Spontaneous Cell Detachment from Temperature Gradients: Getting the Method Ready for Antimicrobial Drug Testing at Cell Culture Level
Abstract
1. Introduction
2. Materials and Methods
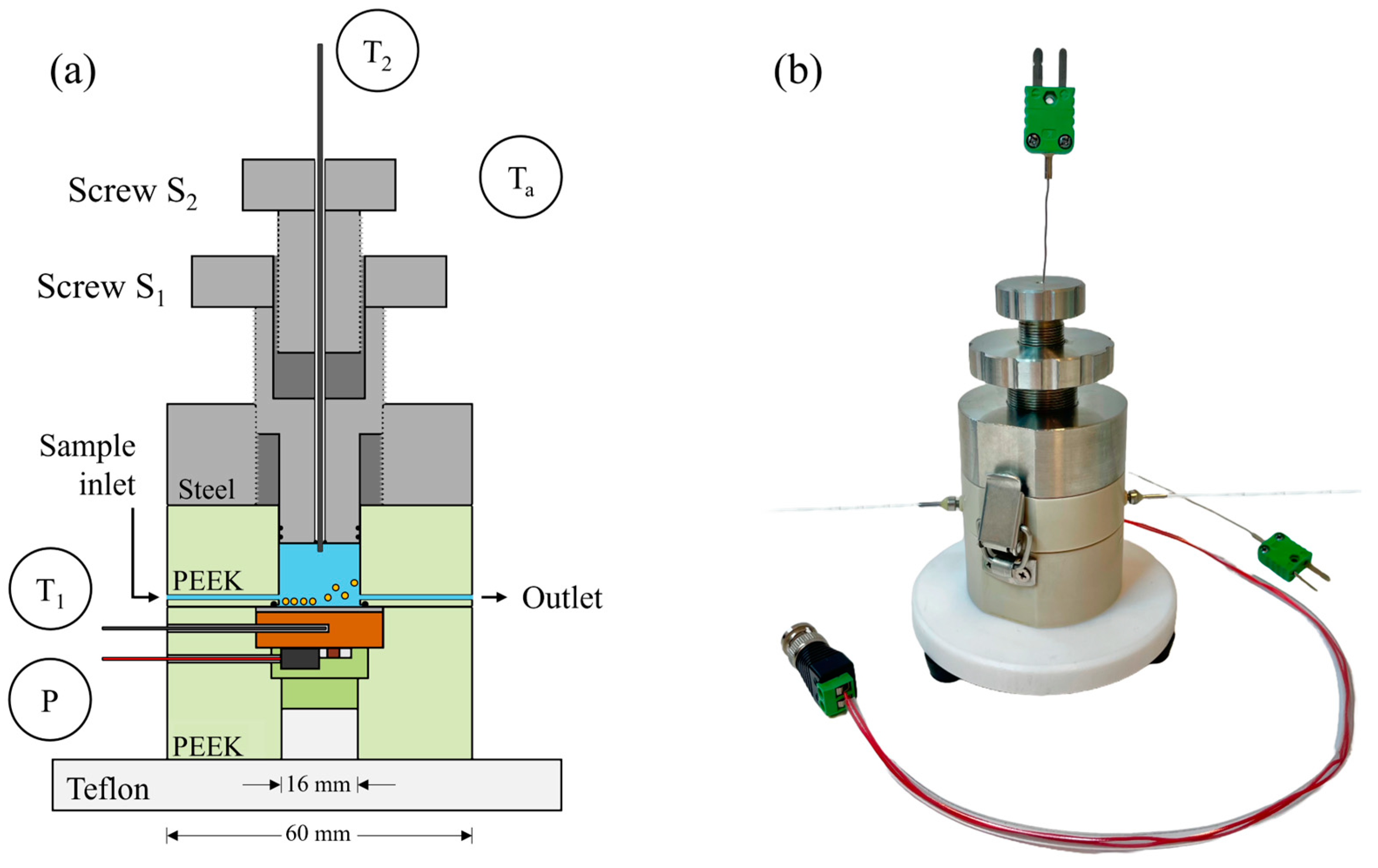
2.1. HTM Sensing Device with a Variable Aspect Ratio
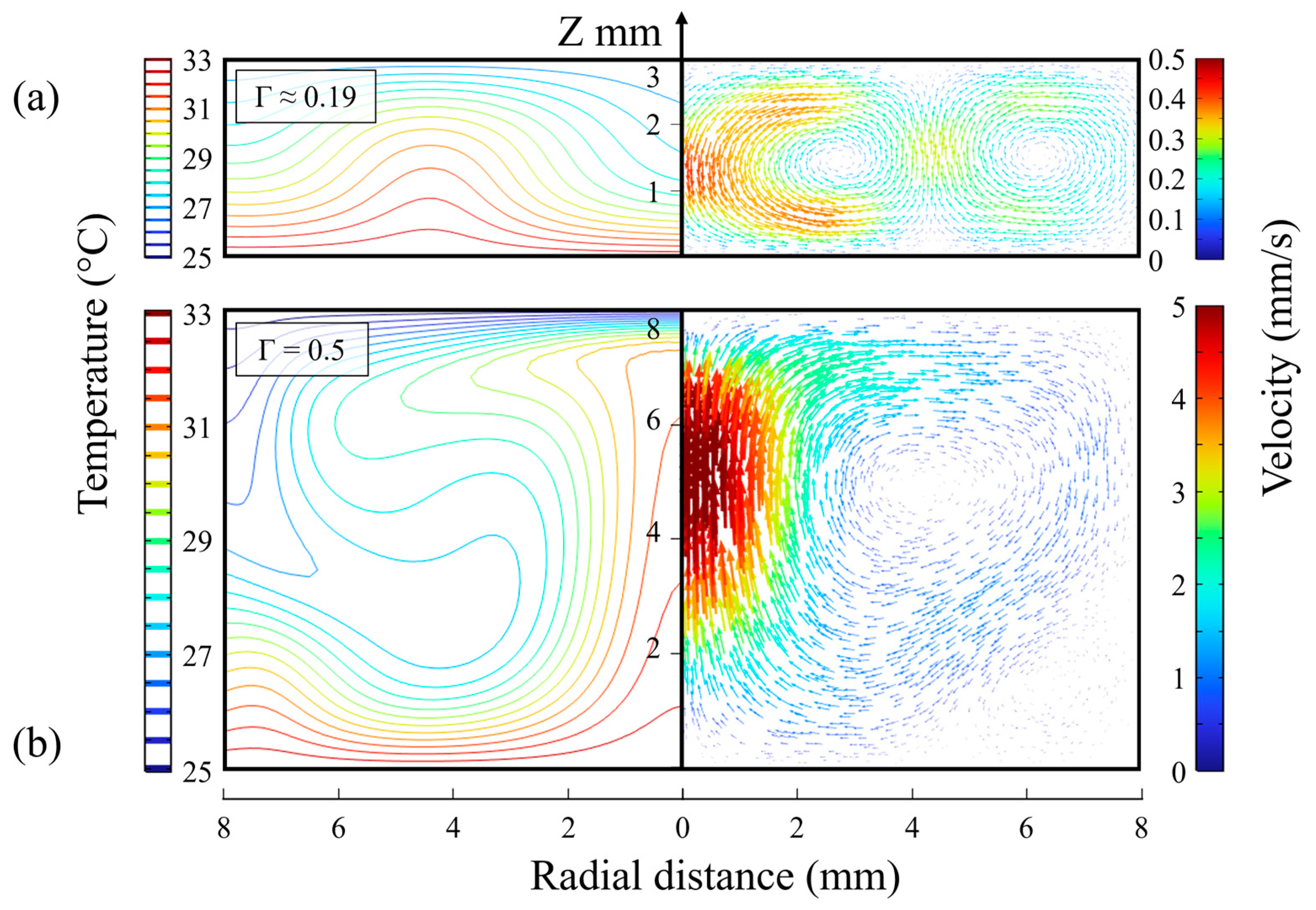
2.2. Heat and Mass Transfer Simulations with COMSOL
2.3. Spontaneous Cell-Detachment Measurements with S. cerevisiae
2.4. Drug Exposure Measurements with Amphotericin B and Povidone-Iodine
2.5. Reference Methods
3. Results
3.1. Thermal Resistance of the Device for Different Aspect Ratios
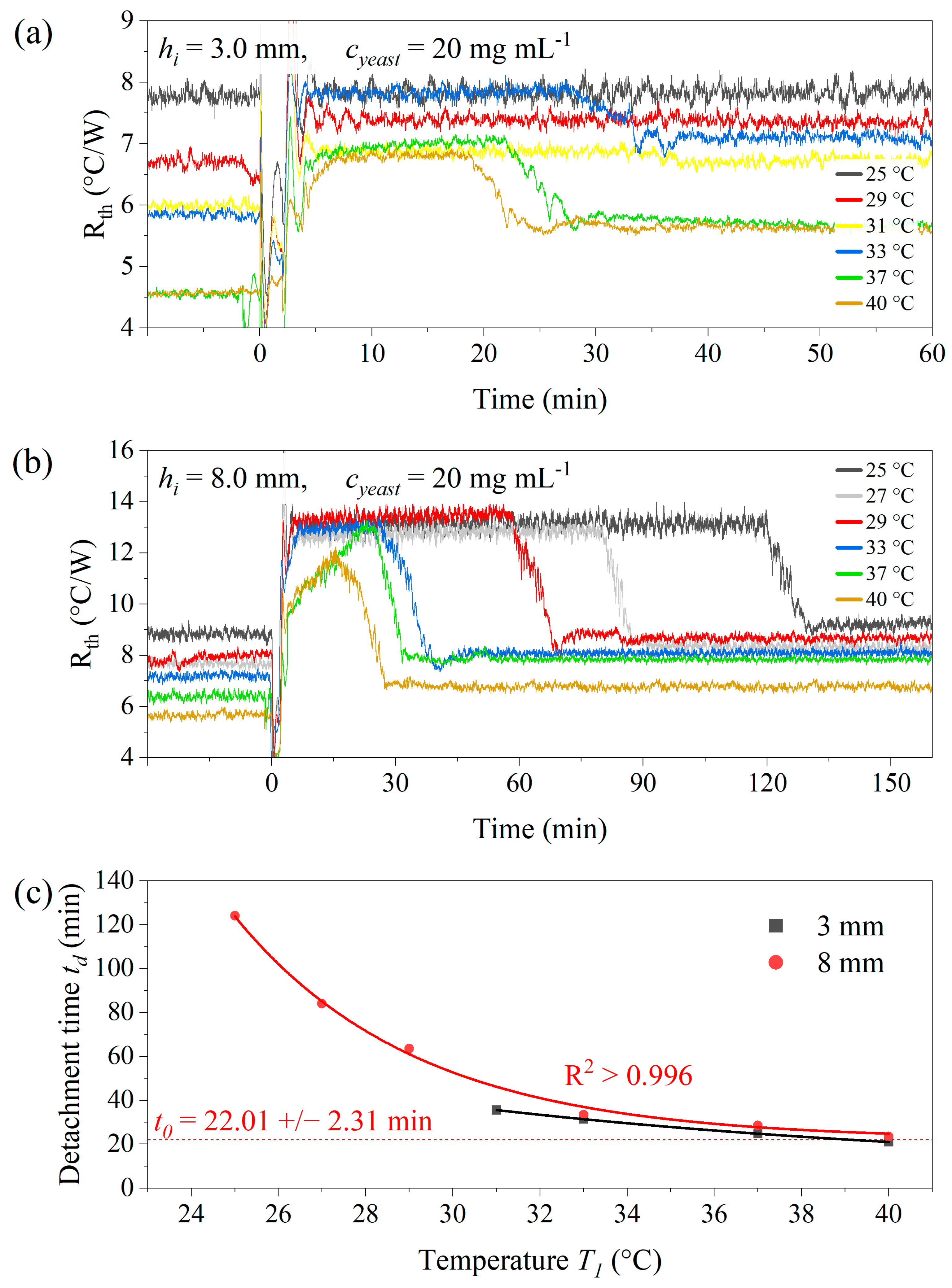
3.2. Temperature-Dependence of the Detachment Time of Yeast Cells
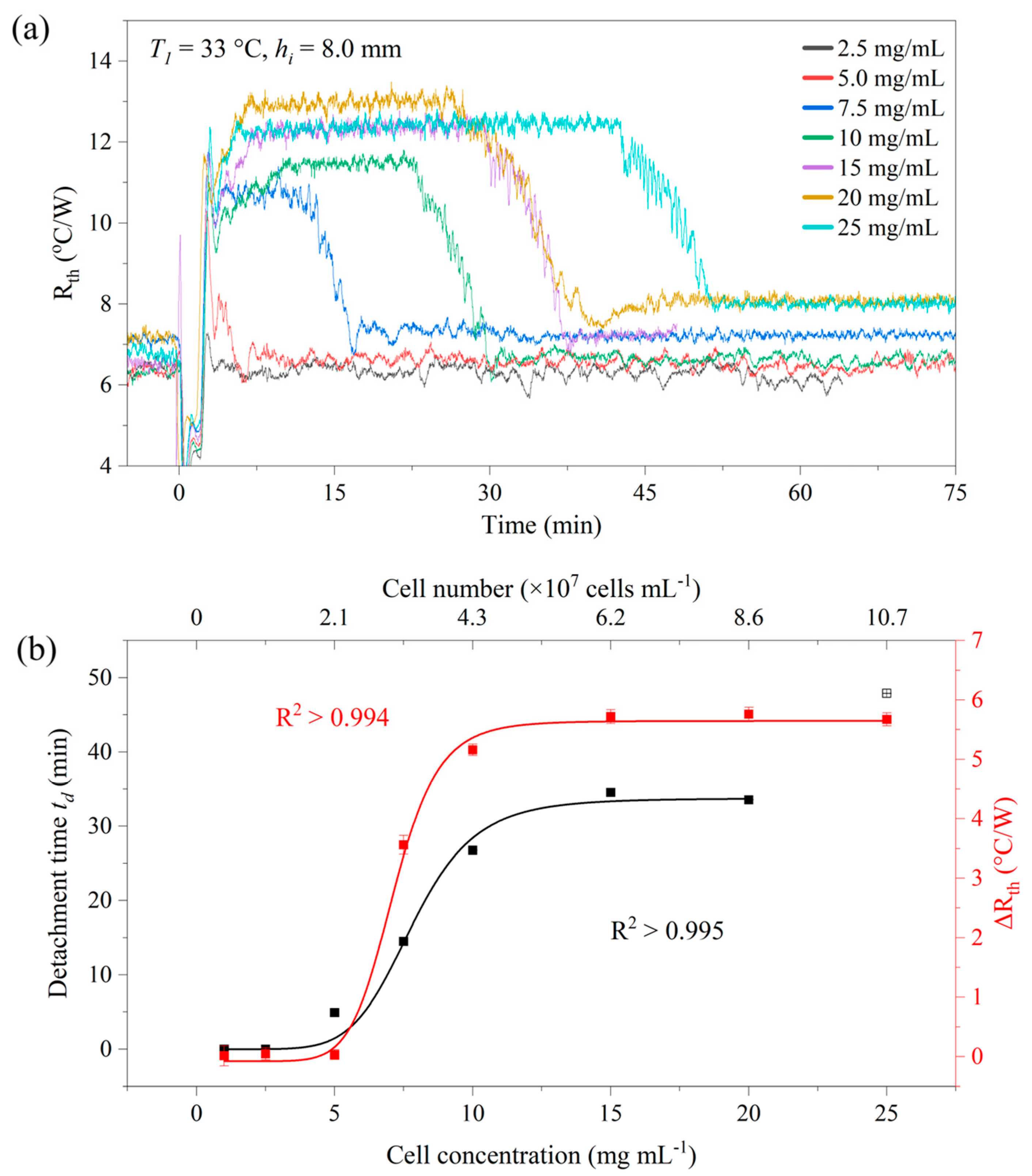
3.3. Detachment Effect with Different Cell Concentrations
3.4. Detachment Effect with Different Aspect Ratios
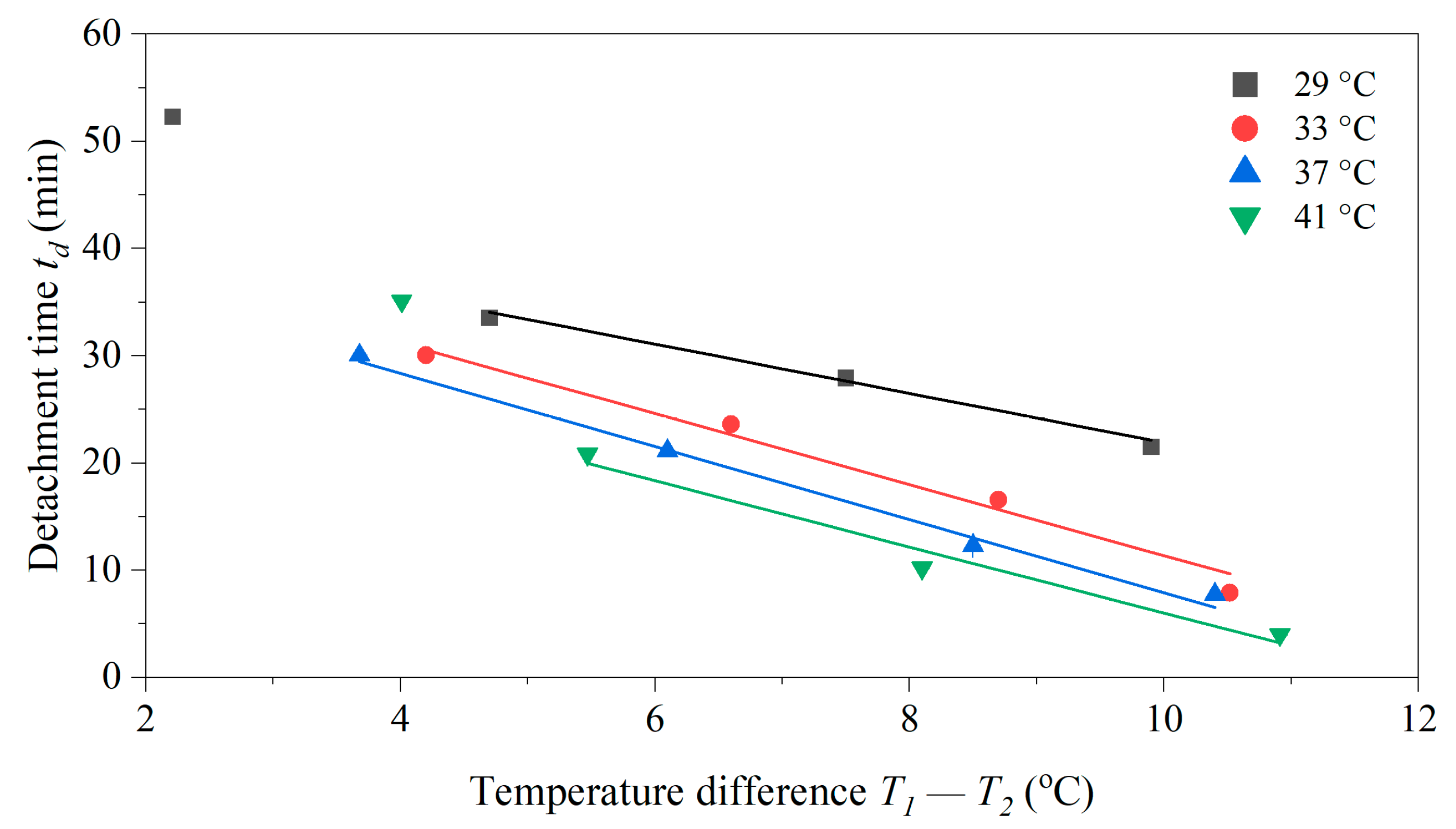
3.5. Role of the Temperature Difference T1 − T2 for the Dwell Time
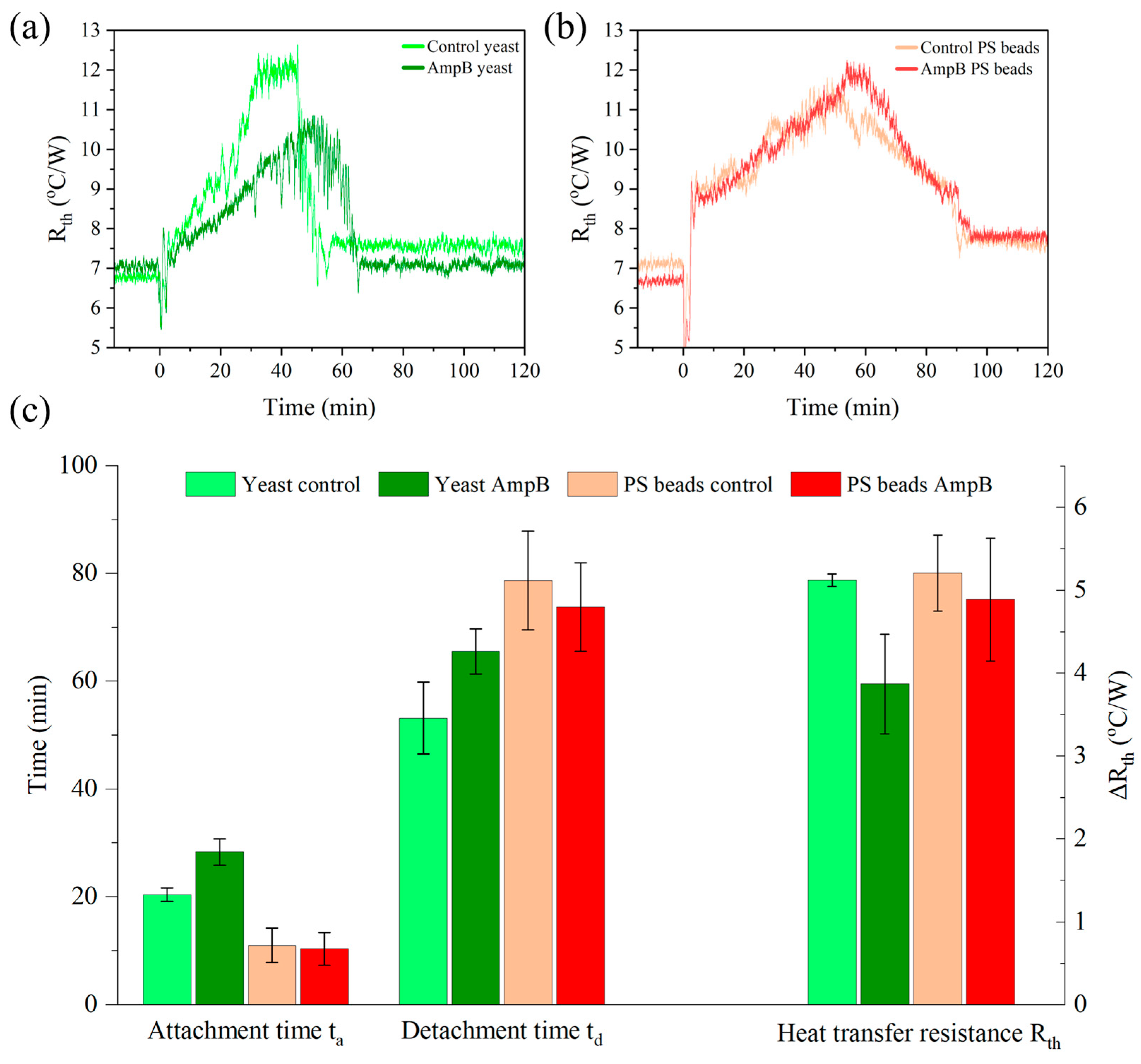
3.6. Detachment Effect in the Presence of the Antimicrobial Amphotericin B
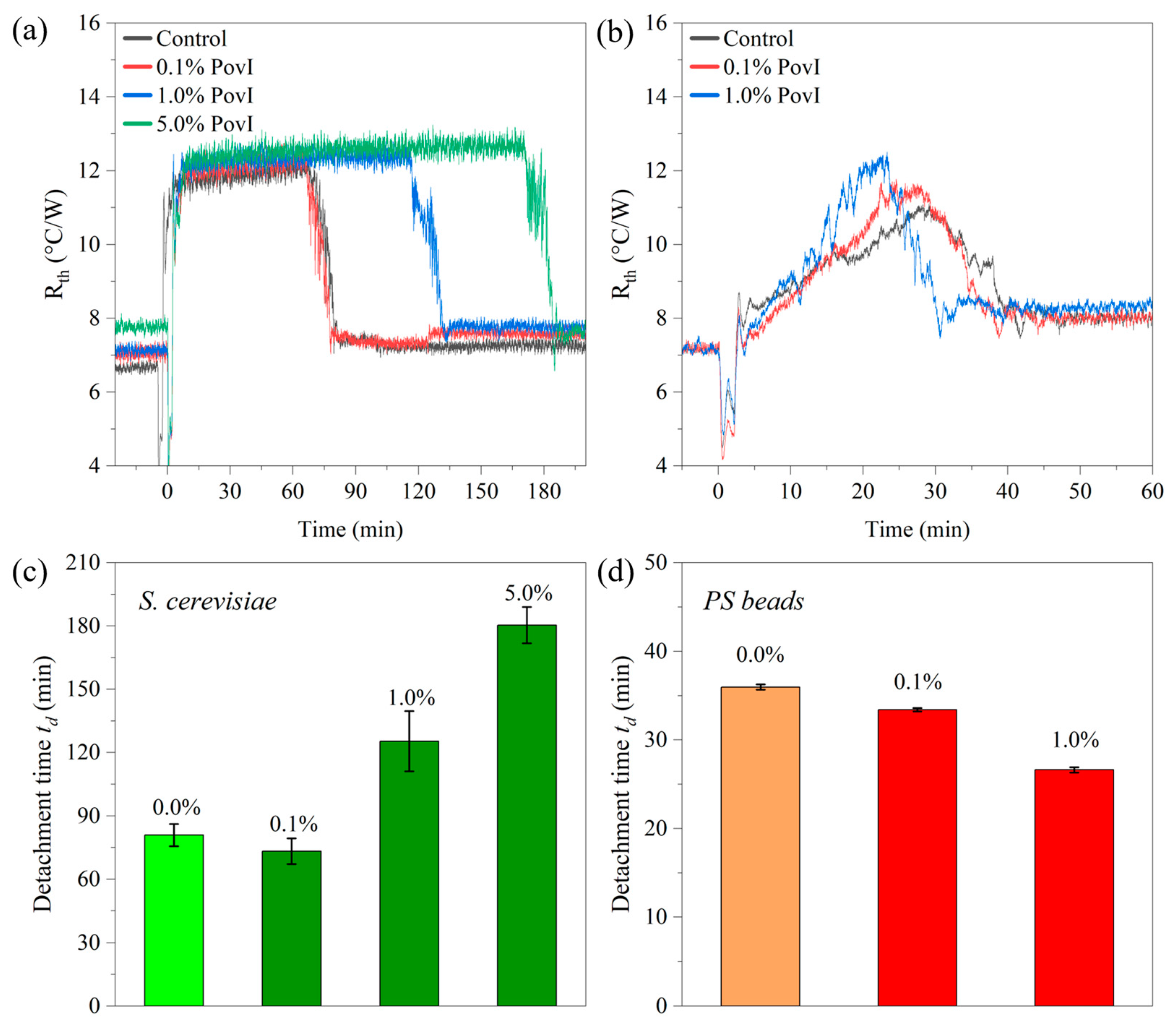
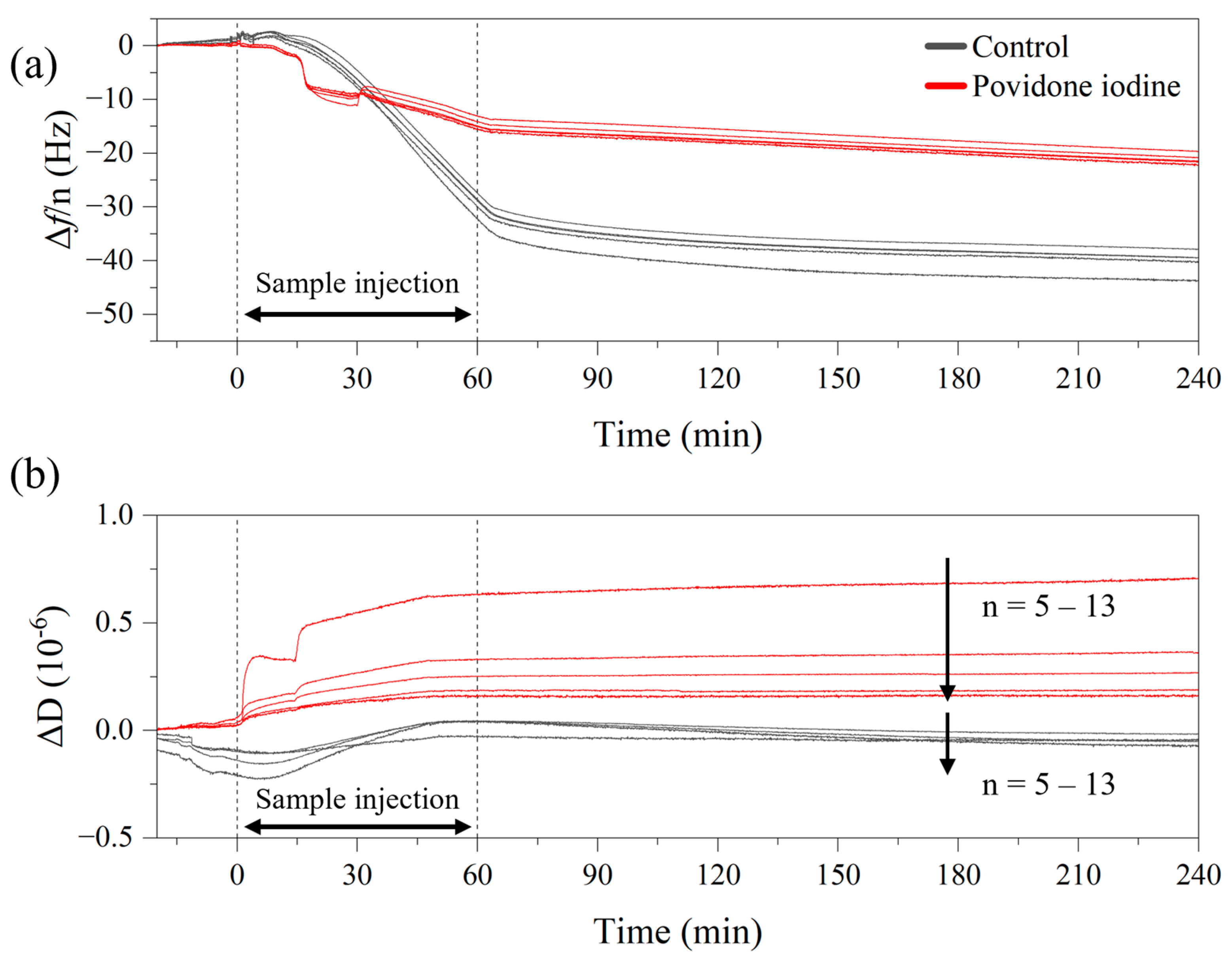
3.7. Detachment Effect in the Presence of the Antiseptic Polyvidone Iodine
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.; Wang, J. Optical Biosensors: An Exhaustive and Comprehensive Review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef] [PubMed]
- Poghossian, A.; Schöning, M.J. Recent Progress in Silicon-Based Biologically Sensitive Field-Effect Devices. Curr. Opin. Electrochem. 2021, 29, 100811. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Özgür, E.; Denizli, A. Recent Advances in Quartz Crystal Microbalance Biosensors Based on the Molecular Imprinting Technique for Disease-Related Biomarkers. Chemosensors 2022, 10, 106. [Google Scholar] [CrossRef]
- Chen, J.; Xu, X.; Zhou, J.; Li, B. Interfacial Thermal Resistance: Past, Present, and Future. Rev. Mod. Phys. 2022, 94, 25002. [Google Scholar] [CrossRef]
- van Grinsven, B.; Vanden Bon, N.; Strauven, H.; Grieten, L.; Murib, M.; Jiménez Monroy, K.L.; Janssens, S.D.; Haenen, K.; Schöning, M.J.; Vermeeren, V.; et al. Heat-Transfer Resistance at Solid–Liquid Interfaces: A Tool for the Detection of Single-Nucleotide Polymorphisms in DNA. ACS Nano 2012, 6, 2712–2721. [Google Scholar] [CrossRef]
- Ahmadi Tabar, F.; Lowdon, J.W.; Caldara, M.; Cleij, T.J.; Wagner, P.; Diliën, H.; Eersels, K.; van Grinsven, B. Thermal Determination of Perfluoroalkyl Substances in Environmental Samples Employing a Molecularly Imprinted Polyacrylamide as a Receptor Layer. Environ. Technol. Innov. 2023, 29, 103021. [Google Scholar] [CrossRef]
- Cornelis, P.; Givanoudi, S.; Yongabi, D.; Iken, H.; Duwé, S.; Deschaume, O.; Robbens, J.; Dedecker, P.; Bartic, C.; Wübbenhorst, M.; et al. Sensitive and Specific Detection of E. coli Using Biomimetic Receptors in Combination with a Modified Heat-Transfer Method. Biosens. Bioelectron. 2019, 136, 97–105. [Google Scholar] [CrossRef]
- Givanoudi, S.; Cornelis, P.; Rasschaert, G.; Wackers, G.; Iken, H.; Rolka, D.; Yongabi, D.; Robbens, J.; Schöning, M.J.; Heyndrickx, M.; et al. Selective Campylobacter Detection and Quantification in Poultry: A Sensor Tool for Detecting the Cause of a Common Zoonosis at its Source. Sens. Actuators B Chem. 2021, 332, 129484. [Google Scholar] [CrossRef]
- Steen Redeker, E.; Eersels, K.; Akkermans, O.; Royakkers, J.; Dyson, S.; Nurekeyeva, K.; Ferrando, B.; Cornelis, P.; Peeters, M.; Wagner, P.; et al. Biomimetic Bacterial Identification Platform Based on Thermal Wave Transport Analysis (TWTA) Through Surface-imprinted Polymers. ACS Infect. Dis. 2017, 3, 388–397. [Google Scholar] [CrossRef]
- Arreguin-Campos, R.; Medeiros Brito, R.M.; Porto, A.R.A.; de Jesus, A.C.P.; Bueno, L.L.; Fujiwara, R.T.; Diliën, H.; Cleij, T.J.; Eersels, K.; van Grinsven, B. Whole-Cell Thermal Sensor for the Detection of P. falciparum-Infected Erythrocytes: Imprinted Polymers as Synthetic Receptors for the Detection of Malaria. ACS Sens. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Singla, P.; Dann, A.J.; McClements, J.; Mark, V.; Sullivan, M.V.; Kim, M.; Stoufer, S.; Dawson, J.A.; Crapnell, R.D.; et al. Sensitive Electrochemical and Thermal Detection of Human Noroviruses Using Molecularly Imprinted Polymer Nanoparticles Generated against a Viral Target. ACS Appl. Mater. Interfaces 2024, 16, 51397–51410. [Google Scholar] [CrossRef] [PubMed]
- McClements, J.; Bar, L.; Singla, P.; Canfarotta, F.; Thomson, A.; Czulak, J.; Johnson, R.E.; Crapnell, R.D.; Banks, C.E.; Payne, B.; et al. Molecularly Imprinted Polymer Nanoparticles Enable Rapid, Reliable, and Robust Point-of-Care Thermal Detection of SARS-CoV-2. ACS Sens. 2022, 7, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.; Bakhshi Sichani, S.; Khorshid, M.; Lieberzeit, P.; Losada-Pérez, P.; Yongabi, D. Bioanalytical Sensors Using the Heat-Transfer Method HTM and Related Techniques. TM Tech. Mess. 2023, 90, 761–785. [Google Scholar] [CrossRef]
- Yongabi, D.; Khorshid, M.; Losada-Pérez, P.; Bakhshi Sichani, S.; Jooken, S.; Stilman, W.; Theßeling, F.; Martens, T.; Van Thillo, T.; Verstrepen, K.; et al. Synchronized, Spontaneous, and Oscillatory Detachment of Eukaryotic Cells: A New Tool for Cell Characterization and Identification. Adv. Sci. 2022, 9, 2200459. [Google Scholar] [CrossRef]
- Vargas-Accarino, E.; Herrera-Montávez, C.; Ramón y Cajal, S.; Aasen, T. Spontaneous Cell Detachment and Reattachment in Cancer Cell Lines: An In Vitro Model of Metastasis and Malignancy. Int. J. Mol. Sci. 2021, 22, 4929. [Google Scholar] [CrossRef]
- Lai, T.; Cao, J.; Ou-Yang, P.; Tsai, C.; Lin, C.; Chen, C.; Tsai, M.; Lee, C. Different methods of detaching adherent cells and their effects on the cell surface expression of Fas receptor and Fas ligand. Sci. Rep. 2022, 12, 5713. [Google Scholar] [CrossRef]
- Bakhshi Sichani, S.; Khorshid, M.; Yongabi, D.; Urbán, C.T.; Schreurs, M.; Verstrepen, K.J.; Lettinga, M.P.; Schöning, M.J.; Lieberzeit, P.; Wagner, P. Design of a Multiparametric Biosensing Platform and Its Validation in a Study on Spontaneous Cell Detachment from Temperature Gradients. ACS Sens. 2024, 9, 3967–3978. [Google Scholar] [CrossRef]
- Lee, N.; Wiegand, S. Thermal Design of a Non-Isothermal Microfluidic Channel for Measuring Thermophoresis. Int. J. Heat Mass Transf. 2024, 231, 125871. [Google Scholar] [CrossRef]
- Helden, L.; Eichhorn, R.; Bechinger, C. Direct Measurement of Thermophoretic Forces. Soft Matter 2015, 11, 2379–2386. [Google Scholar] [CrossRef]
- Kollipara, P.S.; Li, X.; Li, J.; Chen, Z.; Ding, H.; Kim, Y.; Huang, S.; Qin, Z.; Zheng, Y. Hypothermal Opto-Thermophoretic Tweezers. Nat. Commun. 2023, 14, 5133. [Google Scholar] [CrossRef] [PubMed]
- Oyama, K.; Arai, T.; Isaka, A.; Sekiguchi, T.; Itoh, H.; Seto, Y.; Miyazaki, M.; Itabashi, T.; Ohki, T.; Suzuki, M.; et al. Directional Bleb Formation in Spherical Cells under Temperature Gradient. Biophys. J. 2015, 109, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Kogure, A.; Fujii, T.; Kokawa, R.; Deuchi, K.; Kuwana, R.; Takamatsu, H. With Respect to Coefficient of Linear Thermal Expansion, Bacterial Vegetative Cells and Spores Resemble Plastics and Metals, Respectively. J. Nanobiotechnol. 2013, 11, 33. [Google Scholar] [CrossRef]
- Vasala, A.; Hytönen, V.P.; Laitinen, O.H. Modern Tools for Rapid Diagnostics of Antimicrobial Resistance. Front. Cell Infect. Microbiol. 2020, 10, 308. [Google Scholar] [CrossRef]
- Kovács, M.; Tóth, J.; Hetényi, C.; Málnási-Csizmadia, A.; Sellers, J.R. Mechanism of Blebbistatin Inhibition of Myosin II. J. Biol. Chem. 2004, 279, 35557–35563. [Google Scholar] [CrossRef]
- Verheijen, M.; Lienhard, M.; Schrooders, Y.; Clayton, O.; Nudischer, R.; Boerno, S.; Timmermann, B.; Selevsek, N.; Schlapbach, R.; Gmuender, H.; et al. DMSO Induces Drastic Changes in Human Cellular Processes and Epigenetic Landscape In Vitro. Sci. Rep. 2019, 9, 4641. [Google Scholar] [CrossRef]
- Hauser, M.J.B. Synchronisation of Glycolytic Activity in Yeast Cells. Curr. Genet. 2022, 68, 69–81. [Google Scholar] [CrossRef]
- Becker, H.; Heim, U. Hot Embossing as a Method for the Fabrication of Polymer High Aspect Ratio Structures. Sens. Actuators A Phys. 2000, 83, 130–135. [Google Scholar] [CrossRef]
- Connolly, S.; Newport, D.; McGourty, K. The Mechanical Responses of Advecting Cells in Confined Flow. Biomicrofluidics 2020, 14, 31501. [Google Scholar] [CrossRef]
- Li, L.; Pan, Y.; Zhang, S.; Yang, T.; Li, Z.; Wang, B.; Sun, H.; Zhang, M.; Li, X. Quorum Sensing: Cell-to-Cell Communication in Saccharomyces cerevisiae. Front. Microbiol. 2023, 14, 1250151. [Google Scholar] [CrossRef]
- Grela, E.; Zdybicka-Barabas, A.; Pawlikowska-Pawlega, B.; Cytrynska, M.; Wlodarczyk, M.; Grudzinski, W.; Luchowski, R.; Gruszecki, W.I. Modes of the Antibiotic Activity of Amphotericin B Against Candida albicans. Sci. Rep. 2019, 9, 17029. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.C.; Palacios, D.S.; Dailey, I.; Endo, M.M.; Uno, B.E.; Wilcock, B.C.; Burke, M.D. Amphotericin Primarily Kills Yeast by Simply Binding Ergosterol. Proc. Natl. Acad. Sci. USA 2012, 109, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and Other Polyenes—Discovery, Clinical Use, Mode of Action and Drug Resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef]
- Enache-Angoulvant, A.; Hennequin, C. Invasive Saccharomyces Infection: A Comprehensive Review. Clin. Infect. Dis. 2005, 41, 1559–1568. [Google Scholar] [CrossRef]
- Chesnay, A.; Bailly, É.; Desoubeaux, G. Demonstration of the Yeasticidal Efficacy of Povidone-Iodine–Based Commercial Antiseptic Solutions Against Candida auris. J. Med. Mycol. 2021, 31, 101173. [Google Scholar] [CrossRef]
- Lepelletier, D.; Maillard, J.Y.; Pozzetto, B.; Simon, A. Povidone Iodine: Properties, Mechanisms of Action, and Role in Infection Control and Staphylococcus aureus Decolonization. Antimicrob. Agents Chemother. 2020, 64, e00682-20. [Google Scholar] [CrossRef]
- Sauerbrei, A. Bactericidal and Virucidal Activity of Ethanol and Povidone-Iodine. MicrobiologyOpen 2020, 9, e1097. [Google Scholar] [CrossRef]
- Cornelis, P.; Wackers, G.; Thomas, I.; Brand, M.; Putzeys, T.; Gennaro, A.; Wübbenhorst, M.; Ingebrandt, S.; Wagner, P. A Novel Modular Device for Biological Impedance Measurements: The Differential Impedimetric Sensor Cell (DISC). Phys. Status Solidi A 2018, 215, 1701029. [Google Scholar] [CrossRef]
- Webpage Comsol Multiphysics Modelling. Available online: https://www.comsol.com/multiphysics/fluid-flow-heat-transfer-and-mass-transport (accessed on 9 October 2024).
- Stilman, W.; Jooken, S.; Wackers, G.; Cornelis, P.; Khorshid, M.; Yongabi, D.; Akkermans, O.; Dyson, S.; van Grinsven, B.; Cleij, T.; et al. Optimization and Characterization of a Flow Cell for Heat-Transfer-Based Biosensing. Phys. Status Solidi A 2017, 214, 1600758. [Google Scholar] [CrossRef]
- Zeneli, M.; Nikolopoulos, A.; Karellas, S.; Nikolopoulos, N. Numerical Methods for Solid-Liquid Phase-Change Problems. In Ultra-High Temperature Thermal Energy Storage, Transfer and Conversion; Alejandro, D., Ed.; Woodhead Publishing: Cambridge, UK, 2021; pp. 165–199. ISBN 978-0-12-819955-8. [Google Scholar] [CrossRef]
- Górzynska, A.; Kondracka, K.; Korzeniowska-Kowal, A.; Nawrot, U. Antifungal Susceptibility of Saccharomyces cerevisiae Isolated from Clinical Specimens. Pathogens 2024, 13, 248. [Google Scholar] [CrossRef]
- Hartmann, R.; Chong, K.L.; Stevens, R.J.A.M.; Verzicco, R.; Lohse, D. Heat Transport Enhancement in Confined Rayleigh-Bénard Convection Feels the Shape of the Container. Europhys. Lett. 2021, 135, 24004. [Google Scholar] [CrossRef]
- Choi, J.W.; Bischof, J.C. A Quantitative Analysis on the Thermal Properties of Phosphate Buffered Saline with Glycerol at Subzero Temperatures. Int. J. Heat Mass Transf. 2008, 51, 640–649. [Google Scholar] [CrossRef]
- Mancinelli, R.; Botti, A.; Bruni, F.; Ricci, M.A.; Soper, A.K. Hydration of Sodium, Potassium, and Chloride Ions in Solution and the Concept of Structure Maker/Breaker. J. Phys. Chem. B 2007, 111, 13570–13577. [Google Scholar] [CrossRef] [PubMed]
- Johannsmann, D.; Langhoff, A.; Leppin, C. Studying Soft Interfaces with Shear Waves: Principles and Applications of the Quartz Crystal Microbalance (QCM). Sensors 2021, 21, 3490. [Google Scholar] [CrossRef]
- Johannsmann, D.; Reviakine, I. Quartz crystal microbalance with dissipation monitoring for studying soft matter at interfaces. Nat. Rev. Methods Primers 2024, 4, 63. [Google Scholar] [CrossRef]
- Yongabi, D.; Khorshid, M.; Gennaro, A.; Jooken, S.; Duwé, S.; Deschaume, O.; Losada-Pérez, P.; Dedecker, P.; Bartic, C.; Wübbenhorst, M.; et al. QCM-D Study of Time-Resolved Cell Adhesion and Detachment: Effect of Surface Free Energy on Eukaryotes and Prokaryotes. ACS Appl. Mater. Interfaces 2020, 12, 18258–18272. [Google Scholar] [CrossRef]
- Ivanoff Reyes, P.; Yang, K.; Zheng, A.; Li, R.; Li, G.; Lu, Y.; Tsang, C.K.; Zheng, S.X.F. Dynamic Monitoring of Antimicrobial Resistance Using Magnesium Zinc Oxide Nanostructure-Modified Quartz Crystal Microbalance. Biosens. Bioelectron. 2017, 93, 189–197. [Google Scholar] [CrossRef]
- Losada-Pérez, P.; Mertens, N.; de Medio-Vasconcelos, B.; Slenders, E.; Leys, J.; Peeters, M.; van Grinsven, B.; Gruber, J.; Glorieux, C.; Pfeiffer, H.; et al. Phase Transitions of Binary Lipid Mixtures: A Combined Study by Adiabatic Scanning Calorimetry and Quartz Crystal Microbalance with Dissipation Monitoring. Adv. Condens. Matter Phys. 2015, 2015, 479318. [Google Scholar] [CrossRef]
- Broeren, M.; Maas, Y.; Retera, E.; Arents, N. Antimicrobial susceptibility testing in 90 min by bacterial cell count monitoring. Clin. Microbiol. Infect. 2013, 3, 286–291. [Google Scholar] [CrossRef]
- Hayden, R.; Clinton, L.; Hewitt, C.; Koyamatsu, T.; Sun, Y.; Jamison, G.; Perkins, R.; Tang, L.; Pounds, S.; Bankowski, M. Rapid Antimicrobial Susceptibility Testing Using Forward Laser Light Scatter Technology. J. Clin. Microbiol. 2016, 54, 2701–2706. [Google Scholar] [CrossRef]
- Gheorghiu, M. A Short Review on Cell-Based Biosensing: Challenges and Breakthroughs in Biomedical Analysis. J. Biomed. Res. 2020, 35, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Gui, Q.; Lawson, T.; Shan, S.; Yan, L.; Liu, Y. The Application of Whole Cell-Based Biosensors for Use in Environmental Analysis and in Medical Diagnostics. Sensors 2017, 17, 1623. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Renugopalakrishnan, V.; Liepmann, D.; Paulmurugan, R.; Malhotra, B.D. Cell-Based Biosensors: Recent Trends, Challenges and Future Perspectives. Biosens. Bioelectron. 2019, 141, 111435. [Google Scholar] [CrossRef]
- Park, B.K.; Yi, N.; Park, J.; Kim, D. Thermal Conductivity of Single Biological Cells and Relation with Cell Viability. Appl. Phys. Lett. 2013, 102, 203702. [Google Scholar] [CrossRef]
- Inomata, N.; Miyamoto, T.; Okabe, K.; Ono, T. Measurement of Cellular Thermal Properties and Their temperature Dependence Based on Frequency Spectra via an On-Chip-Integrated Microthermistor. Lab Chip 2023, 23, 2411–2420. [Google Scholar] [CrossRef]



| Domain Number | Material | Cp (Jkg−1K−1) | ρ (kgm−3) | k (Wm−1K−1) |
|---|---|---|---|---|
| 1 | Air | 1000 | 1.12 | 0.027 |
| 2 | Copper | 385 | 8700 | 400 |
| 3 | PEEK | 1700 | 1320 | 0.25 |
| 4 | Stainless steel | 445 | 8900 | 90.7 |
| 5 | Teflon | 1500 | 2200 | 0.25 |
| 6 | Viton | 180 | 1800 | 0.104 |
| 7 | Water | 4186 | 1000 | 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbán, C.T.; Bakhshi Sichani, S.; Ueda Modaffore, G.; Glorieux, C.; Gruber, J.; Yongabi, D.; Lettinga, M.P.; Wagner, P. Spontaneous Cell Detachment from Temperature Gradients: Getting the Method Ready for Antimicrobial Drug Testing at Cell Culture Level. Sensors 2025, 25, 2902. https://doi.org/10.3390/s25092902
Urbán CT, Bakhshi Sichani S, Ueda Modaffore G, Glorieux C, Gruber J, Yongabi D, Lettinga MP, Wagner P. Spontaneous Cell Detachment from Temperature Gradients: Getting the Method Ready for Antimicrobial Drug Testing at Cell Culture Level. Sensors. 2025; 25(9):2902. https://doi.org/10.3390/s25092902
Chicago/Turabian StyleUrbán, Csongor Tibor, Soroush Bakhshi Sichani, Gabriela Ueda Modaffore, Christ Glorieux, Jonas Gruber, Derick Yongabi, Minne Paul Lettinga, and Patrick Wagner. 2025. "Spontaneous Cell Detachment from Temperature Gradients: Getting the Method Ready for Antimicrobial Drug Testing at Cell Culture Level" Sensors 25, no. 9: 2902. https://doi.org/10.3390/s25092902
APA StyleUrbán, C. T., Bakhshi Sichani, S., Ueda Modaffore, G., Glorieux, C., Gruber, J., Yongabi, D., Lettinga, M. P., & Wagner, P. (2025). Spontaneous Cell Detachment from Temperature Gradients: Getting the Method Ready for Antimicrobial Drug Testing at Cell Culture Level. Sensors, 25(9), 2902. https://doi.org/10.3390/s25092902








