Abstract
PVC based membranes of disodium salt of porphyrin 3,7,12,17-tetramethyl-8, 13-divinyl 2,18-porphine dipropionic acid (I) as ionophore with sodium tetra phenyl borate (NaTPB) as anion excluder and dibutyl phthalate (DBP), dioctyl phthalate (DOP), dibutyl butyl phosphonate (DBBP), tris(2- ethyl hexyl)phosphate (TEP), tri-n-butylphosphate (TBP) and 1- chloronaphthalene (CN) as plasticizing solvent mediators were prepared and constructed for determination of Zn(II). The PVC based membrane of (I) with DBBP as plasticizer and having anion excluder, NaTPB in the ratio PVC: I: NaTPB: DBBP (150: 10: 2: 200) gave the best results in terms of working concentration range (1.3×10-5-1.0 ×10-1M) with a Nernstian slope (30.0 mV/decade of activity). The useful pH range of the sensor is 3.0 –7.4, beyond which a drift in potential was observed. The response time of the sensor is 10s and the lifetime was about 2 months during which it could be used without any measurable divergence. It had good stability and reproducibility. The membrane worked satisfactorily in non-aqueous medium up to 40% (v/v) non-aqueous content. The selectivity coefficient values indicate that the electrode is highly selective for Zn2+ over a number of other cations except Na+ and Cd2+. Although Na+ and Cd2+ are likely to cause some interference, they would not interfere if present at the concentrations < 1 ×10-5 and < 5 ×10-5 M, respectively. The electrode has been used as an indicator electrode to determine the end point in the potentiometric titration of Zn2+ with EDTA.
Introduction
Ion Selective Electrodes are being widely used in the fields of environmental, industrial, agricultural and medicinal as they offer several advantages over other methods of analysis. The most attractive features of this technique are the speed with which samples can be analyzed, portability of the device, sample non-destruction, online monitoring, cost effectiveness and large measuring range. Some commercialized sensors for alkali and alkaline earth metals, halides, etc. are available, however more efforts are required to develop ion-selective electrodes of commercial standards for heavy metal ions, which are toxic beyond a certain concentration level.
Besides its carcinogens and mutagens nature the toxicity of zinc arises from its synergistic/antagonistic interaction with other heavy metals, particularly its homologue cadmium [1]. Its compounds are widely used in electroplating, pharmaceuticals, paint, rubber, dye, wood preservatives, ointments and batteries so the waste from these industries need to be frequently analyzed. Common zinc compounds found at hazardous waste sites include zinc chloride, zinc oxide, zinc sulfate and zinc sulfide. Besides, it is also present in high protein foods and its large doses can cause fever, chills, pulmonary manifestation, gastroenteritis, vomiting, nausea, anemia and renal failure. In view of its toxicity, the determination of zinc becomes important.
Only few zinc selective electrodes [2,3,4,5,6,7,8,9,10,11,12,13] are reported in literature and most of them have poor sensitivity, selectivity, long response time and short life time [2,3,4,5,6,7,8,9]. An electrode was fabricated by incorporating zinc salts of bi(4-octylphenyl)hydrogen phosphate in PVC matrix [2] but the electrode showed serious interference from some metals. Linnersund and Bhatti [3] tried zinc complex of bis(2-ethylhexyl)phosphate, an extractant, as electroactive material for preparing Zn2+-selective electrodes but it had a very narrow working pH of range 4.5-6.0. Liquid membrane electrodes [4,5] have also been reported.
Lebedeva and Jansons prepared Zn2+-selective electrodes using saturated solutions of Zn-quinoline-8-carbodithioate in chloroform [4]. Kojima and Kamata [5] used tetrabutylthiuram disulfide as the carrier in PVC based membrane electrode. Zinc orthophosphate and zinc mercuric thiocyanate [6] were used by Rocheleaw and Purdy as electroactive material on a carbon support for the fabrication of Zn2+-selective sensors. The electrode worked well but suffered interference from Cu2+, Cd2+ and Pb2+. Another electrode, based on salicylaldoxime-formaldehyde resin, for zinc [7] exhibited a working concentration range of 3.0 μM-0.1 M with a near Nernstian slope. A zinc-selective electrode was used by Obmetho et al.[8] for the determination of zinc in Zinc alloys. Srivastava et al.[9] used a cryptand for the fabrication of zinc selective sensor but it exhibited a non-Nernstian response.
Zn-bis(2,4,4-trimethylpentyl) thiophosphinic acid complex was also used for fabricating Zn2+-selective sensor [10] but it suffers interference from copper. Crown ether based electrode has also been reported [11] in literature for zinc. It exhibited a working concentration range of 70 μM-0.1M with a Nernstian slope of 29.5 mV/decade of activity. Shamsipur et al. [12] reported a zinc-selective sensor based on benzo substituted macrocyclic diamides. An electrode based on 5,6,14,15-dibenzo-1,4-dioxo-8,12-diazacyclopentadecane-5,14-diene showed response for zinc [13]. It has a working concentration range of 5μ M-100 mM in the pH range of 1.5-7.0.
Porphyrins are a class of naturally occuring macrocyclic compounds, which play an important role in the metabolism of living organisms [14]. Recently these have been found to bind selectively to metal ions and are being used to develop selective sensors for different cations. Here we successfully tried disodium salt of 3,7,12,17-Tetramethyl-8,13-divinyl-2,18-porphine-dipropionic acid (I) as an active material in PVC matrix for the fabrication of Zn2+-selective sensor and the results of the investigations are presented in this communication.
Experimental
Reagents
3,7,12,17-Tetramethyl-8,13-divinyl-2,18-porphine-dipropionic acid (disodium salt) was obtained from Aldrich Chemical Company, USA. All reagents used in the investigations were of analytical reagent grade (Merck,India). Doubly distilled water was used for preparing all aqueous solutions. Anion excluder, Sodium tetra phenyl borate (NaTPB) and tri-n-butylphosphate (TBP) from BDH (England), Dibutyl phthalate (DBP) and Dioctyl phthalate (DOP) from Reidal, India, Dibutyl(butyl) phosphonate (DBBP) from Mobil (USA), 1-Chloro naphthalene (CN) and Tris(2-ethyl hexyl) phosphate (TEP) from Merck (Germany) were used.
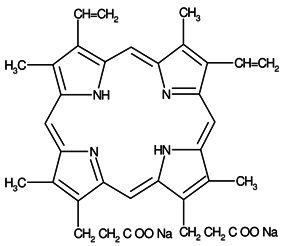
Protoporphyrin (IX) 3,7,12,17-Tetramethyl-8,13-divinyl-2,18-porphine-dipropionic acid disodium salt

Protoporphyrin (IX) 3,7,12,17-Tetramethyl-8,13-divinyl-2,18-porphine-dipropionic acid disodium salt
Apparatus
The potential measurements were carried out at 25±0.1°C on a pH 5652 digital pH meter/millivoltmeter (ECIL, Hyderabad, India) and CVM 301 Century microvoltmeter (Century Instruments, Chandigarh, India). pH measurements were made on a digital pH meter (model pH 5652, ECIL, Hyderabad, India; Glass electrodes as pH electrode and calomel as reference electrode).
Electrode preparation
The method reported by Craggs et al. was adopted for the preparation of membranes [15]. A number of membranes incorporating the active ingredient, anion excluder and plasticizer in different compositions in PVC matrix were fabricated (Table-1). Varying amounts of the ion active phase (I) and an appropriate amount of PVC were dissolved in a minimum amount of THF. The anion excluder, NaTPB and solvent mediators, DBBP, DOP, DBP, CN, TEP and TBP were also added to get membranes of different compositions. The solutions thus obtained, after complete dissolution of various components, were poured into acrylic rings placed on a smooth glass and allowed to evaporate at room temperature. After 48 hours, transparent membranes of 0.5 mm thickness were obtained which were then cut to size and attached to a Pyrex tube with the help of Araldite. The ratio of various membrane ingredients, time of contact and the concentration of equilibrating solution were optimized first so that the membranes develop reproducible, stable and noiseless potentials. Membrane to membrane and batch to batch reproducibility was assured by carefully following the optimum conditions of fabrication.
Potential Measurements
The membranes were equilibrated for 3 days in 0.5 M Zn2+ solution. Potentials were measured by direct potentiometry at 25 ± 0.1°C with the help of ceramic junction calomel electrodes by setting up the following assembly.
- Internal reference electrode (SCE)
- Internal solution (0.1M Zn2+)
- Membrane
- Test solution
- External reference electrode (SCE)
A fixed concentration of Zn2+ solution was taken as internal solution (0.1M) and saturated calomel electrodes (SCE) were used as reference electrodes.
Results and Discussion
Membrane Characteristics
Potential studies on the membrane sensors were carried out with the varying Zn2+ concentration (1.0 × 10-6 to 1.0 × 10-1 M). Table-1 depicts the results of the working concentration range, slope and response time of each membrane. Potentials generated with (blank) membranes (containing only PVC, NaTPB and solvent mediators at [Zn2+] varying from 10-3 to 10-2) were insignificant (5-10 mV). As such, the potentials generated in the proposed sensor are ascribed to the uptake of Zinc ions on the disodium salt of 3,7,12,17-Tetramethyl-8,13-divinyl-2,18-porphine-dipropionic acid.

Table 1.
Composition and response characteristics of Zn2+- selective electrode
| Electrode No. | Components in membranes (% w/w) (I) PVC NaTPB TEP DOP CN TBP DBP DBBP | Working Concentration range (M) | Slope mV/decade of activity | Response Time (s) | ||||||||
| 1. | 10 | 150 | - | - | - | - | - | - | - | 7.9 × 10-4 to 1.0 × 10-1 | 38.2 | 40 |
| 2. | 10 | 150 | 2 | - | - | - | - | - | 200 | 1.3 × 10-5 to 1.0 × 10-1 | 30.0 | 10 |
| 3. | 10 | 150 | 2 | - | 150 | - | - | - | - | 1.7 × 10-4 to 1.0 × 10-1 | 33.0 | 15 |
| 4. | 10 | 150 | 2 | - | - | - | - | 150 | - | 7.9 × 10-5 to 1.0 × 10-1 | 35.0 | 20 |
| 5. | 10 | 150 | 2 | - | - | 150 | - | - | - | 3.1 × 10-5 to 1.0 × 10-1 | 34.0 | 22 |
| 6. | 10 | 150 | 2 | 165 | - | - | - | - | - | 3.9 × 10-4 to 1.0 × 10-1 | 40.0 | 32 |
| 7. | 10 | 150 | 2 | - | - | - | 170 | - | - | 7.0 × 10-4 to 1.0 × 10-1 | 39.0 | 35 |
Effect of Plasticizer
The membrane without plasticizer (Electrode no.1) exhibited a narrow working concentration range (7.9 × 10-4 to 1.0 × 10-1 M) with a slope of 38.2 mV/decade of activity and a response time of 40 seconds. Since the nature of plasticizer influences the dielectric constant of the membrane phase, the mobility of the ionophore and state of ligand [16], it was expected to play a key role in determining the ion-selective characteristics. So various plasticizers (DBBP, DOP, DBP, CN, TEP, TBP) were added in varying amounts to the membranes and ion-selective characteristics were studied. Also, the optimization of permselectivity of the membrane sensor is known to be highly dependent on the incorporation of additional membrane components, therefore Sodium tetra phenyl borate (NaTPB) was also added to the membrane components to increase the conductivity and minimize interference from lipophilic anions [17]. The addition of solvent mediators DBP and CN (Electrode no.4 and 5) improved the working concentration range to 7.9 × 10-5-1.0 × 10-1 M and 3.1 × 10-5-1.0 × 10-1 M and the slopes to 35.0 and 34.0 mV/decade of activity, respectively. For the membrane containing DOP as plasticizer (Electrode no.3), the potential remained linear in the concentration range 1.7 × 10-4-1.0 × 10-1 M with slope 33.0 mV/decade of activity. However, the addition of the solvent mediators TEP and TBP (Electrode no.6 and 7) did not improve the performance of the electrodes (Fig.1).
The membrane having DBBP as plasticizer exhibited best results (Electrode no.2). This sensor gave a Nernstian response (slope 30.0 ± 1.0 mV/ decade of activity) in the concentration range 1.3 × 10-5-1.0 × 10-1 M of Zn2+ and a response time as fast as 10 seconds. Different amounts of (I) were tried with this membrane to get the optimum composition (Fig.2).
The results are summarised in Table-2. It is clear from this table that the increase in the amount of (I) did not improve any of the characteristics of the sensor. Therefore, electrode no. 2 with the optimum composition PVC:(I):NaTPB:DBBP (150:10:2:200) was chosen for all further studies. Repeated monitoring of potentials (20 measurements) on the same portion of the sample with this electrode gave a standard deviation of ± 1.0 mV. The sensing behavior of the membranes did not change when the potentials were recorded from lower to higher concentrations or vice versa. The membrane can be used for over two months, at a stretch, without observing any change in response time, slope and detection limit of the electrode. The difference in potential could be corrected by re-equilibrating the membrane with 0.5 M Zn2+ solution for 2-3 days. When not in use the sensor was kept stored in air.
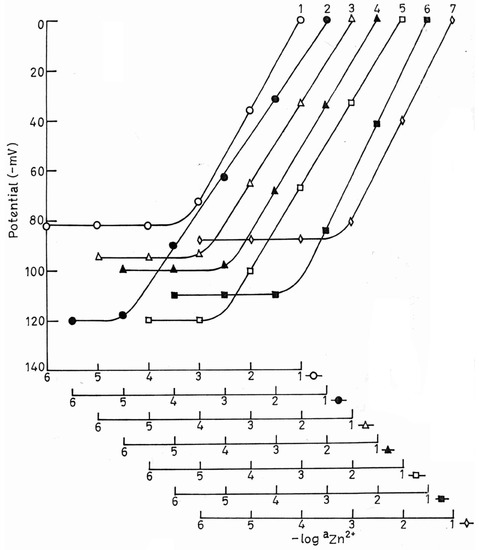
Figure.1.
Variation of potential of PVC based membrane of (I) (no.1) without solvent mediator and (nos. 2,3,4,5,6 and 7) with solvent mediators DBBP, DOP, DBP, CN, TEP and TBP, respectively with Zn2+ concentration.

Table 2.
Response characteristics of the PVC based membranes with different amounts of Ionophore
| Electrode No. | Components of the membranes (% w/w) (I) PVC NaTPB DBBP | Working concentration range (M) | Slope mV/decade of activity | Response Time (s) | |||
| A. | 8 | 150 | 2 | 200 | 5.3 × 10-5 to 1.0 × 10-1 | 28.2 | 15 |
| B. | 9.5 | 150 | 2 | 200 | 1.5 × 10-5 to 1.0 × 10-1 | 29.0 | 12 |
| C. | 10 | 150 | 2 | 200 | 1.3 × 10-5 to 1.0 × 10-1 | 30.0 | 10 |
| D. | 11 | 150 | 2 | 200 | 1.3 × 10-5 to 1.0 × 10-1 | 30.2 | 10 |
| E. | 12 | 150 | 2 | 200 | 1.3 × 10-5 to 1.0 × 10-1 | 30.0 | 10 |
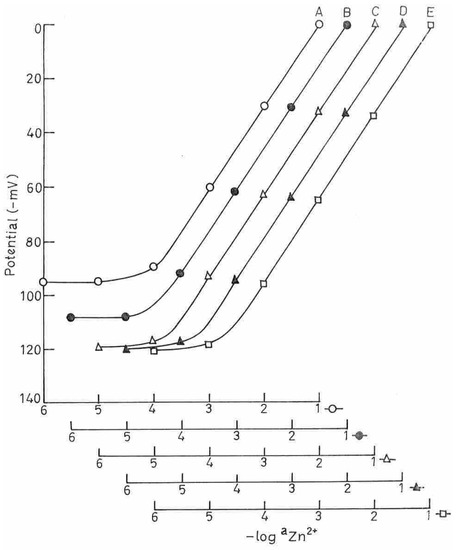
Fig.2.
Variation of potential of PVC based membranes with varying amount of ionophore (A-8.0, B-9.5, C-10.0, D-11.0, E-12.0 (% w/w)
pH Dependence and Non-aqueous Effect
One of the ions present in aqueous solution is the hydrogen ion. It interferes, in many instances, with the functioning. In view of this, it is necessary to find the optimum pH range where the electrode without interference from the hydrogen ions. pH dependence of the membrane electrode works has been tested by using 1.0 ×10-2 and 1.0 ×10-3 M Zn2+ solutions over a pH range 1 to 8 (Fig.3). pH was adjusted by the addition of small drops of hydrochloric acid (0.1 M) and/or sodium hydroxide (0.1 M). As could be seen from fig.3, the potentials stay constant from pH 3.0 to 7.4. Beyond this pH, a drift in potential is observed which is due to the formation of some hydroxy complexes of Zn2+ at higher pH while at lower pH, there could be protonation of (I) in the membrane which results in a loss of their complexing ability with the metal ion. Thus the working pH range of the proposed assembly may be taken from 3.0 -7.4.
The functioning of the sensor was also investigated in partially medium using methanol-water, ethanol-water and acetone-water mixture (Table-3). It was found that the sensor assembly works well in non-aqueous media having a 40% (v/v) alcoholic content without showing any appreciable change in the working concentration range or slope. However, above 40% potentials show drift with time. It is worth mentioning that the lifetime of the membrane did not alter in non-aqueous solutions.
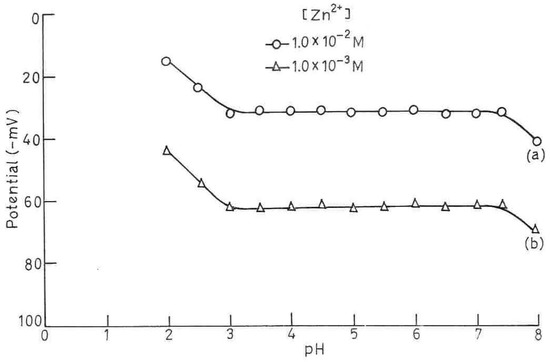
Figure 3.
Effect of pH on potential; [Zn2+]= 1.0 × 10-2 M (a) and 1.0 × 10-3 M (b) for sensor no.2.

Table 3.
Effect of partially non-aqueous medium on the working of Zn2+ sensor (Electrode no. 2)
| Non- aqueous Content (% v/v) | Slope (mV/decade of activity) | Working Concentration Range (M) |
| 0 | 30.0 | 1.3 × 10-5 to 1.0 × 10-1 |
| Methanol | ||
| 30 | 30.2 | 1.3 × 10-5 to 1.0 × 10-1 |
| 40 | 29.3 | 1.5 × 10-5 to 1.0 × 10-1 |
| 45 | 25.2 | 2.5 × 10-4 to 1.0 × 10-1 |
| Ethanol | ||
| 30 | 29.6 | 1.5 × 10-5 to 1.0 × 10-1 |
| 40 | 29.0 | 1.6 × 10-5 to 1.0 × 10-1 |
| 45 | 24.5 | 3.6 × 10-4 to 1.0 × 10-1 |
| Acetone | ||
| 30 | 30.2 | 1.3 × 10-5 to 1.0 × 10-1 |
| 40 | 29.5 | 1.4 × 10-5 to 1.0 × 10-1 |
| 45 | 24.6 | 1.6 × 10-4 to 1.0 × 10-1 |
Potentiometric Selectivity
The selectivity coefficient values for the proposed sensor were evaluated by the modified form of the Fixed Interference Method [18] as well as by the Matched Potential Method [19] at 1.0 × 10-2 M concentration of interfering ions (B). The selectivity coefficient values (Table-4) clearly indicate that the electrode is moderatly selective to Zn2+ over a number of cations (except for Na+ and Cd2+). As the selectivity coefficient values are higher for Na+ and Cd2+, they are likely to cause some interference, but these ions may also not cause any interference at low concentrations. To determine the maximum interfering concentration level of these two metal ions in the estimation of Zn2+, some mixed run studies were carried out. It can be seen from Fig.4 that Na+ at concentration ≤1.0 × 10-5 M does not cause any deviation in the original plot of pure Zn2+ solution. Thus, electrode can tolerate Na+ up to concentration ≤ 1.0 × 10-5 M over the entire working concentration range. If Na+ concentration exceeds this limit, the sensor can be used to determine Zn2+ in the reduced concentration ranges of 5.6 × 10-5-1.0 × 10-1 and 3.1 × 10-4-1.0 × 10-1 in the presence of Na+ at 1.0 × 10-4 and 1.0 × 10-3 M respectively. Similarly Cd2+ can be tolerated up to concentration ≤ 5.0 × 10-5 M over the whole working concentration ranges of 6.3 × 10-5- 1.0 × 10-1 and 5.0 × 10-4-1.0 × 10-1 M in the presence of 1.0 × 10-4 and 1.0 × 10-3 M Cd2+, respectively (Fig.5).
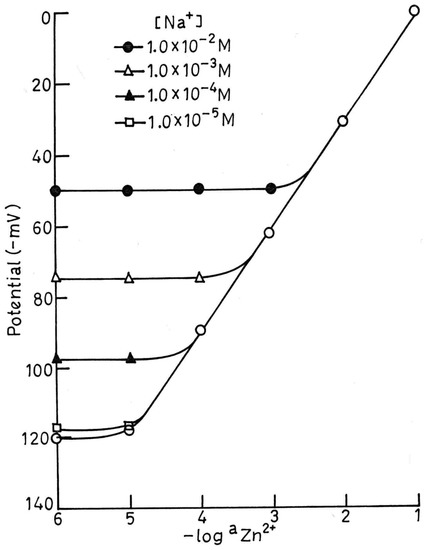
Fig.4.
Variation of potential with Zn2+ concentration in presence of different concentration of Na+.
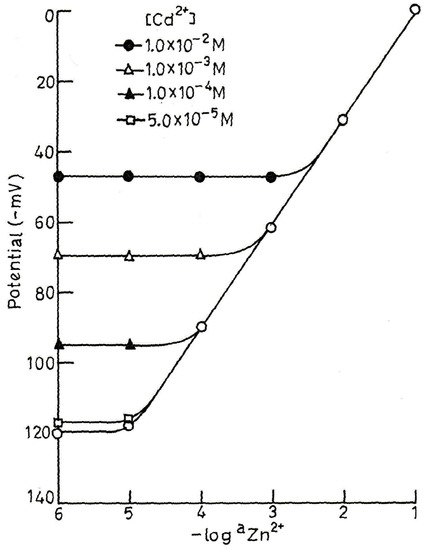
Fig.5.
Variation of potential with Zn2+ concentration in presence of different concentration of Cd2+.
Analytical Applications
The analytical applicability of the sensor was tested by using it as an indicator electrode in the potentiometric titration of Zn2+ solution. 10 ml of 1.0 × 10-3 M Zn2+ solution was titrated against 1.0 × 10-2 M EDTA solution. The curve is not a standard sigmoid shaped (Fig.6) which may be due to Na+ interference as disodium salt of EDTA was used. However, a very good inflection point, corresponds to Zn-EDTA stoichiometry is observed which shows that this sensor can be used for the determination of Zn2+ potentiometrically.
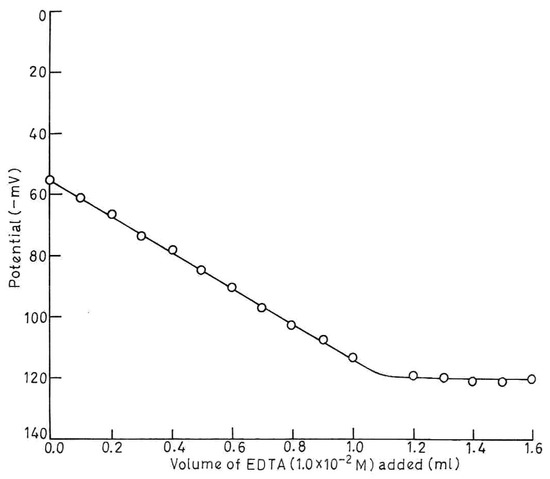
Fig.6.
Potentometric titration of Zn2+ ions (1.0 × 10-3 M, 10 ml) with EDTA (1.0 × 10-2 M).

Table 4.
Potentiometric selectivity coefficient values , in the presence of 1.0 ×10-2 mol dm-3 concentration of interfering ions (B), observed for Zn2+ (sensor no. 2) using Fixed Interference and Matched Potential Method
| Interfering ions (B) | Selectivity Coefficient | ||
| Fixed Interference Method | Matched Potential Method | ||
| With exponent | Without exponent | ||
| Li+ | 31.6 × 10-1 | 3.1 × 10-2 | 0.17 |
| Na+ | 25.1 | 2.5 × 10-1 | 0.65 |
| K+ | 25.1 × 10-1 | 2.5 × 10-2 | 0.20 |
| NH4+ | 31.6 × 10-1 | 3.1 × 10-2 | 0.16 |
| Cs+ | 28.1 × 10-1 | 2.8 × 10-2 | 0.18 |
| Tl+ | 17.7 × 10-1 | 1.7 × 10-2 | 0.10 |
| Ag+ | 44.6 × 10-1 | 4.4 × 10-2 | 0.20 |
| Ca2+ | 1.2 × 10-2 | 1.2 × 10-2 | 0.13 |
| Sr2+ | 6.3 × 10-2 | 6.3 × 10-2 | 0.25 |
| Cu2+ | 5.9 × 10-2 | 5.9 × 10-2 | 0.22 |
| Ni2+ | 5.6 × 10-2 | 5.6 × 10-2 | 0.20 |
| Cd2+ | 3.1 × 10-1 | 3.1 × 10-1 | 0.60 |
| Hg2+ | 6.3 × 10-2 | 6.3 × 10-2 | 0.24 |
| Co2+ | 4.4 × 10-2 | 4.4 × 10-2 | 0.20 |
| Pb2+ | 7.0 × 10-2 | 7.0 × 10-2 | 0.25 |
| Fe3+ | 1.7 × 10-2 | 7.9 × 10-2 | 0.25 |
| Al3+ | 1.9 × 10-3 | 8.9 × 10-2 | 0.31 |
| Cr3+ | 1.9 × 10-3 | 8.9 × 10-2 | 0.28 |
Conclusion
The plasticized PVC-based membrane incorporating disodium salt of 3,7,12,17-Tetramethyl-8,13-divinyl-2,18-porphine-dipropionic acid as an ionophore, DBBP as solvent mediator and NaTPB as anion excluder in a PVC matrix in the ratio of 10:150:2:200 (w/w) (Ionophore:PVC:NaTPB:DBBP) could be used to determine Zn2+ in the concentration range 1.3 × 10-5 to 1.0 × 10-1 M with a slope of 30.0 mV/decade of activity. The sensor works in a wide pH range 3.0-7.4 with a response time of 10 seconds. The selectivity of the electrode towards Zn2+ is quite good over other cations and the lifetime of the assembly is more than 2 months in aqueous and non-aqueous medium also. In addition, the membrane sensor can be used as an indicator electrode in potentiometric titration involving Zinc (II) ions against EDTA.
Acknowledgements
The authors are highly thankful to Department of Science & Technology, Govt. of India, New Delhi, and DAAD (German Academic Exchange Program) for providing funds to undertake the work.
References
- Moore, J.W.; Ramamoorthy, S. Heavy Metals in Natural Waters: Applied Monitoring and Impact Assessment; Springer: New York, 1984; pp. 182–204. [Google Scholar]
- Gorton, L.; Fiedler, U. Zinc Sensitive Polymeric Membrane Electrode. Anal. Chim. Acta. 1977, 90, 233–236. [Google Scholar] [CrossRef]
- Linnersund, U.F.; Bhatti, K.M. Development of Polymeric Membranes for the Zinc-Ion Selective Electrodes. Anal. Chim. Acta. 1979, 111, 57–70. [Google Scholar] [CrossRef]
- Lebedeva, O.A.; Jansons, E. Cd and Zn-Selective Liquid Electrodes on the Basis of Quinoline-8-Carbodithioates. Latv. PSR Zinat. Akad. Vestis. Kim. Ser. 1987, 4, 483–485. [Google Scholar]
- Kojima, R.; Kamata, S. Zinc Selective Membrane Electrode Using Tetrabutyl Thiuram Disulfide Neutral Carrier. Anal. Sci. 1994, 10, 409–412. [Google Scholar] [CrossRef]
- Rocheleaw, M.J.; Purdy, W.C. Investigation of Materials for Making a Carbon Support Zinc-Selective Electrode. Talanta. 1990, 37, 307–311. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Vardhan, H.; Singh, M.; Rao, G.N.; Srivastava, S. A New Chelating Ion-Exchange Resin Based Sensor for Zinc Ions. Anal. Proc. 1995, 32, 173–178. [Google Scholar] [CrossRef]
- Obmetho, A.A.; Rakhmanko, E.M.; Lomako, V.L.; Starvobinets, G.L. Determination of Zinc in Alloys by a Ion-Selective Electrode. Zh. Anal. Khim. 1988, 43, 444–452. [Google Scholar]
- Srivastava, S.K.; Gupta, V.K.; Jain, S. PVC-based 2,2,2-Cryptand Sensor for Zinc Ions. Anal. Chem. 1996, 68, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Jain, A.K.; Singh, L.P.; Khurana, U. Zn2+ Sensor Based on Zn-bis(2,4,4-trimethylpentyl) dithiophosphinic acid Complex in PVC Matrix. Electrochim. Acta. 1998, 43, 2047–2052. [Google Scholar] [CrossRef]
- Gupta, V.K. A PVC based 12-Crown-4 Membrane Potentiometric Sensor for Zinc(II) Ions. Sens. Actuators B. 1999, 55, 195–200. [Google Scholar] [CrossRef]
- Shamsipur, M.; Rouhani, S.; Ganjali, M.R.; Sharghi, H.; Eshghi, H. Zinc Selective Membrane Potentiometric Sensor Based on a Recently Synthesized Benzosubstituted Macrocyclic Diamide. Sens. Actuators B. 1999, 59, 30–34. [Google Scholar] [CrossRef]
- Fakhari, A.R.; Alaghemand, M.; Shamsipur, M. Zinc-Selective Membrane Electrode Based on 5,6,14,15-dibenzo-1,4-dioxa-8,12-diazacyclopentadecane-5,14-diene. Anal. Lett. 2001, 34, 2169–2174. [Google Scholar]
- Gupta, V.K.; Chandra, S.; Chauhan, D.K.; Mangla, R. Membranes of 5,10,15,20-Tetrakis(4- Methoxyphenyl Porphyrinatocobalt (TMOPP-Co) (I) as MoO42- Selective Sensors. Sensors. 2002, 2, 164–174. [Google Scholar] [CrossRef]
- Craggs, A.; Moody, G.J.; Thomas, J.D.R. Procedure for the Construction of All Solid-State PVC Membrane Electrode. J. chem. Educ. 1974, 51, 541–546. [Google Scholar] [CrossRef]
- Bakker, E.; Buhlman, P.; Pretsch, E. Carrier-Based Ion Selective Electrodes and Bulk Optodes.1.General Characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [PubMed]
- Christon, G. D. Analytical Chemistry; John Wiley Pte. Ltd.: Singapore, 1994; Chapter 4; p. 331. [Google Scholar]
- Sa’ez de Viteri, F.J.; Diamond, D. Determination and Application of Ion Selective Electrode Model Parameters Using Flow Injuction and Simplex Optimization. Analyst. 1994, 119, 749–758. [Google Scholar] [CrossRef]
- Gadzekpo, V.P.Y.; Christian, G.D. Determination of Selectivity Coefficients of Ions Selective Electrodes by a Matched Potential Method. Anal. Chim. Acta. 1984, 164, 279–282. [Google Scholar] [CrossRef]
- Sample Availability: Available from the authors.
© 2003 by MDPI (http://www.mdpi.net). Reproduction is permitted for noncommercial purposes.