Identification of Sarin Simulant DMMP Based on a Laminated MOS Sensor Using Article Swarm Optimization-Backpropagation Neural Network
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Film Materials
2.1.1. Preparation of Co@SnO2 Slurry
2.1.2. Preparation of Pt@CeLaCoNiOx Slurry
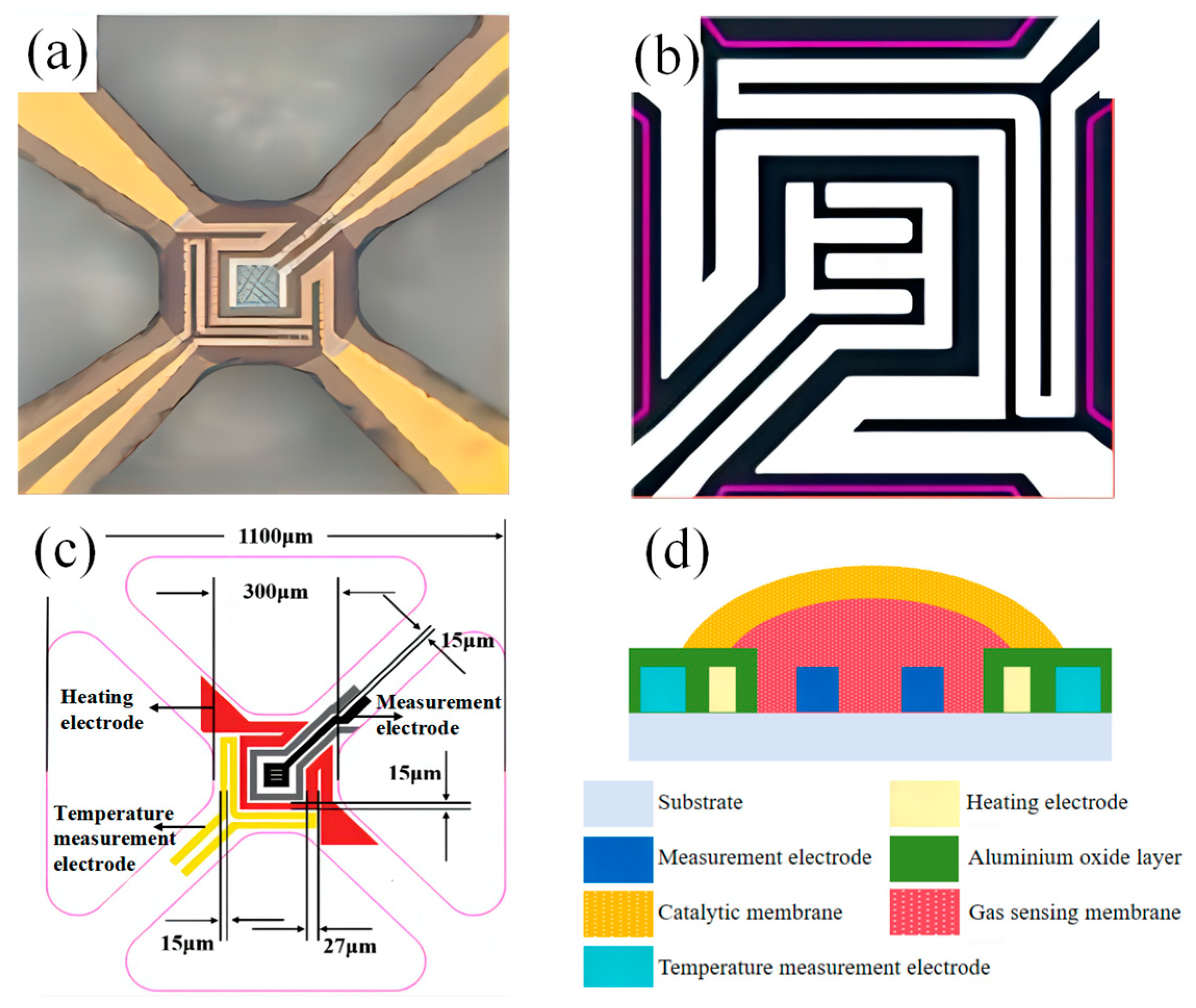
2.2. Preparation of the Catalytic Film/Gas Sensitive Film MOS Sensor
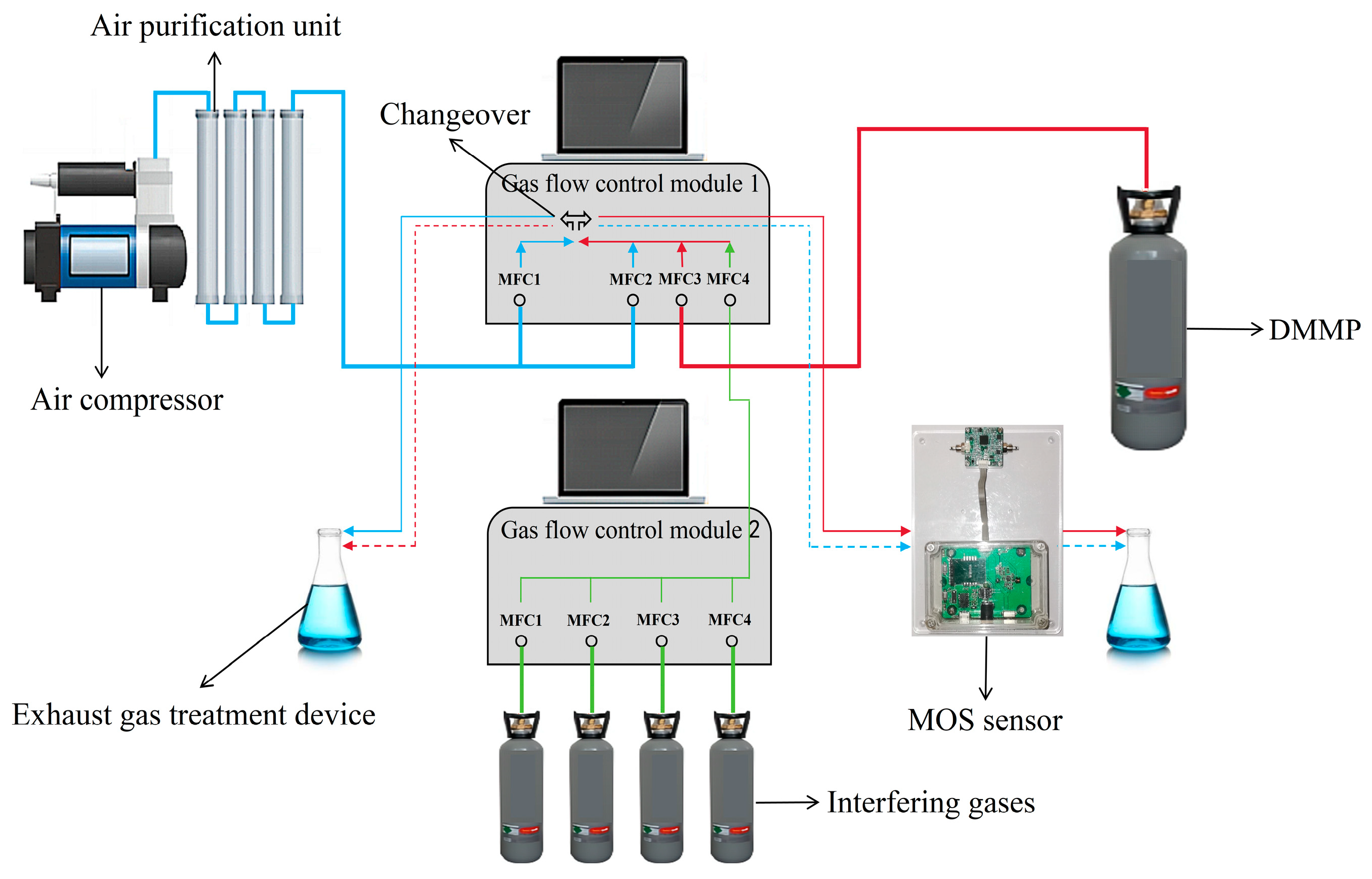
2.3. Test Platform
3. Results and Discussion
3.1. Characterization of Material
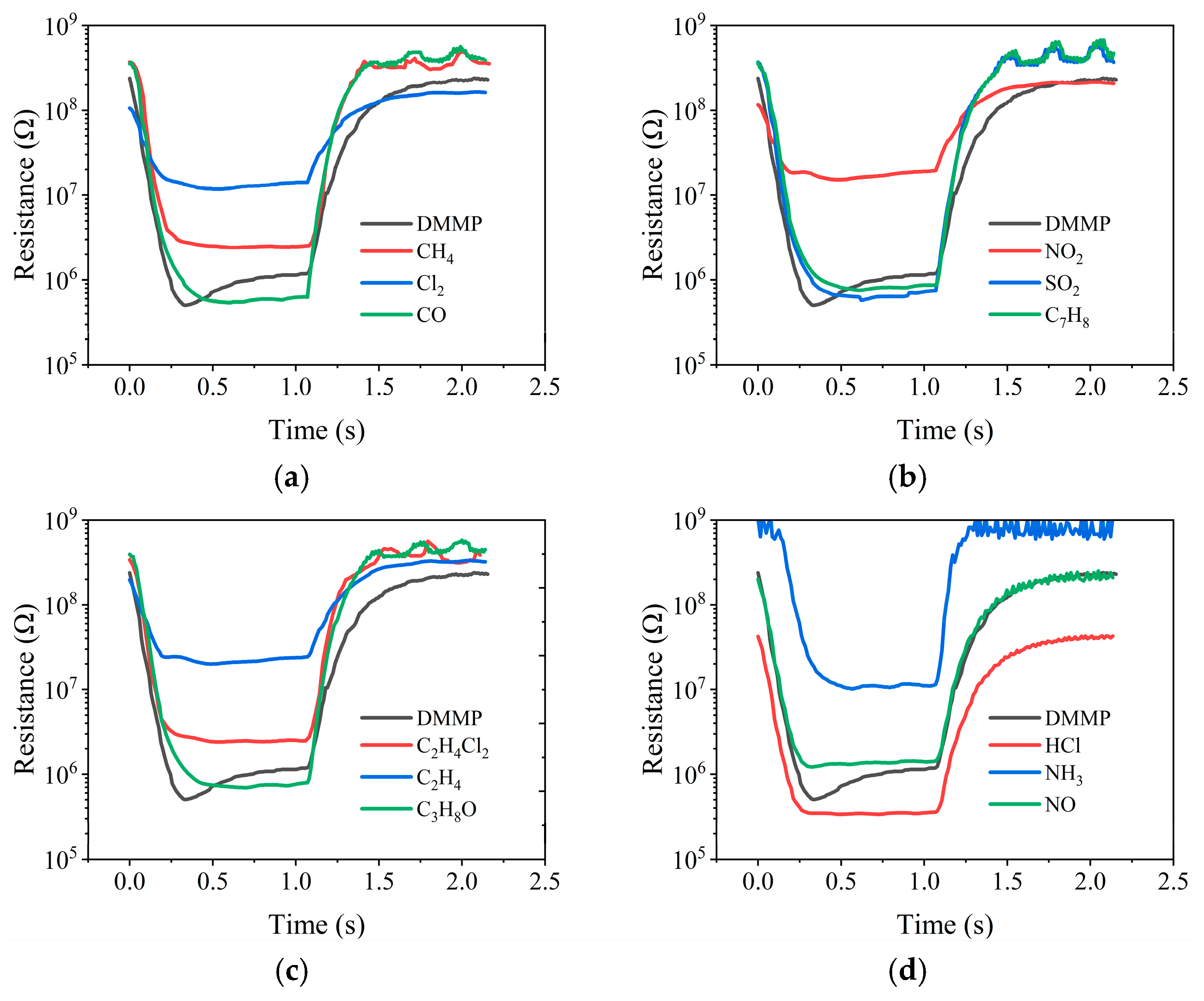
3.2. Sensing Performance
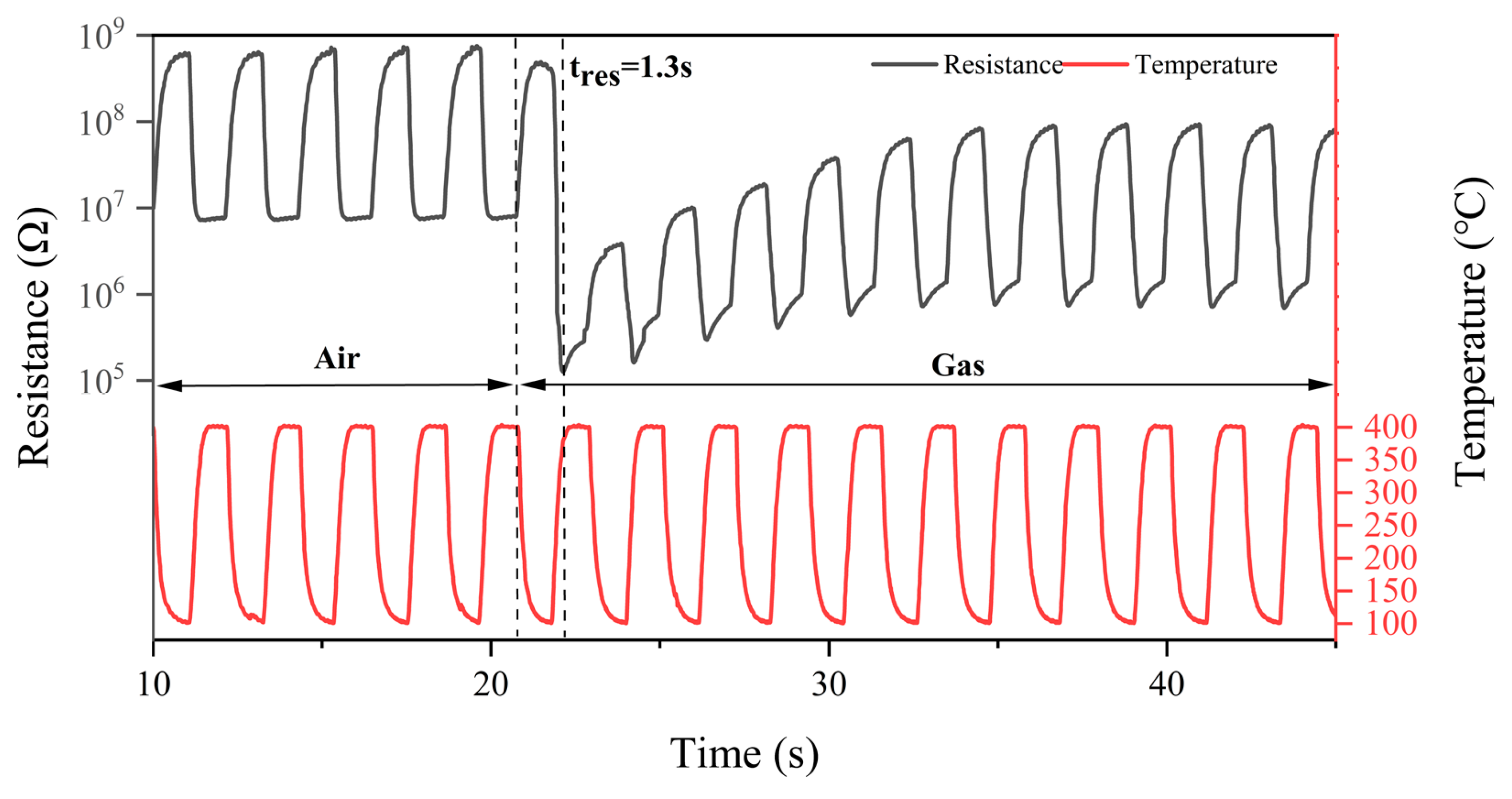
4. Definition of Peak Height
4.1. Sample Data Collection and Categorisation
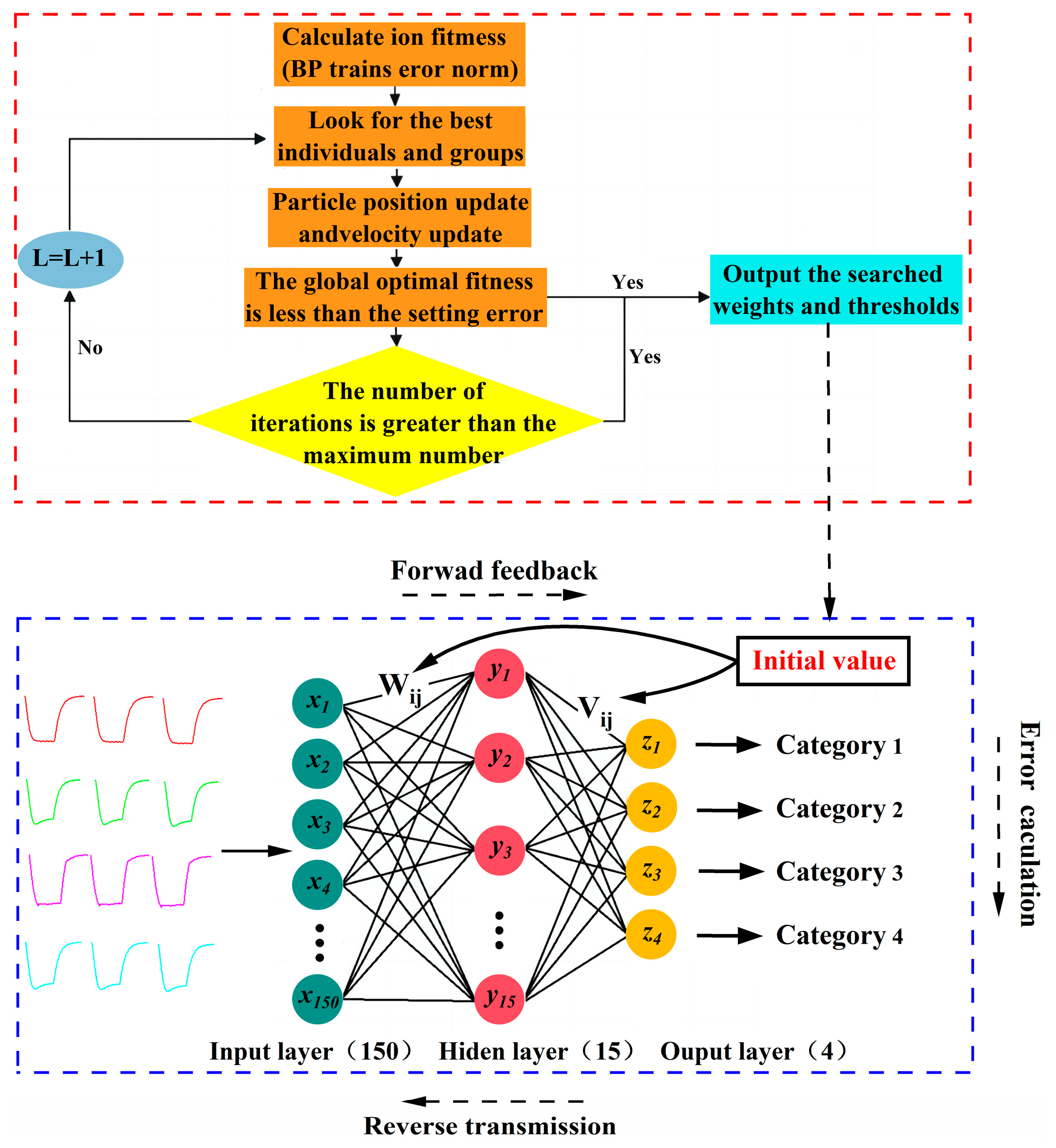
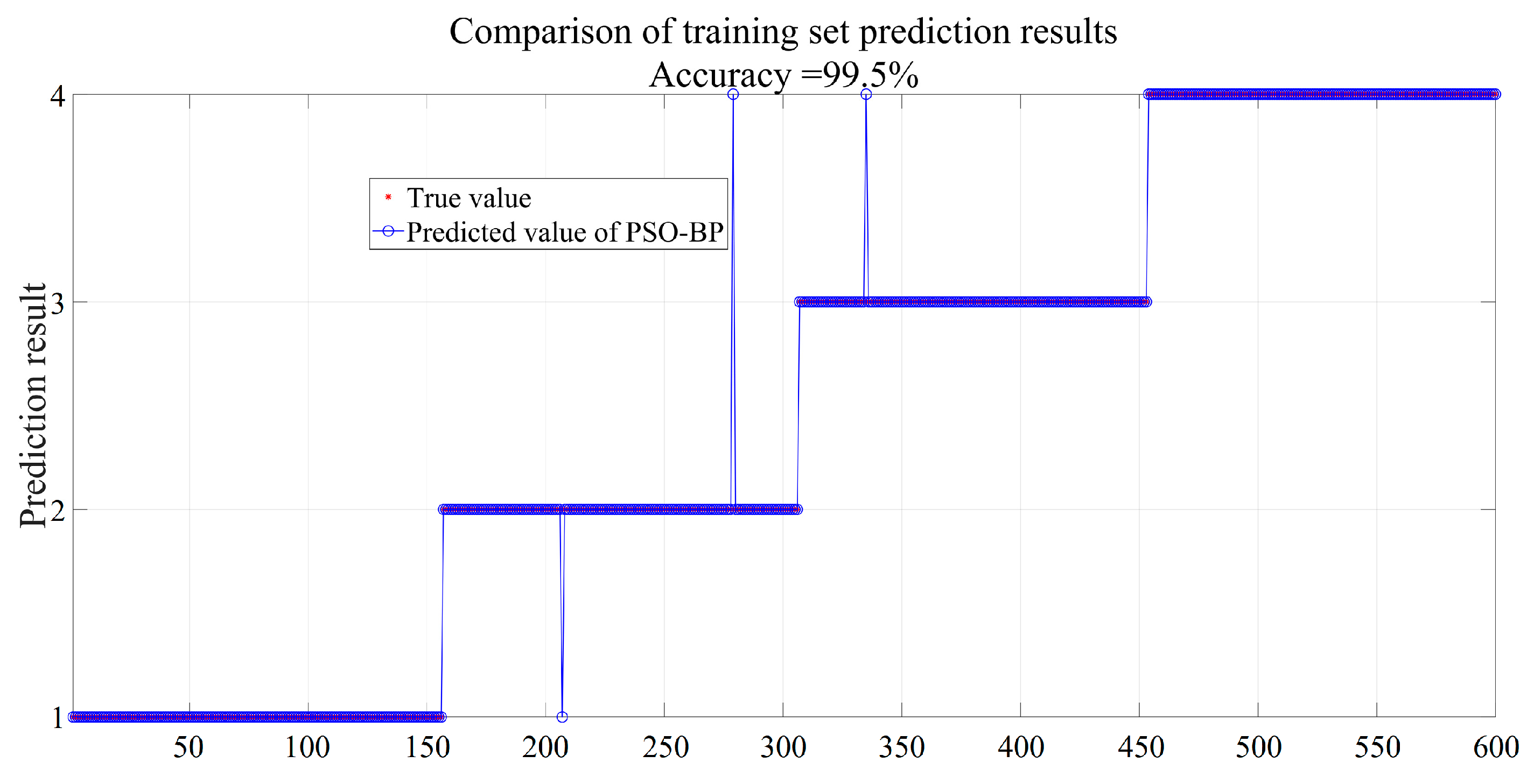
4.2. Prediction of Gas Categories Based on the PSO-BP
4.3. Concentration Impact
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emelianova, A.; Reed, A.; Basharova, E.A.; Kolesnikov, A.L.; Gor, G.Y. Closer Look at Adsorption of Sarin and Simulants on Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2023, 15, 18559–18567. [Google Scholar] [CrossRef]
- Witkiewicz, Z.; Jasek, K.; Grabka, M. Semiconductor gas sensors for detecting chemical warfare agents and their simulants. Sensors 2023, 23, 3272. [Google Scholar] [CrossRef]
- Chen, F.; Yang, M.; Wang, X.; Song, Y.; Guo, L.; Lu, G. Template-free synthesis of cubic-rhombohedral-In2O3 flower for ppb level acetone detection. Sens. Actuators B Chem. 2019, 290, 459–466. [Google Scholar] [CrossRef]
- Guo, L.; Chen, F.; Kou, X.; Wang, C.; Sun, Y.; Lu, G. Ultra-sensitive sensing platform based on Pt-ZnO-In2O3 nanofibers for detection of acetone. Sens. Actuators B Chem. 2018, 272, 185–194. [Google Scholar] [CrossRef]
- Xie, J.; Wang, H.; Lin, Y.; Zhou, Y.; Wu, Y. Highly sensitive humidity sensor based on quartz crystal microbalance coated with ZnO colloid spheres. Sens. Actuators B Chem. 2013, 177, 1083–1088. [Google Scholar] [CrossRef]
- Bao, S.; Li, K.; Ning, P.; Peng, J.; Tang, L. Highly effective removal of mercury and lead ions from wastewater by mercaptoamine-functionalised silica-coated magnetic nano-adsorbents: Behaviours and mechanisms. Appl. Surf. Sci. 2017, 393, 457–466. [Google Scholar] [CrossRef]
- Wang, M.; Hou, T.; Shen, Z.; Zhao, X.; Ji, H. MOF-derived Fe2O3: Phase control and effects of phase composition on gas sensing performance. Sens. Actuators B Chem. 2019, 292, 171–179. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, G.; Cheng, M.; Gao, Y.; Zhao, L.; Li, S.; Lu, G. The preparation of reduced graphene oxide-encapsulated α-Fe2O3 hybrid and its outstanding NO2 gas sensing properties at room temperature. Sens. Actuators B Chem. 2018, 261, 252–263. [Google Scholar] [CrossRef]
- Patil, L.A.; Bagul, S.D.; Patil, D.G. DMMP sensing performance of undoped and al doped nanocrystalline ZnO thin films prepared by ultrasonic atomization and pyrolysis method. J. Nanostruct. 2017, 7, 309–322. [Google Scholar]
- Alali, K.T.; Liu, J.; Aljebawi, K.; Liu, P.; Chen, R.; Li, R.; Wang, J. Electrospun np WO3/CuO heterostructure nanofibers as an efficient sarin nerve agent sensing material at room temperature. J. Alloys Compd. 2019, 793, 31–41. [Google Scholar] [CrossRef]
- Li, Q.; Chen, D.; Miao, J.; Lin, S.; Yu, Z.; Cui, D.; Chen, X. Highly sensitive sensor based on ordered porous ZnO nanosheets for ethanol detecting application. Sens. Actuators B Chem. 2021, 326, 128952. [Google Scholar] [CrossRef]
- Jiang, B.; Lu, J.; Han, W.; Sun, Y.; Wang, Y.; Cheng, P.; Lu, G. Hierarchical mesoporous zinc oxide microspheres for ethanol gas sensor. Sens. Actuators B Chem. 2022, 357, 131333. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, J.; Xu, L.; Liu, T.; Liu, Y.; Wang, X.; Zhao, C. Fabrication of lettuce-like ZnO gas sensor with enhanced H2S gas sensitivity. Crystals 2020, 10, 145. [Google Scholar] [CrossRef]
- Alali, K.T.; Liu, J.; Chen, R.; Liu, Q.; Zhang, H.; Li, J.; Wang, J. HFIP-Functionalized Co3O4 Micro-Nano-Octahedra/rGO as a Double-film Sensing Material for Chemical Warfare Agents. Chem.–A Eur. J. 2019, 25, 11892–11902. [Google Scholar] [CrossRef]
- Wang, T.; Ma, H.; Jiang, W.; Zhang, H.; Zeng, M.; Yang, J.; Yang, Z. Type discrimination and concentration prediction towards ethanol using a machine learning–enhanced gas sensor array with different morphology-tuning characteristics. Phys. Chem. Chem. Phys. 2021, 23, 23933–23944. [Google Scholar] [CrossRef]
- Tang, W.; Wang, J. Mechanism for toluene detection of flower-like ZnO sensors prepared by hydrothermal approach: Charge transfer. Sens. Actuators B Chem. 2015, 207, 66–73. [Google Scholar] [CrossRef]
- Liu, Y.D.; Zhao, S.Y.; You, L.J.; Xu, Y.; Si, R.J.; Zhang, S.P. Improving the Selectivity of Metal Oxide Semiconductor Sensors for Mustard Gas Simulant 2-Chloroethyl Ethyl Sulfide by Combining the Laminated Structure and Temperature Dynamic Modulation. Sensors 2025, 25, 525. [Google Scholar] [CrossRef]
- Li, T.; Tsyshevsky, R.; Algrim, L.; McEntee, M.; Durke, E.M.; Eichhorn, B.; Karwacki, C.; Zachariah, M.R.; Kuklja MMRodriguez, E.E. Understanding dimethyl methylphosphonate adsorption and decomposition on mesoporous CeO2. ACS Appl. Mater. Interfaces 2021, 13, 54597–54609. [Google Scholar] [CrossRef]
- Mitchell, M.B.; Sheinker, V.N.; Mintz, E.A. Adsorption and decomposition of dimethyl methylphosphonate on metal oxides. J. Phys. Chem. B 1997, 101, 11192–11203. [Google Scholar] [CrossRef]
- Sadeghi, M.; Yekta, S. Adsorption and neutralization chemistry of dimethyl methyl phosphonate (DMMP) as an organo-phosphorous pollutant (OPP) on the surface of nano-structured Co3O4 and MnCo2O4 catalysts. Iran. Chem. Commun. 2016, 4, 21–41. [Google Scholar]
- Lee, S.C.; Choi, H.Y.; Lee, S.J.; Lee, W.S.; Huh, J.S.; Lee, D.D.; Kim, J.C. The development of SnO2-based recoverable gas sensors for the detection of DMMP. Sens. Actuators B Chem. 2009, 137, 239–245. [Google Scholar] [CrossRef]
- Dhar, A.; Vekariya, R.L.; Sharma, P. Kinetics and mechanistic study of n-alkane hydroisomerization reaction on Pt-doped γ-alumina catalyst. Petroleum 2017, 3, 489–495. [Google Scholar] [CrossRef]
- Hosoya, Y.; Itagaki, Y.; Aono, H.; Sadaoka, Y. Ozone detection in air using SmFeO3 gas sensor. Sens. Actuators B Chem. 2005, 108, 198–201. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, X.; Shen, J.; Hong, H.; Lin, H.; Liao, B.Q. New methods based on back propagation (BP) and radial basis function (RBF) artificial neural networks (ANNs) for predicting the occurrence of haloketones in tap water. Sci. Total Environ. 2021, 772, 145534. [Google Scholar] [CrossRef]
- Zhang, J.R.; Zhang, J.; Lok, T.M.; Lyu, M.R. A hybrid particle swarm optimization–back-propagation algorithm for feedforward neural network training. Appl. Math. Comput. 2007, 185, 1026–1037. [Google Scholar] [CrossRef]
- Masoumi, A.; Jabari, F.; Ghassem Zadeh, S.; Mohammadi-Ivatloo, B. Long-term load forecasting approach using dynamic feed-forward back-propagation artificial neural network. In Optimization of Power System Problems: Methods, Algorithms and MATLAB Codes; Springer: Berlin/Heidelberg, Germany, 2020; pp. 233–257. [Google Scholar]
- Panda, S.; Panda, G. Fast and improved backpropagation learning of multi-film artificial neural network using adaptive activation function. Expert Syst. 2020, 37, e12555. [Google Scholar] [CrossRef]
- Liu, F.; Huo, W.; Han, Y.; Yang, S.; Li, X. Study on network security based on PCA and BP neural network under green communication. IEEE Access 2020, 8, 53733–53749. [Google Scholar] [CrossRef]
- Pan, X.; Zhou, W.; Lu, Y.; Sun, N. Prediction of network traffic of smart cities based on DE-BP neural network. IEEE Access 2019, 7, 55807–55816. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, M.; Yin, Y.; Zhang, Q.; Cai, Z. Prediction algorithm for network security situation based on bp neural network optimized by sa-soa. Int. J. Perform. Eng. 2020, 16, 1171. [Google Scholar]
- Yu, F.; Xu, X. A short-term load forecasting model of natural gas based on optimized genetic algorithm and improved BP neural network. Appl. Energy 2014, 134, 102–113. [Google Scholar] [CrossRef]
- Fan, Z.; Zi-xuan, X.; Ming-hu, W. State of health estimation for Li-ion battery using characteristic voltage intervals and genetic algorithm optimized back propagation neural network. J. Energy Storage 2023, 57, 106277. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, R.; Cheng, Z. Air quality prediction using improved PSO-BP neural network. IEEE Access 2020, 8, 99346–99353. [Google Scholar] [CrossRef]
- Li, G.; Tan, Z.; Xu, W.; Xu, F.; Wang, L.; Chen, J.; Wu, K. A particle swarm optimization improved BP neural network intelligent model for electrocardiogram classification. BMC Med. Inform. Decis. Mak. 2021, 21, 99. [Google Scholar] [CrossRef] [PubMed]
- Abdolrasol, M.G.; Hussain, S.S.; Ustun, T.S.; Sarker, M.R.; Hannan, M.A.; Mohamed, R.; Milad, A. Artificial neural networks based optimization techniques: A review. Electronics 2021, 10, 2689. [Google Scholar] [CrossRef]
- Mulumba, D.M.; Liu, J.; Hao, J.; Zheng, Y.; Liu, H. Application of an optimized PSO-BP neural network to the assessment and prediction of underground coal mine safety risk factors. Appl. Sci. 2023, 13, 5317. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, Y.; Yang, W.; Ma, T.; Zhang, S.; Liang, T. Improved Selectivity of CeMnOx/Pt@SnO2 Laminated MOS Sensor for Hydrogen Cyanide Under Temperature Dynamic Modulation. Nanomaterials 2025, 15, 155. [Google Scholar] [CrossRef]





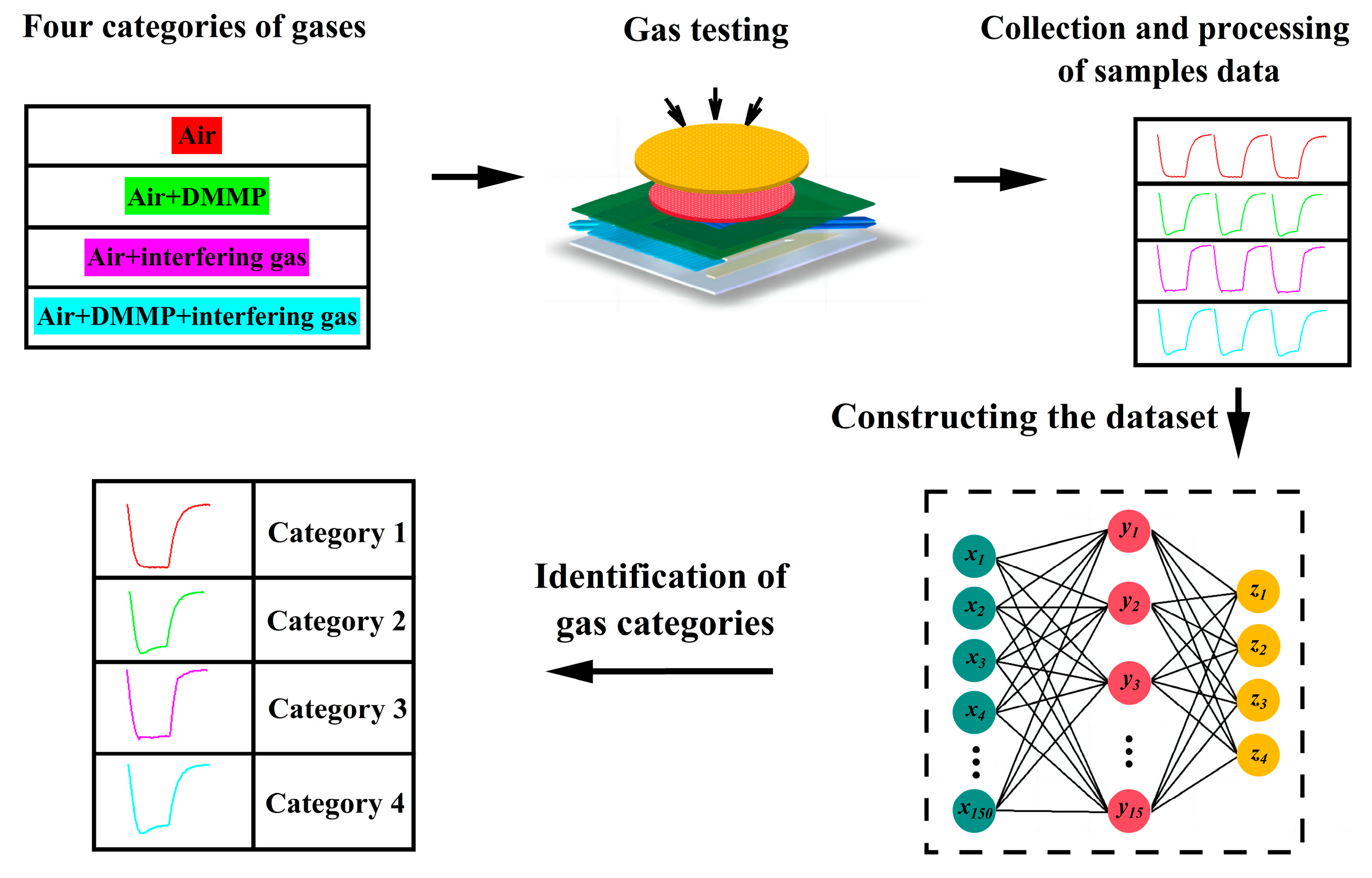



| Categories | Composition of Gases | Amount of Samples |
|---|---|---|
| 1 | Air | 200 |
| 2 | Air+0.5 mg/m3 DMMP | 200 |
| 3 | Air+0.5 mg/m3 interfering gas | 200 |
| 4 | Air+0.5 mg/m3 DMMP+0.5 mg/m3 interfering gas | 200 |
| Categories | Composition of Gases | Amount of Samples |
|---|---|---|
| Dataset 1 | ||
| 1 | Air | 200 |
| 2 | Air+0.1 mg/m3 DMMP | 200 |
| 3 | Air+0.5 mg/m3 interfering gas | 200 |
| 4 | Air+0.1 mg/m3 DMMP+0.5 mg/m3 interfering gas | 200 |
| Dataset 2 | ||
| 1 | Air | 200 |
| 2 | Air+0.2 mg/m3 DMMP | 200 |
| 3 | Air+0.5 mg/m3 interfering gas | 200 |
| 4 | Air+0.2 mg/m3 DMMP+0.5 mg/m3 interfering gas | 200 |
| Dataset 3 | ||
| 1 | Air | 200 |
| 2 | Air+0.5 mg/m3 DMMP | 200 |
| 3 | Air+0.5 mg/m3 interfering gas | 200 |
| 4 | Air+0.5 mg/m3 DMMP+0.5 mg/m3 interfering gas | 200 |
| Dataset 4 | ||
| 1 | Air | 200 |
| 2 | Air+1 mg/m3 DMMP | 200 |
| 3 | Air+0.5 mg/m3 interfering gas | 200 |
| 4 | Air+1 mg/m3 DMMP+0.5 mg/m3 interfering gas | 200 |
| Dataset 5 | ||
| 1 | Air | 200 |
| 2 | Air+2 mg/m3 DMMP | 200 |
| 3 | Air+0.5 mg/m3 interfering gas | 200 |
| 4 | Air+2 mg/m3 DMMP+0.5 mg/m3 interfering gas | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, T.; Qi, Y.; Cao, S.; Yan, R.; Gu, J.; Liu, Y. Identification of Sarin Simulant DMMP Based on a Laminated MOS Sensor Using Article Swarm Optimization-Backpropagation Neural Network. Sensors 2025, 25, 2734. https://doi.org/10.3390/s25092734
Liang T, Qi Y, Cao S, Yan R, Gu J, Liu Y. Identification of Sarin Simulant DMMP Based on a Laminated MOS Sensor Using Article Swarm Optimization-Backpropagation Neural Network. Sensors. 2025; 25(9):2734. https://doi.org/10.3390/s25092734
Chicago/Turabian StyleLiang, Ting, Yelin Qi, Shuya Cao, Rui Yan, Jin Gu, and Yadong Liu. 2025. "Identification of Sarin Simulant DMMP Based on a Laminated MOS Sensor Using Article Swarm Optimization-Backpropagation Neural Network" Sensors 25, no. 9: 2734. https://doi.org/10.3390/s25092734
APA StyleLiang, T., Qi, Y., Cao, S., Yan, R., Gu, J., & Liu, Y. (2025). Identification of Sarin Simulant DMMP Based on a Laminated MOS Sensor Using Article Swarm Optimization-Backpropagation Neural Network. Sensors, 25(9), 2734. https://doi.org/10.3390/s25092734




