Hydrothermal Synthesis of Zinc Stannate Nanoparticles for the Electrochemical Detection of Organophosphate Pesticide—Parathion-Ethyl
Abstract
1. Introduction
2. Experimental Sections
2.1. Chemical and Reagents
2.2. Instrumentation
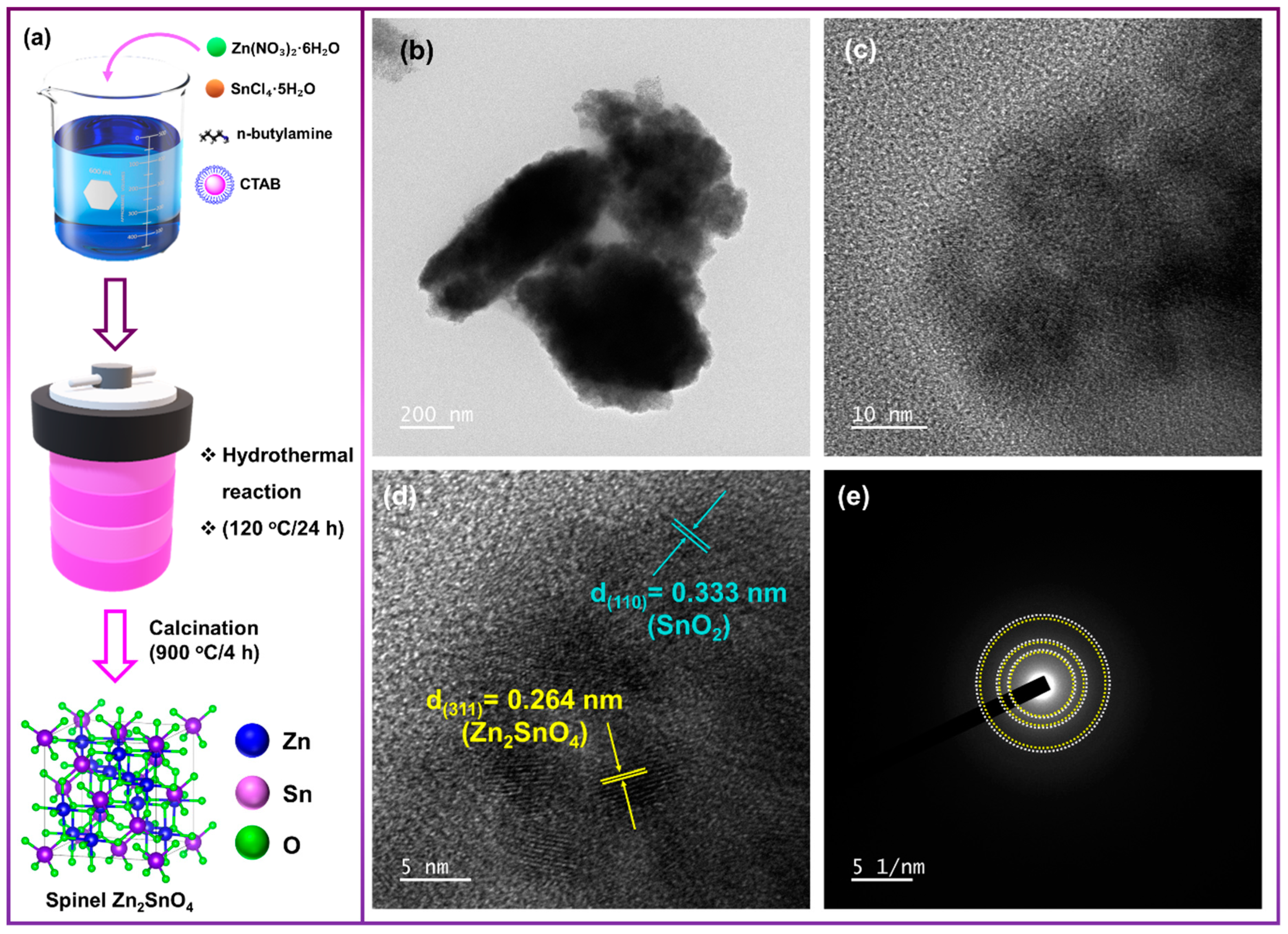
2.3. Synthesis of Electrocatalyst Zn2SnO4/SnO2 by Hydrothermal Method
2.4. Electrode Surface Modifications
2.5. Optimization of the Detection System
3. Results and Discussions
3.1. Transmission Electron Microscopy (TEM) Analysis
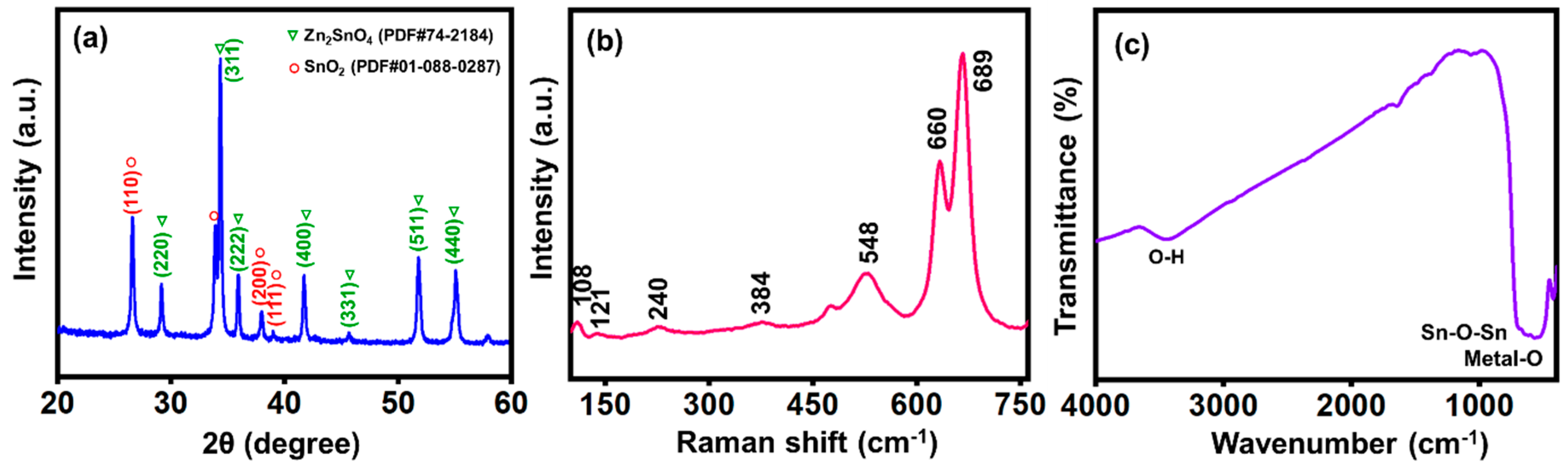
3.2. X-Ray Diffraction (XRD) Analysis
3.3. Raman Spectroscopy Analysis
3.4. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
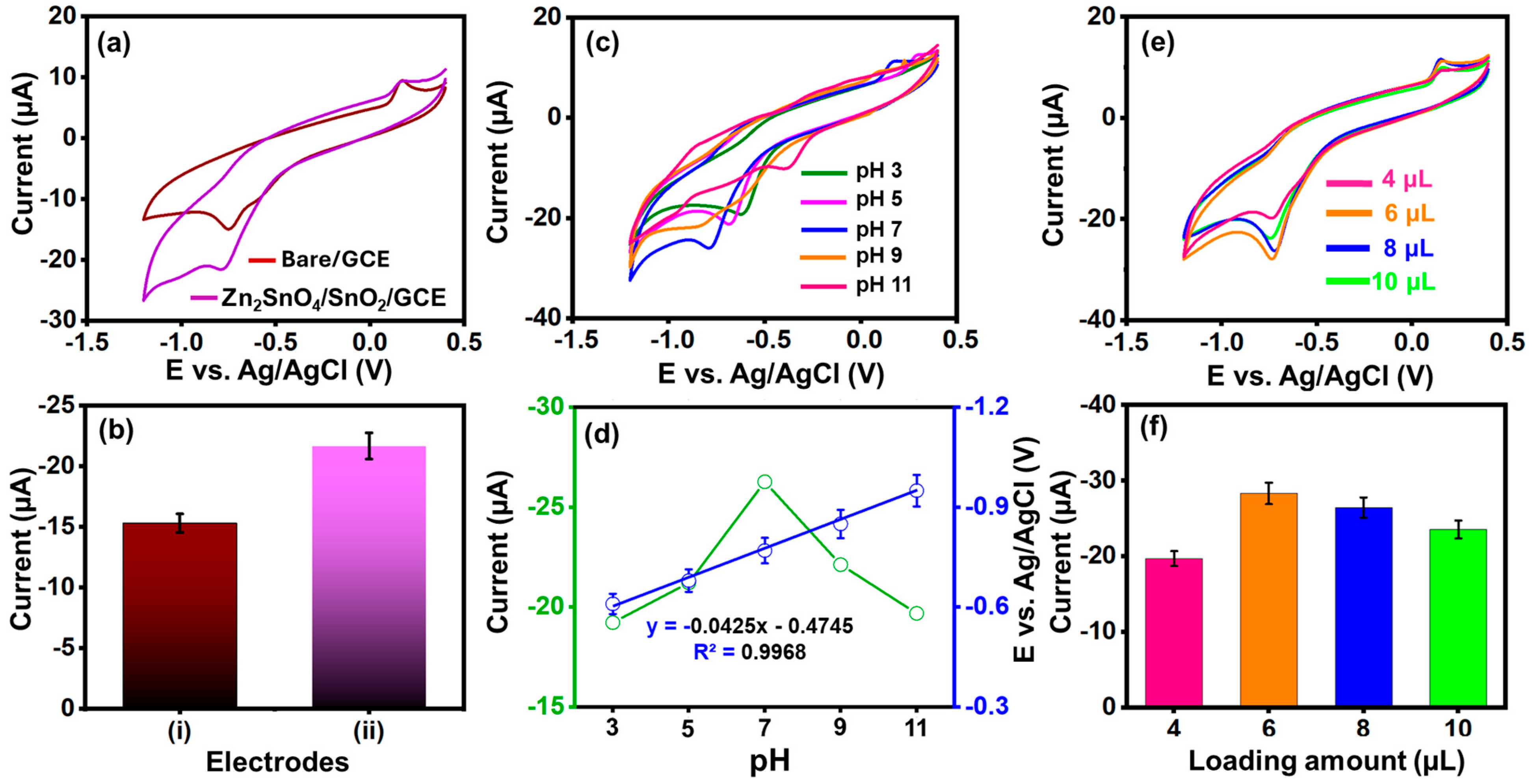
3.5. Electro-Catalytic Behaviors of Zn2SnO4/SnO2 Through Redox Probe
3.6. Electrochemical Behaviors of Zn2SnO4/SnO2/GCE Towards EP Detection
3.7. Effect of Supporting Electrolyte
3.8. Effect of Catalyst Loading Amount
3.9. Impact of Different Concentration and Scan Rates
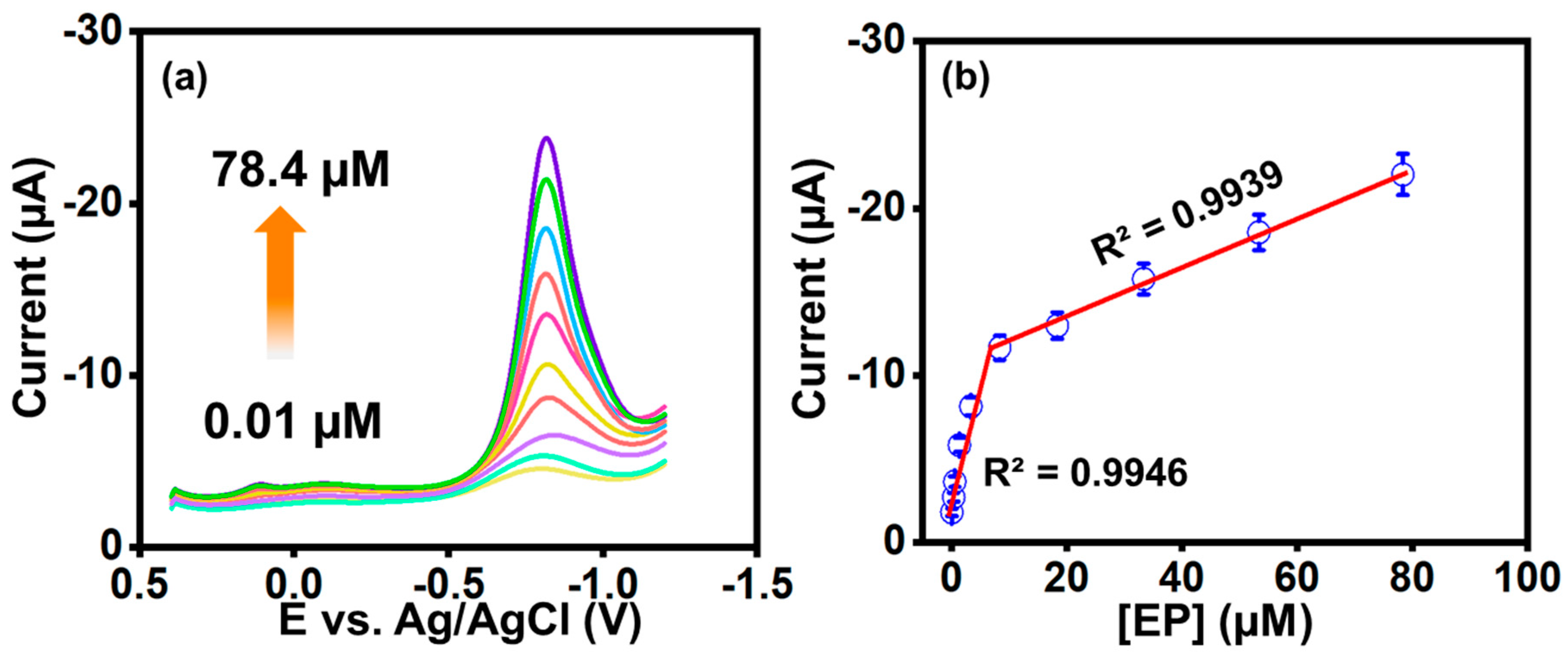
3.10. Differential Pulse Voltammetry (DPV) Analysis
3.11. Selectivity, Repeatability, and Reproducibility Study
3.12. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kogularasu, S.K.; Sriram, B.; Chang-Chien, G.P.; Wang, S.F.; Ahammad, F.N.U.; Billey, W.; Platero, J.S.; Soundappan, T.; Sekhar, P. Advances in Electrochemical Sensors: Improving Food Safety, Quality, and Traceability. ECS Sens. Plus 2024, 3, 020605. [Google Scholar]
- Huang, B.; Zhang, W.D.; Chen, C.H.; Yu, Y.X. Electrochemical determination of methyl parathion at a Pd/MWCNTs-modified electrode. Microchim. Acta 2010, 171, 57–62. [Google Scholar] [CrossRef]
- Wei, L.; Huang, X.; Yan, F.; Zheng, L.; Wang, J.; Xie, L.; Ya, Y. Al-doped mesoporous cellular foam modified electrode as sensor for the detection of parathion pesticide. Int. J. Electrochem. Sci. 2019, 14, 1986–1996. [Google Scholar] [CrossRef]
- Piovesan, J.V.; Haddad, V.F.; Pereira, D.F.; Spinelli, A. Magnetite nanoparticles/chitosan-modified glassy carbon electrode for non-enzymatic detection of the endocrine disruptor parathion by cathodic square-wave voltammetry. J. Electroanal. Chem. 2018, 823, 617–623. [Google Scholar] [CrossRef]
- Mutharani, B.; Ranganathan, P.; Chen, S.M.; Karuppiah, C. Enzyme-free electrochemical detection of nanomolar levels of the organophosphorus pesticide paraoxon-ethyl by using a poly(N-isopropyl acrylamide)-chitosan microgel decorated with palladium nanoparticles. Microchim. Acta 2019, 186, 167. [Google Scholar]
- Wei, X.H.; Li, C.; Wang, C.; Lin, S.; Wu, J.; Guo, M.J. Rapid and destructive adsorption of paraoxon-ethyl toxin via a self-detoxifying hybrid electrospun nanofibrous membrane. Chem. Eng. J. 2018, 351, 31–39. [Google Scholar] [CrossRef]
- Zhao, L.; Deng, C.; Xue, S.; Liu, H.; Hao, L.; Zhu, M. Multifunctional g-C3N4/Ag NPs intercalated GO composite membrane for SERS detection and photocatalytic degradation of paraoxon-ethyl. Chem. Eng. J. 2020, 402, 126223. [Google Scholar]
- Deo, R.P.; Wang, J.; Block, I.; Mulchandani, A.; Joshi, K.A.; Trojanowicz, M.; Scholz, F.; Chen, W.; Lin, Y. Determination of organophosphate pesticides at a carbon nanotube/organophosphorus hydrolase electrochemical biosensor. Anal. Chim. Acta 2005, 530, 185–189. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Mulchandani, A.; Mulchandani, P.; Chen, W. Remote biosensor for in-situ monitoring of organophosphate nerve agents. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 1999, 11, 866–869. [Google Scholar]
- Eyer, F.; Meischner, V.; Kiderlen, D.; Thiermann, H.; Worek, F.; Haberkorn, M.; Felgenhauer, N.; Zilker, T.; Eyer, P. Human parathion poisoning: A toxicokinetic analysis. Toxicol. Rev. 2003, 22, 143–163. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Wang, T.J.; Ahmed, F. Construction of two-dimensional molybdenum carbide based electrocatalyst for real-time monitoring of parathion-ethyl. J. Environ. Chem. Eng. 2021, 9, 106537. [Google Scholar] [CrossRef]
- Pilvenyte, G.; Ratautaite, V.; Boguzaite, R.; Ramanavicius, S.; Chen, C.F.; Viter, R.; Ramanavicius, A. Molecularly imprinted polymer-based electrochemical sensors for the diagnosis of infectious diseases. Biosensors 2023, 13, 620. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Durairaj, S.; Prins, S.; Chen, A. Nanomaterial-based electrochemical sensors and biosensors for the detection of pharmaceutical compounds. Biosens. Bioelectron. 2021, 175, 112836. [Google Scholar] [CrossRef] [PubMed]
- Meskher, H.; Ragdi, T.; Thakur, A.K.; Ha, S.; Khelfaoui, I.; Sathyamurthy, R.; Sharshir, S.W.; Pandey, A.K.; Saidur, R.; Singh, P.; et al. A review on CNTs-based electrochemical sensors and biosensors: Unique properties and potential applications. Crit. Rev. Anal. Chem. 2024, 54, 2398–2421. [Google Scholar] [CrossRef]
- Radha, A.; Wang, S.F. Strontium stannate nanoparticles decorated on graphitic carbon nitride sheets for the electrochemical detection of 3-nitro-L-tyrosine. Environ. Sci. Nano 2024, 11, 4337–4347. [Google Scholar] [CrossRef]
- Akila, B.; Kanna Sharma, T.S.; Sakthinathan, S.; Jana, J.; George, M.; Chiu, T.W.; Choi, W.M. Synthesis of Functionalized Boron Nitride Decorated on Erbium Vanadate (ErVO4) Composite for Electrochemical Detection of Nitrofurazone in Environmental Samples. ACS Sustain. Chem. Eng. 2024, 12, 6473–6484. [Google Scholar] [CrossRef]
- Saha, C.; Bhushan, M.; Singh, L.R. Pesticide sensing using electrochemical techniques: A comprehensive review. J. Iran. Chem. Soc. 2023, 20, 243–256. [Google Scholar] [CrossRef]
- Sharma, T.S.K.; Jana, J.; Bhamu, K.C.; Song, J.; Sivaselvam, S.; Van Tam, T.; Kang, S.G.; Chung, J.S.; Hur, S.H.; Choi, W.M. Rational synthesis of alkaline earth metal vanadates: Structural origin of MgVO3 honeycomb lattice system and its electrochemical analysis for the detection of sulfadiazine. Chem. Eng. J. 2023, 464, 142673. [Google Scholar] [CrossRef]
- Alagumalai, K.; Shanmugam, R.; Chen, S.M.; Thirumalraj, B.; Haidyrah, A.S.; Karuppiah, C. Impact of gadolinium oxide with functionalized carbon nanosphere: A portable advanced electrocatalyst for pesticide detection in aqueous environmental samples. Talanta 2022, 238, 123028. [Google Scholar] [CrossRef]
- Hu, J.; Xiong, X.; Guan, W.; Tan, C. Hollow mesoporous SnO2/Zn2SnO4 heterojunction and RGO decoration for high-performance detection of acetone. ACS Appl. Mater. Interfaces 2022, 14, 55249–55263. [Google Scholar] [CrossRef]
- Young, D.L.; Williamson, D.L.; Coutts, T.J. Structural characterization of zinc stannate thin films. J. Appl. Phys. 2002, 91, 1464–1471. [Google Scholar] [CrossRef]
- Sukanya, R.; Balamurugan, K.; Chen, S.M.; Rajakumaran, R.; Muthupandi, K.; Shim, J.J.; Breslin, C.B. Fabrication of a selective sensor amplification probe modified with multi-component Zn2SnO4/SnO2 heterostructured microparticles as a robust electrocatalyst for electrochemical detection of antibacterial drug secnidazole. Materials 2021, 14, 6700. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, W.; Zhu, Z.; Sun, P.; Ma, J.; Lu, G. Phase investigation on zinc–tin composite crystallites. RSC Adv. 2013, 3, 12084–12087. [Google Scholar] [CrossRef]
- Ali, M.B.; Barka-Bouaifel, F.; Elhouichet, H.; Sieber, B.; Addad, A.; Boussekey, L.; Férid, M.; Boukherroub, R. Hydrothermal synthesis, phase structure, optical and photocatalytic properties of Zn2SnO4 nanoparticles. J. Colloid Interface Sci. 2015, 457, 360–369. [Google Scholar]
- Zhu, H.; Yang, D.; Yu, G.; Zhang, H.; Jin, D.; Yao, K. Hydrothermal synthesis of Zn2SnO4 nanorods in the diameter regime of sub-5 nm and their properties. J. Phys. Chem. B 2006, 110, 7631–7634. [Google Scholar] [CrossRef]
- Zeng, J.; Xin, M.; Li, K.; Wang, H.; Yan, H.; Zhang, W. Transformation process and photocatalytic activities of hydrothermally synthesized Zn2SnO4 nanocrystals. J. Phys. Chem. C 2008, 112, 4159–4167. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, F.; Sun, L.; Li, Y.; Liao, L.; Guan, Y.; Lao, J.; Yang, Y.; Zhou, T.; Wang, Y.; et al. A highly active Z-scheme SnS/Zn2SnO4 photocatalyst fabricated for methylene blue degradation. RSC Adv. 2022, 12, 31985–31995. [Google Scholar] [CrossRef]
- Shangguan, C.; Wei, X.; Dong, M.; Li, Y.; Zhu, L.; Wang, J.; You, R. Micro-spherical SnO2/Zn2SnO4 heterojunction for sensitive and selective detection of hydrogen sulfide. Appl. Surf. Sci. 2024, 669, 160518. [Google Scholar] [CrossRef]
- Liu, R.; Du, W.; Chen, Q.; Gao, F.; Wei, C.; Sun, J.; Lu, Q. Fabrication of Zn2 SnO4/SnO2 hollow spheres and their application in dye-sensitized solar cells. RSC Adv. 2013, 3, 2893–2896. [Google Scholar] [CrossRef]
- Xu, C.; Wu, K.; Hu, S.; Cui, D. Electrochemical detection of parathion at a glassy-carbon electrode modified with hexadecane. Anal. Bioanal. Chem. 2002, 373, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Electrochemical sensor for determination of parathion based on electropolymerization poly(safranine) film electrode. Int. J. Electrochem. 2011, 2011, 986494. [Google Scholar] [CrossRef]



| Materials | Linear Ranges (µM) | LOD (µM) | Ref. |
|---|---|---|---|
| CS-FeO | 0.34–4.46 | 0.187 µM | [4] |
| AA-P3TAA | 0.5–100 | 0.5 µM | [6] |
| Silver nanoparticle-modified electrode | 0.0412–7.90 | 0.0412 µM | [30] |
| Carbon nanoparticles and halloysite nanoclay modified CPE | 0.0012–4.81 | 0.00038 µM | [31] |
| Zn2SnO4/SnO2/GCE | 0.01–8.7; 8.7–78.4 | 0.0059 µM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagismathi, L.; Wang, S.-F. Hydrothermal Synthesis of Zinc Stannate Nanoparticles for the Electrochemical Detection of Organophosphate Pesticide—Parathion-Ethyl. Sensors 2025, 25, 2837. https://doi.org/10.3390/s25092837
Vagismathi L, Wang S-F. Hydrothermal Synthesis of Zinc Stannate Nanoparticles for the Electrochemical Detection of Organophosphate Pesticide—Parathion-Ethyl. Sensors. 2025; 25(9):2837. https://doi.org/10.3390/s25092837
Chicago/Turabian StyleVagismathi, Loganathan, and Sea-Fue Wang. 2025. "Hydrothermal Synthesis of Zinc Stannate Nanoparticles for the Electrochemical Detection of Organophosphate Pesticide—Parathion-Ethyl" Sensors 25, no. 9: 2837. https://doi.org/10.3390/s25092837
APA StyleVagismathi, L., & Wang, S.-F. (2025). Hydrothermal Synthesis of Zinc Stannate Nanoparticles for the Electrochemical Detection of Organophosphate Pesticide—Parathion-Ethyl. Sensors, 25(9), 2837. https://doi.org/10.3390/s25092837




