Gas-Sensing Study and Applications of Triboelectric Nanogenerator-Powered CuO-Modified CeO2 Nanomaterials for Ammonia Sensor at Room Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
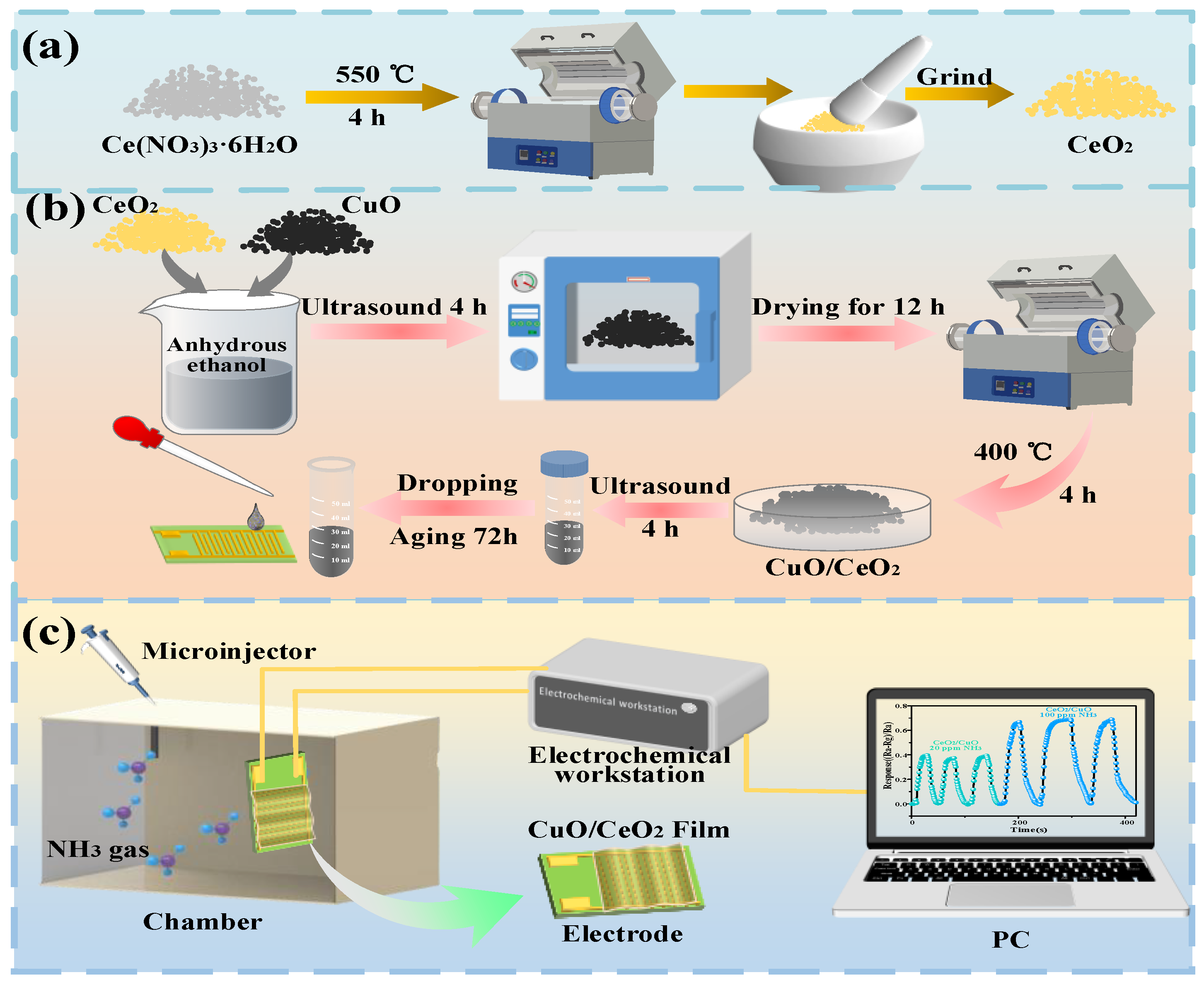
2.2. Preparation of Composite Materials
2.3. Preparation of Gas-Sensitive Sensors
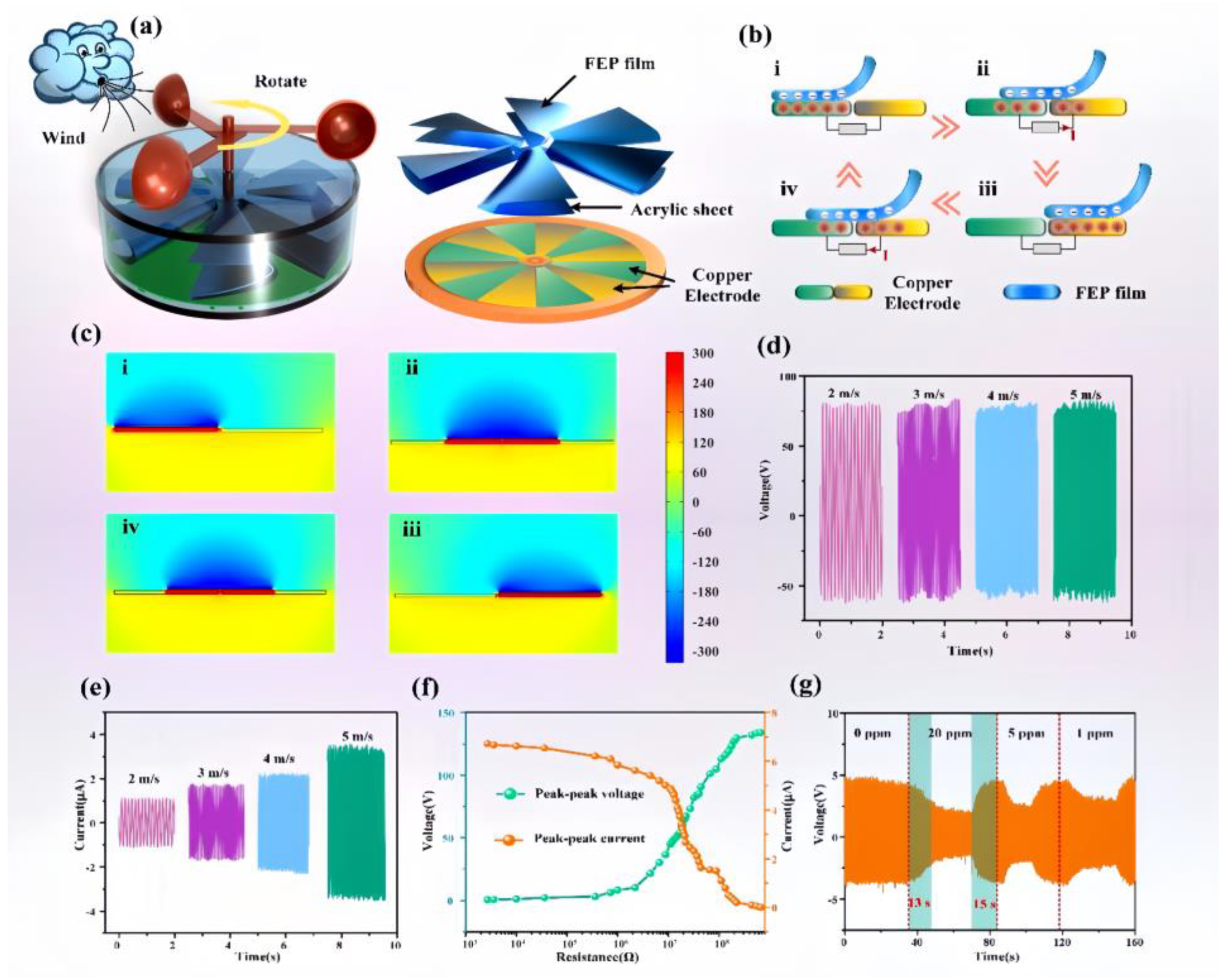
2.4. NH3-Sensing and Measuring Device
3. Results and Discussion
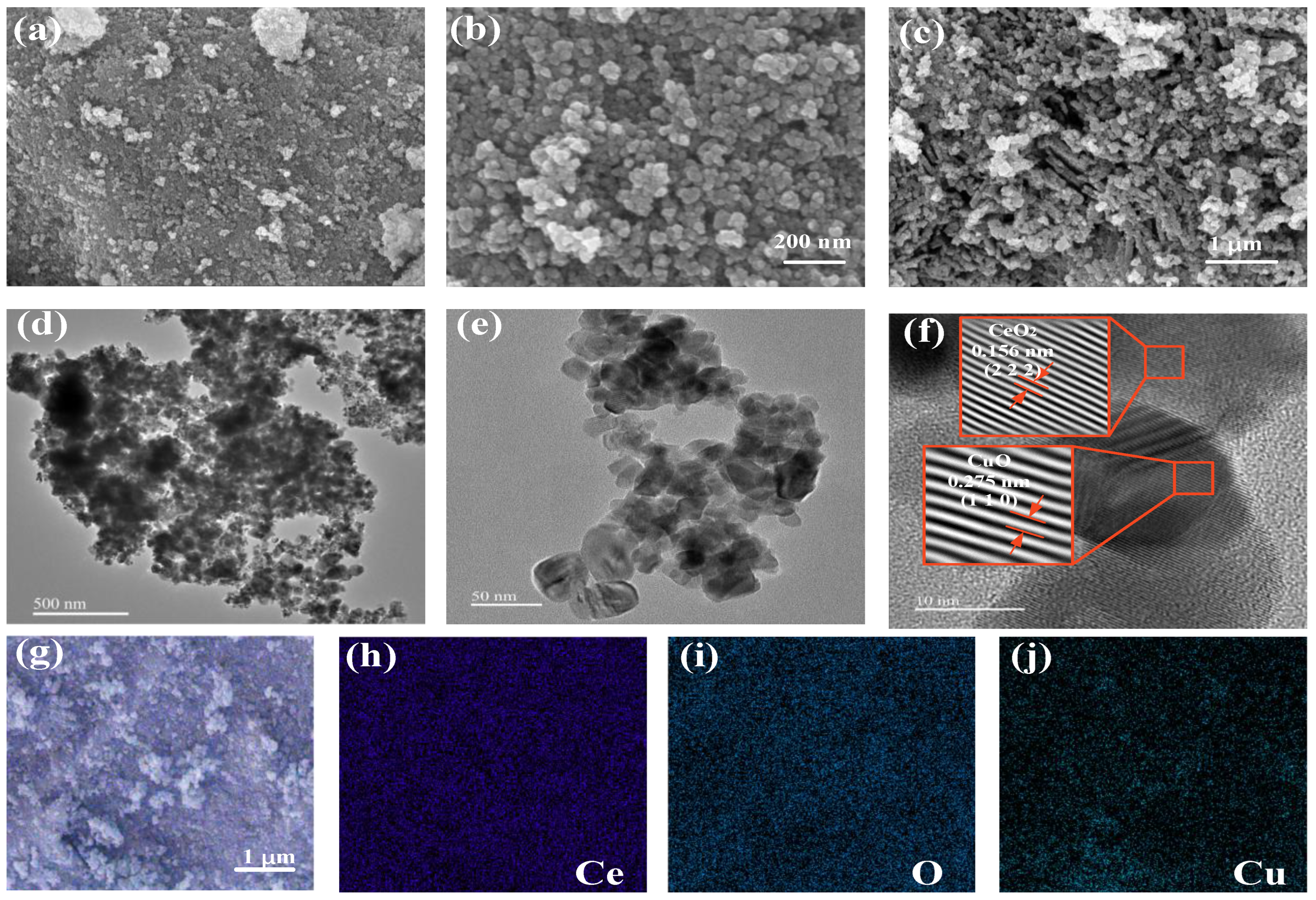
3.1. Characterization Results
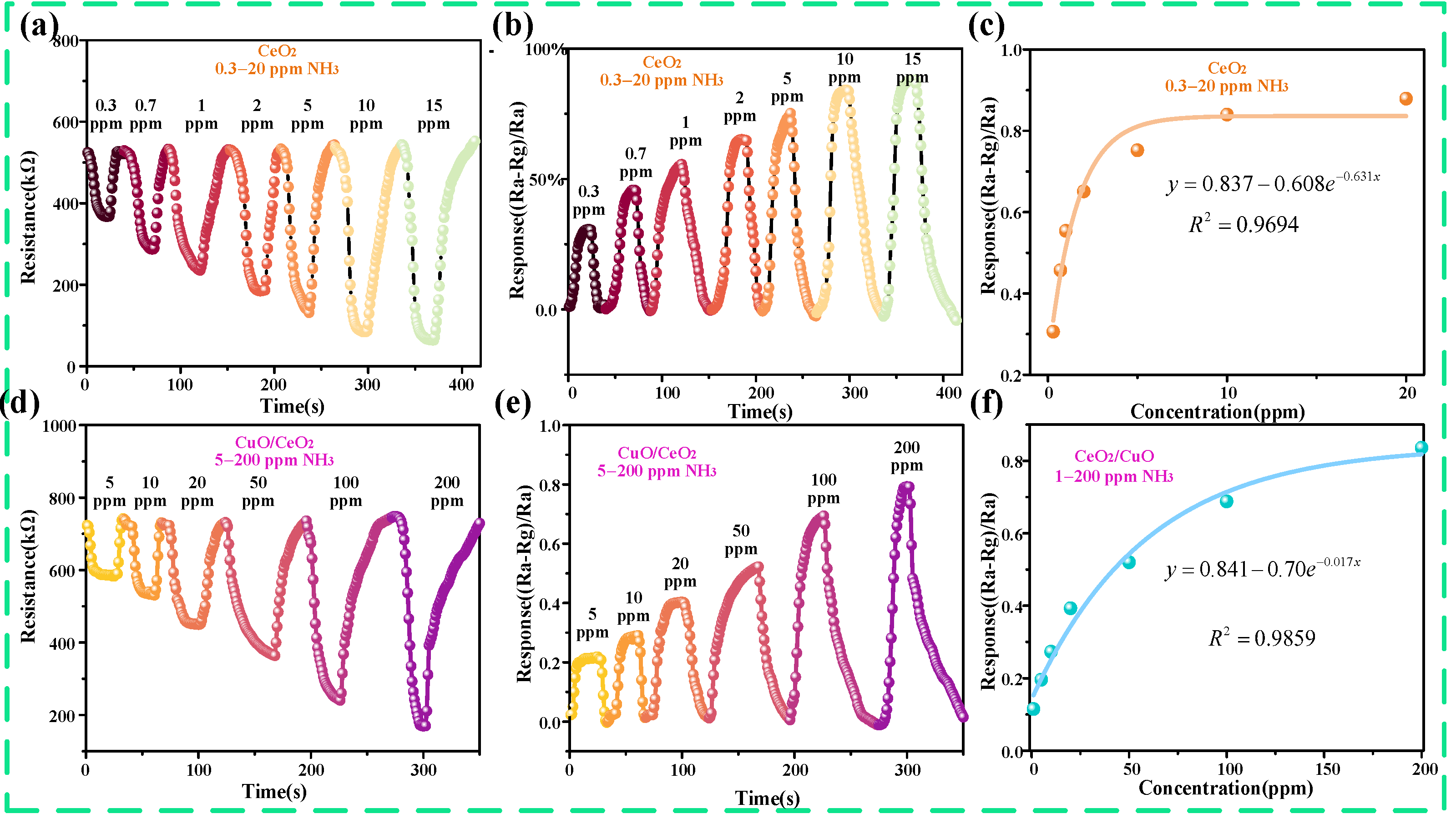
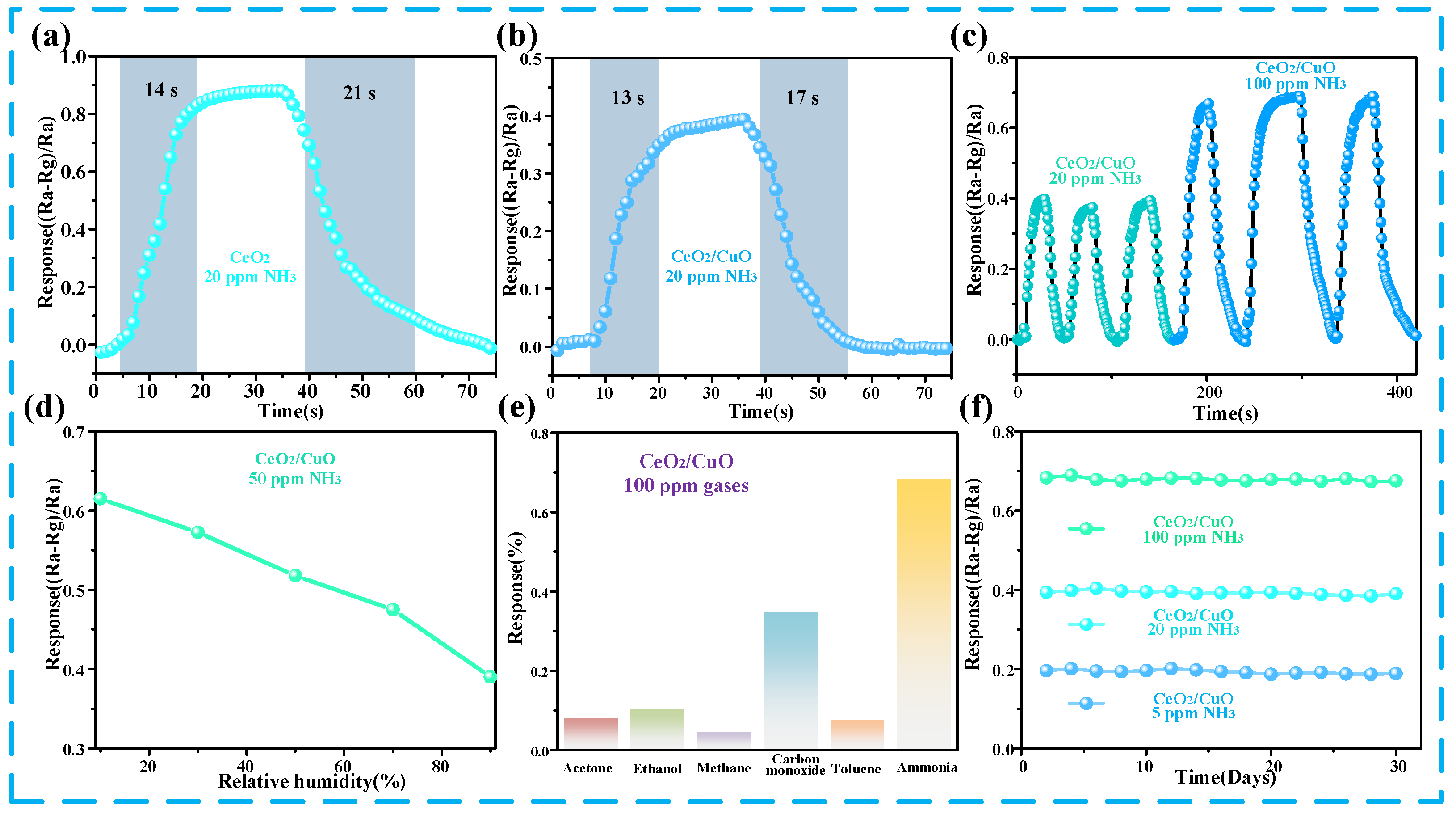
3.2. Ammonia Gas-Sensing Performance of CuO/CeO2 Sensor
3.3. Mechanism of the CuO/CeO2 Sensor
3.4. Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giraldo, J.P.; Kruss, S. Nanosensors for monitoring plant health. Nat. Nanotechnol. 2023, 18, 107–108. [Google Scholar] [CrossRef]
- Gui, Y.; Wang, Y.; He, S.; Yang, J. Self-powered smart agriculture real-time sensing device based on hybrid wind energy harvesting triboelectric-electromagnetic nanogenerator. Energy Convers. Manag. 2022, 269, 116098. [Google Scholar] [CrossRef]
- Jararweh, Y.; Fatima, S.; Jarrah, M.; AlZu’bi, S. Smart and sustainable agriculture: Fundamentals, enabling technologies, and future directions. Comput. Electr. Eng. 2023, 110, 108799. [Google Scholar] [CrossRef]
- Gao, Y.; Serrenho, A.C. Greenhouse gas emissions from nitrogen fertilizers could be reduced by up to one-fifth of current levels by 2050 with combined interventions. Nat. Food 2023, 4, 170–178. [Google Scholar] [CrossRef]
- Sompouviset, T.; Ma, Y.; Sukkaew, E.; Zheng, Z.; Zhang, A.; Zheng, W.; Li, Z.; Zhai, B. The Effects of Plastic Mulching Combined with Different Fertilizer Applications on Greenhouse Gas Emissions and Intensity, and Apple Yield in Northwestern China. Agriculture 2023, 13, 1211. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Yan, B.; Chen, L.; Shutes, B.; Wang, M.; Lyu, J.; Zhang, F. Ammonia volatilization, greenhouse gas emissions and microbiological mechanisms following the application of nitrogen fertilizers in a saline-alkali paddy ecosystem. Geoderma 2023, 433, 116460. [Google Scholar] [CrossRef]
- Piccolla, C.D.; Hesterberg, D.; Muraoka, T.; Novotny, E.H. Optimizing pyrolysis conditions for recycling pig bones into phosphate fertilizer. Waste Manag. 2021, 131, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.; Hendratna, A.; Scott, N.; Gao, S. Biochar enhancement of nitrification processes varies with soil conditions. Sci. Total Environ. 2023, 887, 164146. [Google Scholar] [CrossRef]
- Hussain, M.; Malik, R.N.; Taylor, A.; Puettmann, M. Hazardous pollutants emissions and environmental impacts from fuelwood burned and synthetic fertilizers applied by tobacco growers in Pakistan. Environ. Technol. Innov. 2017, 7, 169–181. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Cheng, F.-Y.; Chen, C.-L.; Wu, D.-H.; Chen, T.-Y.; Liao, K.-F.; Lay, W.-L.; Zhang, Y.-T. A high-resolution inventory of ammonia emissions from agricultural fertilizer application and crop residue in Taiwan. Atmos. Environ. 2023, 309, 119920. [Google Scholar] [CrossRef]
- Pan, B.; Lam, S.K.; Mosier, A.; Luo, Y.; Chen, D. Ammonia volatilization from synthetic fertilizers and its mitigation strategies: A global synthesis. Agric. Ecosyst. Environ. 2016, 232, 283–289. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Dong, J.; Li, Y.; Wang, X.; Gan, C.; Lin, Y.; Liao, H. Improved ammonia emission inventory of fertilizer application for three major crops in China based on phenological data. Sci. Total Environ. 2023, 896, 165225. [Google Scholar] [CrossRef]
- Wu, G.; Du, H.; Pakravan, K.; Kim, W.; Cha, Y.L.; Chiang, S.-T.; Beidaghi, M.; Zhang, X.; Kim, S.H.; Pan, X.; et al. Polyaniline/Ti3C2Tx functionalized mask sensors for monitoring of CO2 and human respiration rate. Chem. Eng. J. 2023, 475, 146228. [Google Scholar] [CrossRef]
- Wang, X.; Gong, L.; Li, Z.; Yin, Y.; Zhang, D. A room temperature ammonia gas sensor based on cerium oxide/MXene and self-powered by a freestanding-mode triboelectric nanogenerator and its multifunctional monitoring application. J. Mater. Chem. A 2023, 11, 7690–7701. [Google Scholar] [CrossRef]
- You, J.-J.; Liu, H.; Zhang, R.-R.; Pan, Q.-F.; Sun, A.-L.; Zhang, Z.-M.; Shi, X.-Z. Development and application of tricolor ratiometric fluorescence sensor based on molecularly imprinted nanoparticles for visual detection of dibutyl phthalate in seawater and fish samples. Sci. Total Environ. 2022, 848, 157675. [Google Scholar] [CrossRef]
- Tong, C.; Fan, L.; Cai, G.; Shi, S.; Yang, Y.; Guo, Y. Design of a sustainable light-up flavonol probe for dual-ratiometric fluorescent sensing and visual differentiating ammonia and hydrazine. Food Chem. 2023, 421, 136216. [Google Scholar] [CrossRef]
- Liu, C.; Liao, W.; Xu, Y.; Wu, D.; Gao, Z.; Xie, J. Characterizing NH3 emissions from a sheep feedlot and a manure composting facility in North China with the inverse dispersion technique. Agric. For. Meteorol. 2023, 339, 109580. [Google Scholar] [CrossRef]
- Baier, D.; Priamushko, T.; Weinberger, C.; Kleitz, F.; Tiemann, M. Selective discrimination between CO and H2 with copper–ceria-resistive gas sensors. ACS Sens. 2023, 8, 1616–1623. [Google Scholar] [CrossRef]
- Jin, C.; Kim, S.; Kim, D.E.; Mirzaei, A.; Roh, J.W.; Choi, S.-W.; Choi, M.S. Gadolinium-Doped CeO2 Gas Sensor for H2S Sensing. Korean J. Met. Mater. 2023, 61, 414–421. [Google Scholar] [CrossRef]
- Takte, M.A.; Ingle, N.N.; Dole, B.N.; Tsai, M.-L.; Hianik, T.; Shirsat, M.D. A stable and highly-sensitive flexible gas sensor based on Ceria (CeO2) nano-cube decorated rGO nanosheets for selective detection of NO2 at room temperature. Synth. Met. 2023, 297, 117411. [Google Scholar] [CrossRef]
- Choudhury, S.; Kanth, S.; Saxena, V.; Gupta, J.; Betty, C.A. Nanostructured CeO2 ultrathin film deposited by the Langmuir Blodgett technique for highly sensitive and specific detection of sub ppm level NO2 gas at room temperature. J. Mater. Chem. C 2023, 11, 11620–11630. [Google Scholar] [CrossRef]
- Pandit, N.A.; Ahmad, T. ZrO2/CeO2-Heterostructured Nanocomposites for Enhanced Carbon Monoxide Gas Sensing. ACS Appl. Nano Mater. 2023, 6, 7299–7309. [Google Scholar] [CrossRef]
- Dogra, N.; Singh, M.; Kumar, A.; Sharma, S. Selective room-temperature ammonia sensing using CeO2-multiwalled carbon nanotube composite. Appl. Phys. A 2022, 129, 24. [Google Scholar] [CrossRef]
- Qian, S.; Zhang, H.; Sun, X.; Tan, D.; Tian, F.; Zhang, T.; Cui, Y.; Chen, X.; Liang, X.; Cai, Z.; et al. Mesoporous silica-encapsulated Co-doped ceria nanodots with enhanced peroxidase-like activity enable intensive and long-lasting chemiluminescence for glutathione detection. Sens. Actuators B Chem. 2023, 383, 133609. [Google Scholar] [CrossRef]
- Dhanawade, R.N.; Pawar, N.S.; Chougule, M.A.; Hingangavkar, G.M.; Jadhav, Y.M.; Nimbalkar, T.M.; Navale, Y.H.; Chavan, G.T.; Jeon, C.-W.; Patil, V.B. Highly sensitive and selective PAni-CeO2 nanohybrid for detection of NH3 biomarker at room temperature. J. Mater. Sci. Mater. Electron. 2023, 34, 781. [Google Scholar] [CrossRef]
- He, S.; Gui, Y.; Wang, Y.; Yang, J. A self-powered β-Ni(OH)2/MXene based ethanol sensor driven by an enhanced triboelectric nanogenerator based on β-Ni(OH)2@PVDF at room temperature. Nano Energy 2023, 107, 108132. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, J.-H.; Kim, S.S. CuO–TiO2 p–n core–shell nanowires: Sensing mechanism and p/n sensing-type transition. Appl. Surf. Sci. 2018, 448, 489–497. [Google Scholar] [CrossRef]
- Yan, S.; Song, W.; Wu, D.; Jin, S.; Dong, S.; Hao, H.; Gao, W. Assembly of In2O3 nanoparticles decorated NiO nanosheets heterostructures and their enhanced gas sensing characteristics. J. Alloys Compd. 2022, 896, 162887. [Google Scholar] [CrossRef]
- Wang, F.; Zhong, H.; Chen, Z.; Wang, D.; Lai, Z.; Deng, Y.; Wang, X. Porous 2D CuO nanosheets for efficient triethylamine detection at low temperature. Chin. Chem. Lett. 2023, 34, 107392. [Google Scholar] [CrossRef]
- Gupta, S.; Zou, H. Implementing an analytical model to elucidate the impacts of nanostructure size and topology of morphologically diverse zinc oxide on gas sensing. Chemosensors 2025, 13, 38. [Google Scholar] [CrossRef]
- Cheng, J.; Zheng, D.; Qiao, J.; Xu, R.; Yu, G.; Dai, C.; Liu, N.; Wang, N.; Chen, B. Elucidating the crystal-facet dependent catalytic performance of coupled flake-CuO/Cu-zeolite hybrid catalysts for coal-gas-SCR. Appl. Surf. Sci. 2023, 616, 156437. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.; Qin, P.; Wei, G.; Chen, C. Comparing the inhibitory effects of CuO-rGO, CuO NPs, and CuCl2 on the oomycete Phytophthora sojae: Insights from phenotypic and transcriptomic analyses. Environ. Sci. Nano 2023, 10, 2299–2311. [Google Scholar] [CrossRef]
- Meng, F.; Yang, Z.; Yuan, Z.; Zhang, H.; Zhu, H. Hydrothermal synthesis of CuO/rGO nanosheets for enhanced gas sensing properties of ethanol. Ceram. Int. 2023, 49, 5595–5603. [Google Scholar] [CrossRef]
- Zhou, J.; Ikram, M.; Rehman, A.U.; Wang, J.; Zhao, Y.; Kan, K.; Zhang, W.; Raziq, F.; Li, L.; Shi, K. Highly selective detection of NH3 and H2S using the pristine CuO and mesoporous In2O3@CuO multijunctions nanofibers at room temperature. Sens. Actuators B Chem. 2018, 255, 1819–1830. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, P.; Niu, G.; Luo, D.; Wang, Q.; Wang, F. Rapid and Efficient Detection of NH3 at Room Temperature Using CuO/WS2 Nanohybrids. IEEE Sens. J. 2022, 22, 12539–12546. [Google Scholar] [CrossRef]
- Zang, C.; Ma, K.; Yano, Y.; Li, S.; Yamahara, H.; Seki, M.; Iizuka, T.; Tabata, H. SnO2–CuO Heterostructured Nanofibers for Enhanced NH3-Gas-Sensing Performance and Potential Application in Breath Analysis. IEEE Sens. J. 2023, 23, 17925–17931. [Google Scholar] [CrossRef]
- Xu, P.; Shen, H.; Li, J.; Zhang, C.; Guan, D. Power bonding diagram model and parameter analysis of contact-separation mode triboelectric nanogenerator. Energy 2023, 279, 127946. [Google Scholar] [CrossRef]
- Rani, G.M.; Wu, C.-M.; Motora, K.G.; Umapathi, R. Waste-to-energy: Utilization of recycled waste materials to fabricate triboelectric nanogenerator for mechanical energy harvesting. J. Clean. Prod. 2022, 363, 132532. [Google Scholar] [CrossRef]
- Gui, Y.; He, S.; Wang, Y.; Yang, J. MOF-derived porous Ni/C material for high-performance hybrid nanogenerator and self-powered wearable sensor. Compos. Part A Appl. Sci. Manuf. 2023, 168, 107492. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Ta, Q.T.H.; Tran, N. Review on the transformation of biomechanical energy to green energy using triboelectric and piezoelectric based smart materials. J. Clean. Prod. 2022, 371, 133702. [Google Scholar] [CrossRef]
- Zhan, T.; Zou, H.; Zhang, H.; He, P.; Liu, Z.; Chen, J.; He, M.; Zhang, Y.; Wang, Z.L. Smart liquid-piston based triboelectric nanogenerator sensor for real-time monitoring of fluid status. Nano Energy 2023, 111, 108419. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, X.; Lv, J.; Li, G.; Yang, P.; Ma, Y.; Zou, H.; Wang, Z.L.J.E. Fluid-based triboelectric nanogenerators: Unveiling the prolific landscape of renewable energy harvesting and beyond. Energy Environ. Sci. 2024, 17, 3700–3738. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Wang, B.; Pei, S. Hydrothermal synthesis of SnO2-CuO composite nanoparticles as a fast-response ethanol gas sensor. J. Alloys Compd. 2021, 886, 161299. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide Semiconductor Gas Sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Li, P.; Wang, B.; Qin, C.; Han, C.; Sun, L.; Wang, Y. Band-gap-tunable CeO2 nanoparticles for room-temperature NH3 gas sensors. Ceram. Int. 2020, 46 Pt B, 19232–19240. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Gui, Y.; Huang, H. Gas-Sensing Study and Applications of Triboelectric Nanogenerator-Powered CuO-Modified CeO2 Nanomaterials for Ammonia Sensor at Room Temperature. Sensors 2025, 25, 2753. https://doi.org/10.3390/s25092753
Ding J, Gui Y, Huang H. Gas-Sensing Study and Applications of Triboelectric Nanogenerator-Powered CuO-Modified CeO2 Nanomaterials for Ammonia Sensor at Room Temperature. Sensors. 2025; 25(9):2753. https://doi.org/10.3390/s25092753
Chicago/Turabian StyleDing, Junsheng, Yingang Gui, and Hua Huang. 2025. "Gas-Sensing Study and Applications of Triboelectric Nanogenerator-Powered CuO-Modified CeO2 Nanomaterials for Ammonia Sensor at Room Temperature" Sensors 25, no. 9: 2753. https://doi.org/10.3390/s25092753
APA StyleDing, J., Gui, Y., & Huang, H. (2025). Gas-Sensing Study and Applications of Triboelectric Nanogenerator-Powered CuO-Modified CeO2 Nanomaterials for Ammonia Sensor at Room Temperature. Sensors, 25(9), 2753. https://doi.org/10.3390/s25092753






