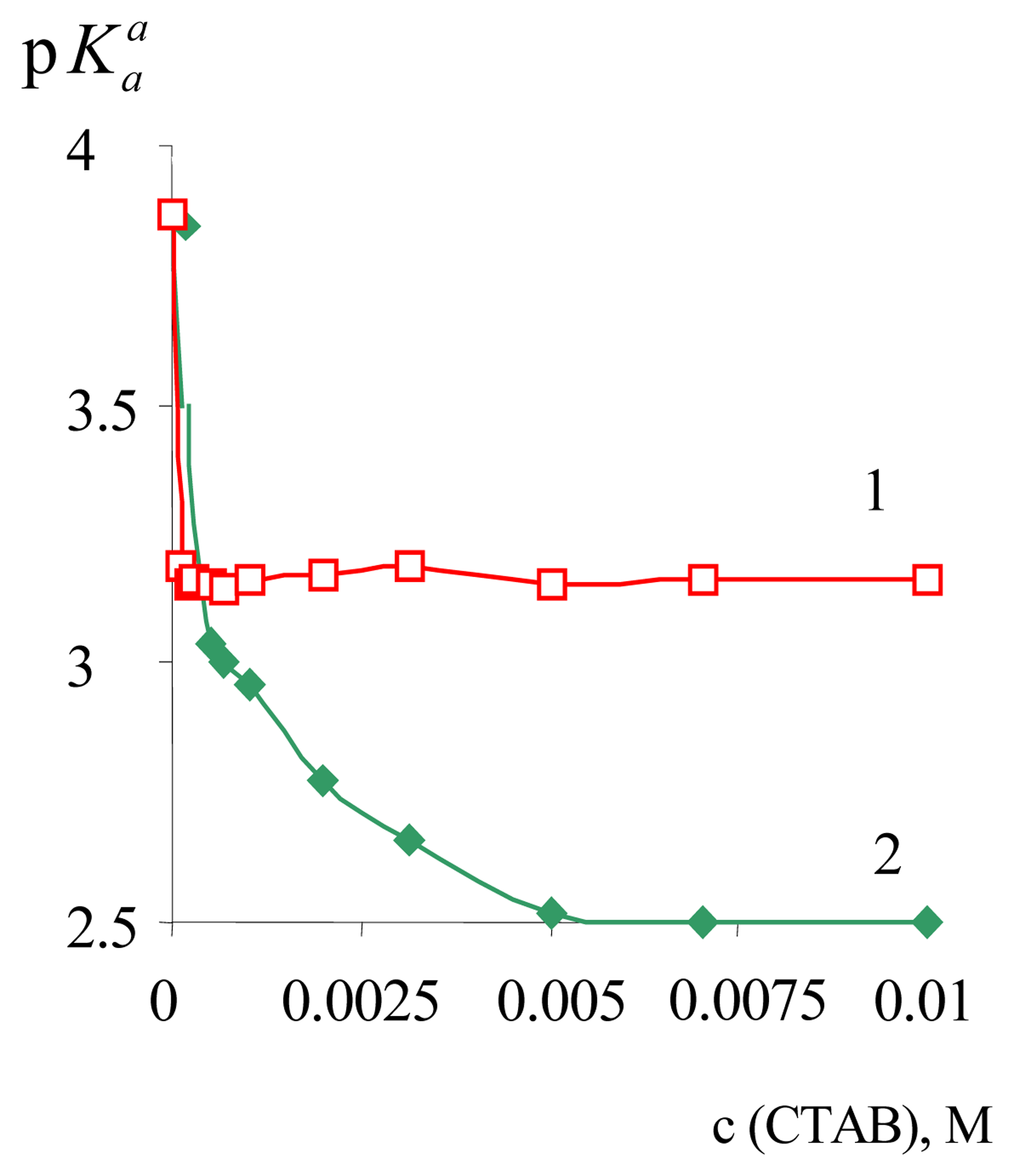
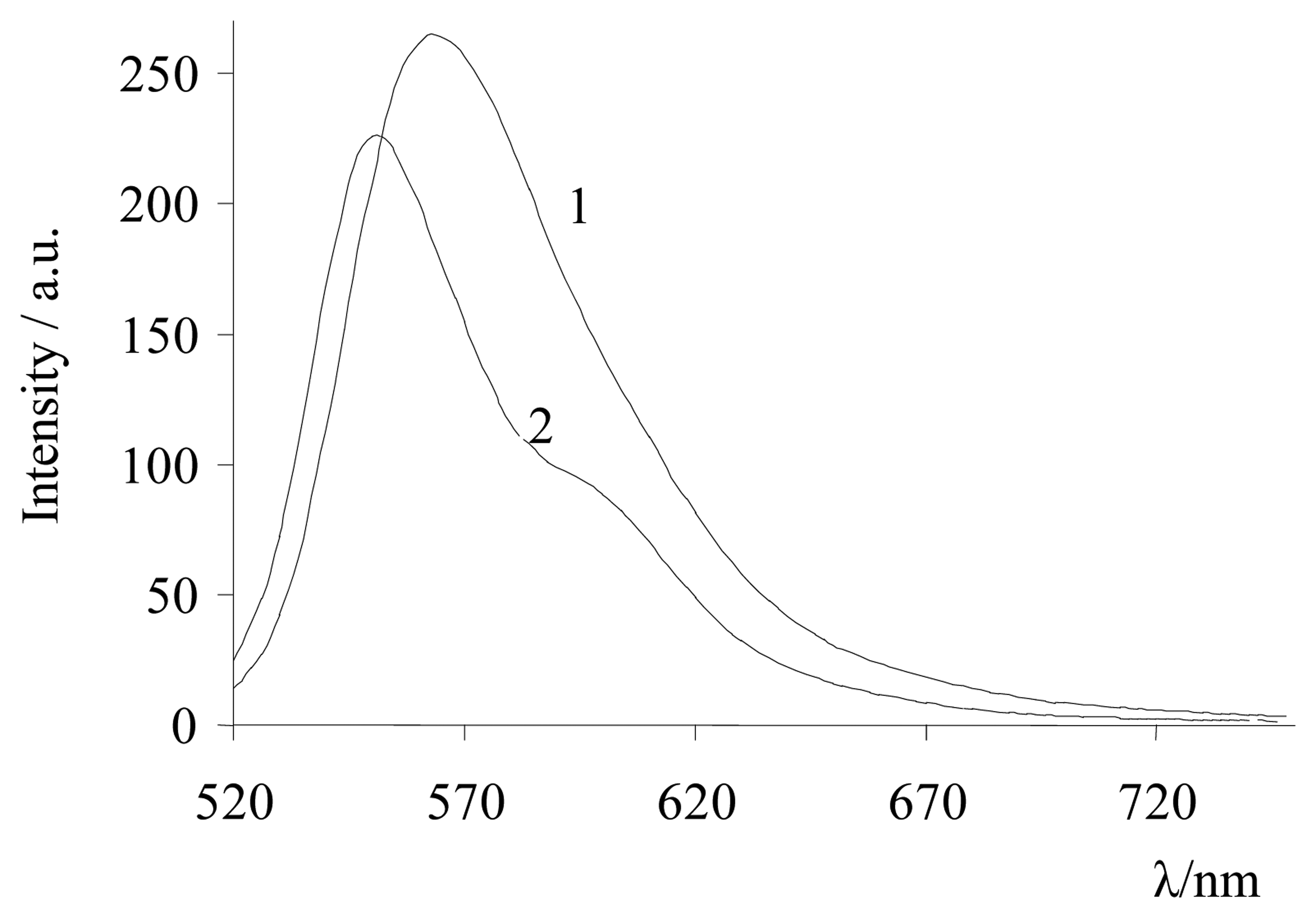
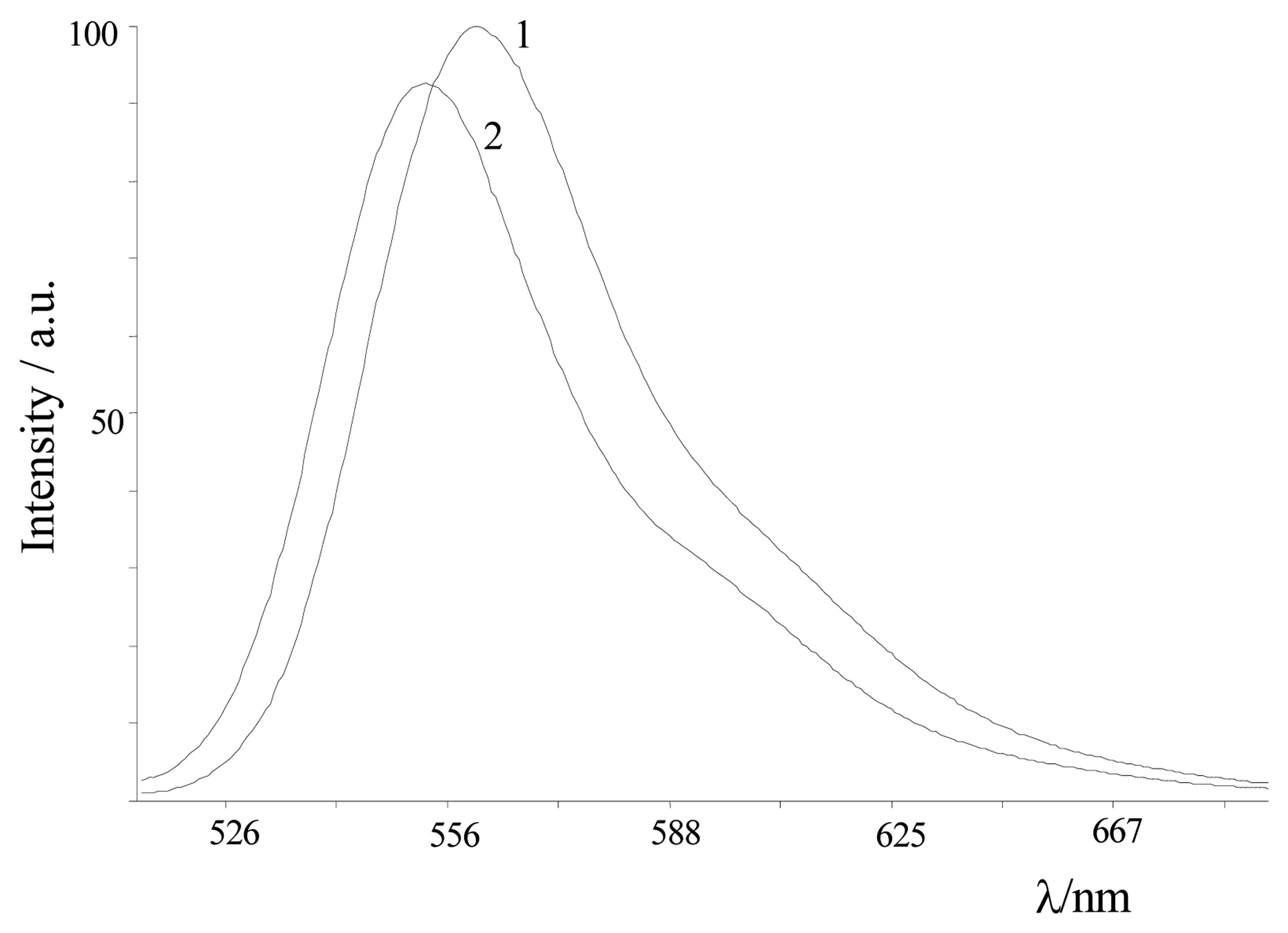
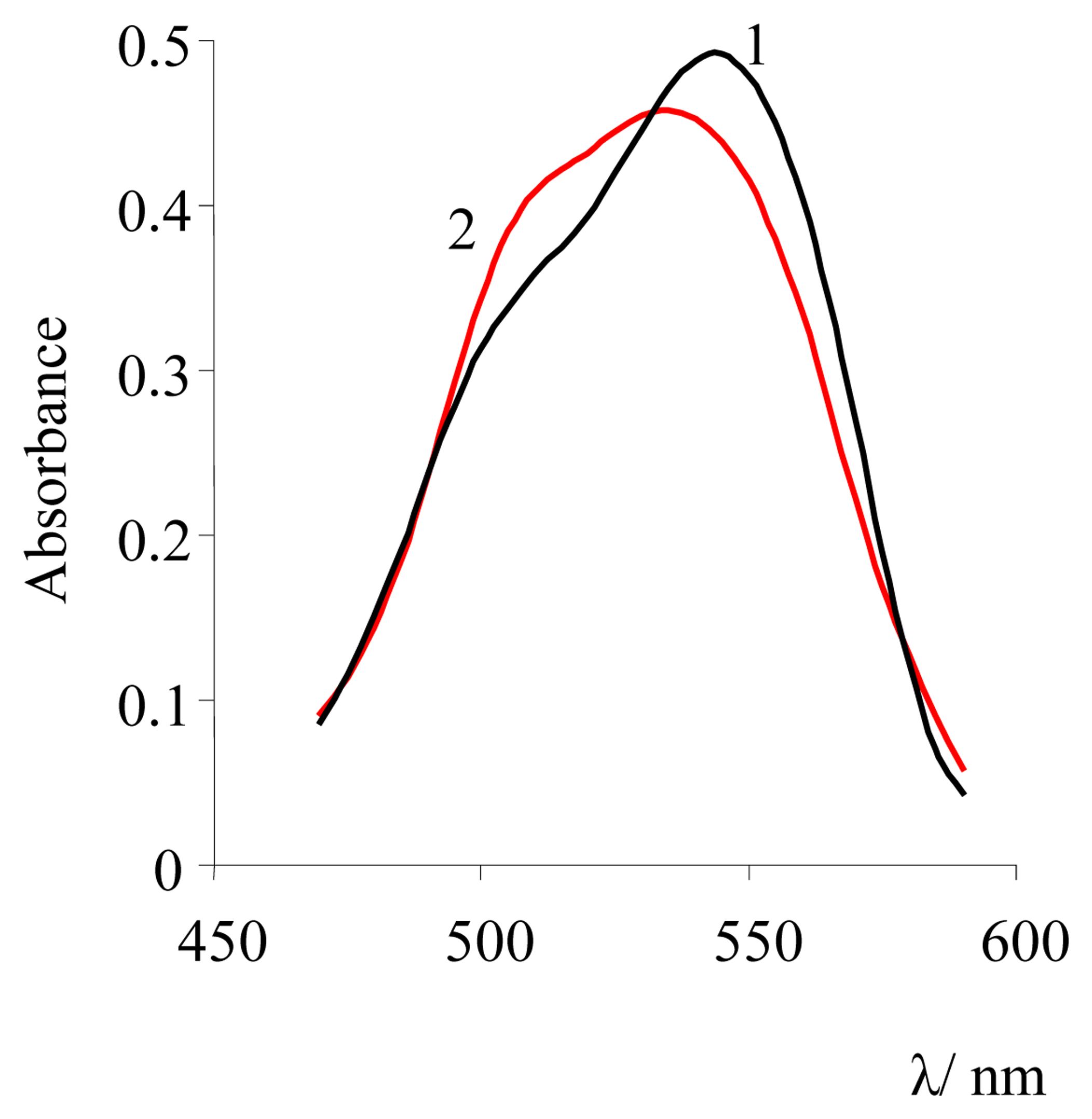
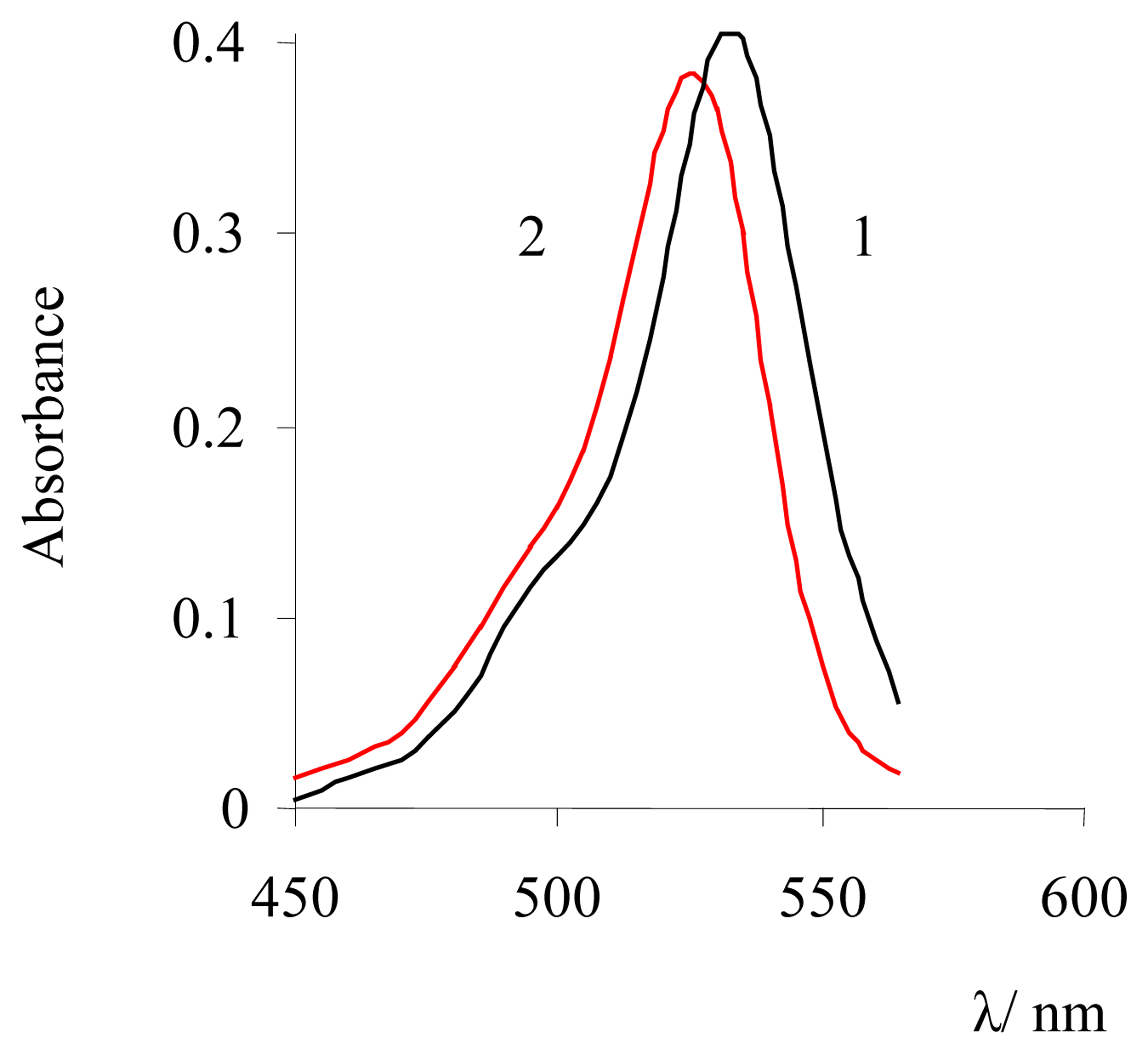
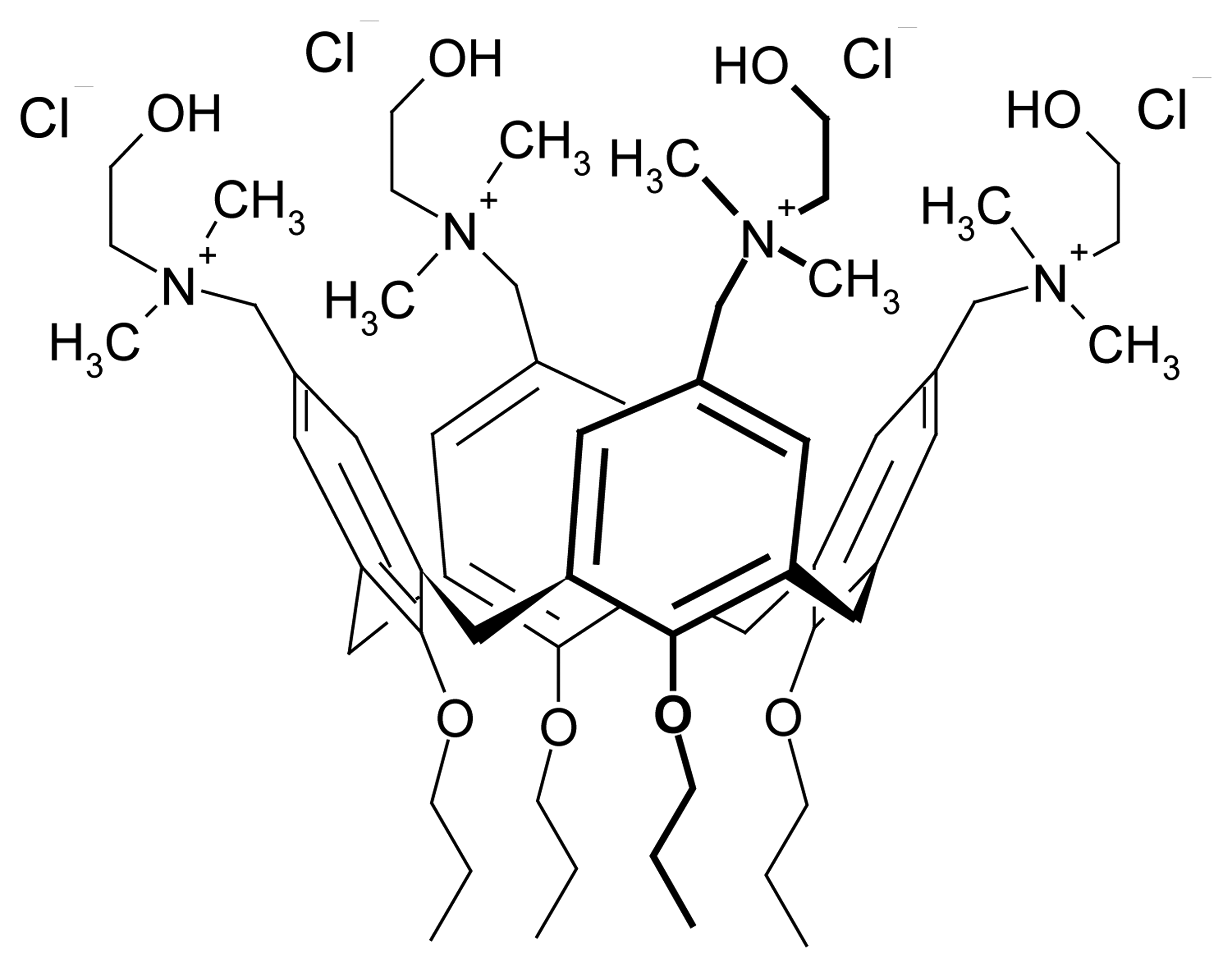
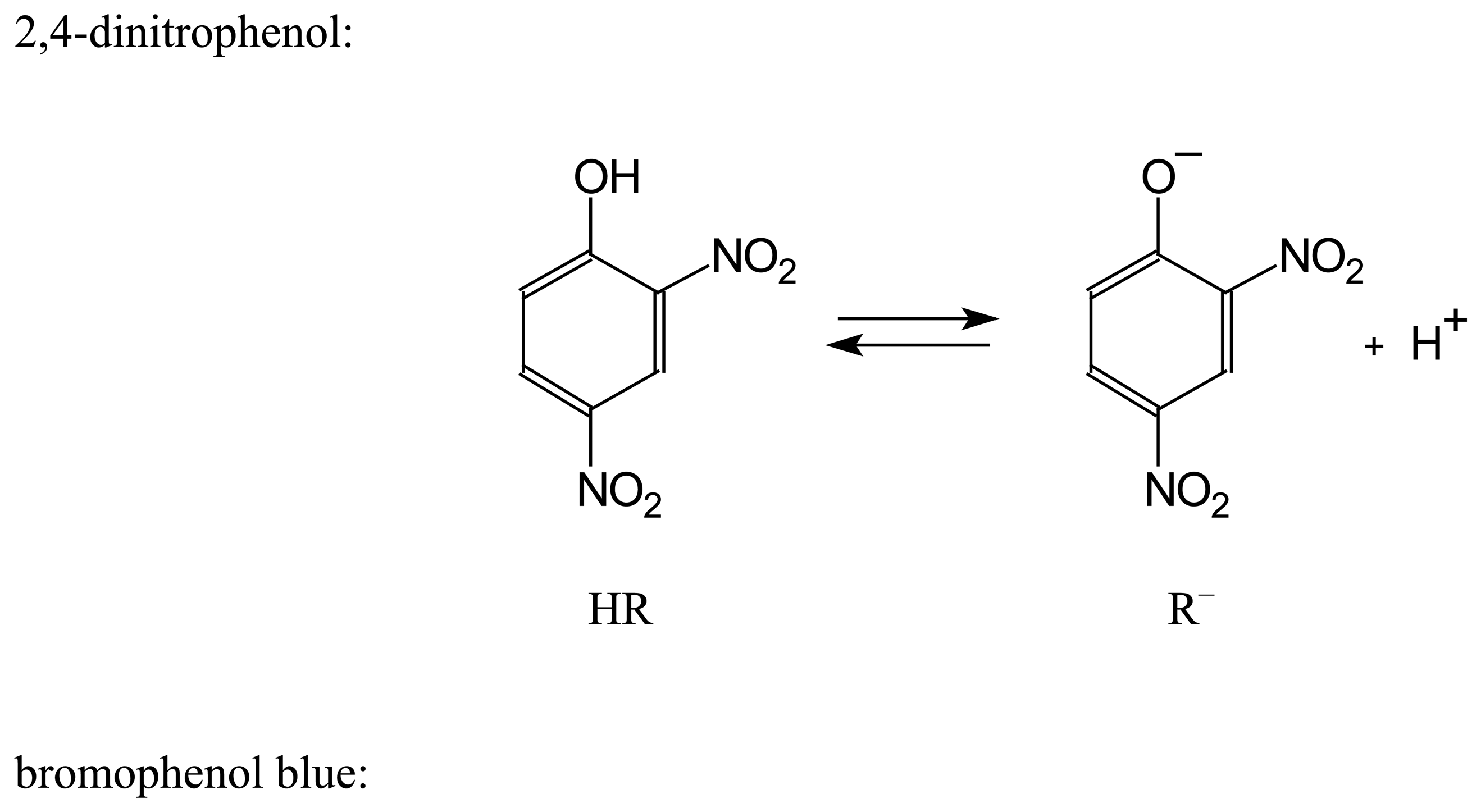
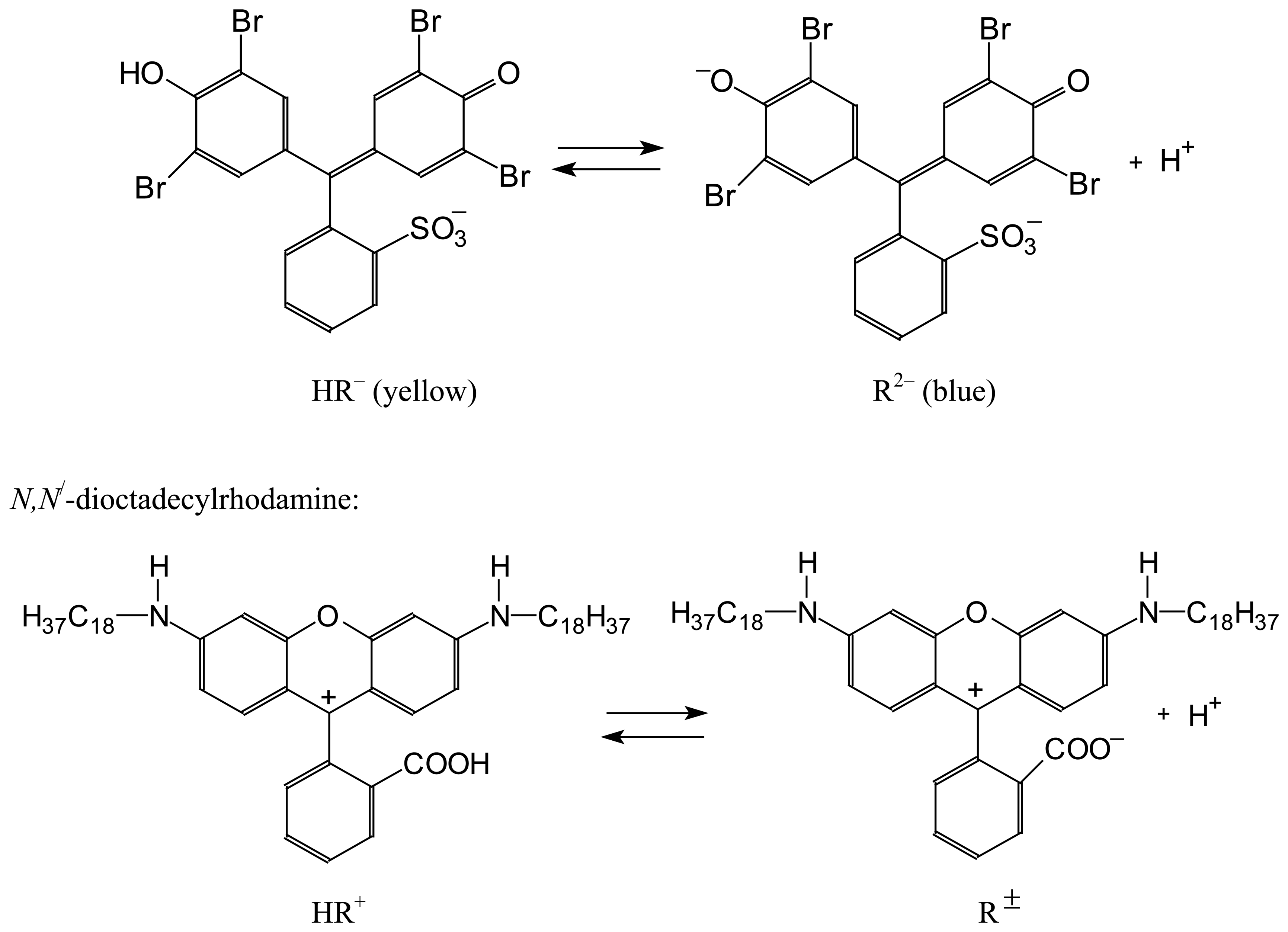

| Dye | |||||
|---|---|---|---|---|---|
| in calixarene solution | in CTAB solution | ||||
| α-DNF | 3.81±0.02 | 2.88±0.05 | –0.93 | 2.46±0.02 | –1.35 |
| BPB | 4.05±0.02 | 2.59±0.02 | –1.46 | 2.83±0.02 a | –1.22 |
| DODR | 3.35±0.01 b | 2.58±0.03 c | –0.77 | 2.49±0.07 | –0.86 |
© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Mchedlov-Petrossyan, N.O.; Vilkova, L.N.; Vodolazkaya, N.A.; Yakubovskaya, A.G.; Rodik, R.V.; Boyko, V.I.; Kalchenko, V.I. The Nature of Aqueous Solutions of a Cationic Calix[4]arene: A Comparative Study of Dye–Calixarene and Dye–Surfactant Interactions. Sensors 2006, 6, 962-977. https://doi.org/10.3390/s6080962
Mchedlov-Petrossyan NO, Vilkova LN, Vodolazkaya NA, Yakubovskaya AG, Rodik RV, Boyko VI, Kalchenko VI. The Nature of Aqueous Solutions of a Cationic Calix[4]arene: A Comparative Study of Dye–Calixarene and Dye–Surfactant Interactions. Sensors. 2006; 6(8):962-977. https://doi.org/10.3390/s6080962
Chicago/Turabian StyleMchedlov-Petrossyan, N. O., L. N. Vilkova, N. A. Vodolazkaya, A. G. Yakubovskaya, R. V. Rodik, V. I. Boyko, and V. I. Kalchenko. 2006. "The Nature of Aqueous Solutions of a Cationic Calix[4]arene: A Comparative Study of Dye–Calixarene and Dye–Surfactant Interactions" Sensors 6, no. 8: 962-977. https://doi.org/10.3390/s6080962
APA StyleMchedlov-Petrossyan, N. O., Vilkova, L. N., Vodolazkaya, N. A., Yakubovskaya, A. G., Rodik, R. V., Boyko, V. I., & Kalchenko, V. I. (2006). The Nature of Aqueous Solutions of a Cationic Calix[4]arene: A Comparative Study of Dye–Calixarene and Dye–Surfactant Interactions. Sensors, 6(8), 962-977. https://doi.org/10.3390/s6080962




