High-Performance Sn2+-Doped CuFe2O4-Based Resistance Gas Sensor for the Detection of the Sarin Simulant DMMP
Abstract
Highlights
- A 2 mol% Sn2+-doped CuFe2O4 sensor was produced for DMMP detection.
- The sensor demonstrated suitable sensing performance and selectivity toward DMMP.
- The superior response–recovery characteristics and enhanced moisture resistance compared to the undoped sensor are relevant in gas-sensing applications.
- Sarin poses a serious threat to human health, necessitating the development of an appropriate sensing technology.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Material Synthesis and Preparation
2.2.1. Synthesis and Preparation of CuFe2O4 Doped with Different Ratios of Sn4+
2.2.2. Synthesis and Preparation of CuFe2O4 Doped with Different Ratios of Sn2+
2.3. Characterization of the Prepared Materials
2.4. Manufacturing and Testing of Gas Sensors
3. Results and Discussion
3.1. Structure and Morphology
3.2. Evaluation of the Gas-Sensing Performance
3.3. Mechanistic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barreca, D.; Maccato, C.; Gasparotto, A. Metal Oxide Nanosystems As Chemoresistive Gas Sensors for Chemical Warfare Agents: A Focused Review. Adv. Mater. Interfaces 2022, 9, 2102525. [Google Scholar] [CrossRef]
- Lee, S.C.; Kim, S.Y.; Lee, W.S.; Jung, S.Y.; Hwang, B.W.; Ragupathy, D.; Lee, D.D.; Lee, S.Y.; Kim, J.C. Effects of textural properties on the response of a SnO2-based gas sensor for the detection of chemical warfare agents. Sensors 2011, 11, 6893–6904. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; D’Cruz, R.; Faruqi, S.; Singh, K.K.; Varma, S.; Singh, M.; Karthik, V. Chemical warfare agents. Environ. Toxicol. Pharmacol. 2008, 26, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.H.; Yun, K.Y.; Cha, G.Y. Gas sensing characteristics of ZnO-doped SnO2 sensors for simulants of the chemical agents. Eco-Mater. Process. Des. VI 2005, 486–487, 9–12. [Google Scholar]
- Fehlen, P.; Thomas, G.; Gonzalez-Posada, F.; Guise, J.; Rusconi, F.; Cerutti, L.; Taliercio, T.; Spitzer, D. Gas sensing of organophosphorous compounds with III–V semiconductor plasmonics. Sens. Actuators B Chem. 2023, 376, 132987. [Google Scholar] [CrossRef]
- Rodrigues, J.; ShimpiIndium, N.G. Indium doped SnO2/polyaniline nanocomposites as a DMMP gas sensor at room temperature. Polym. Bull. 2024, 81, 8993–9007. [Google Scholar] [CrossRef]
- Kim, J.I.; Cho, K.H.; Lee, J.H. Compact, wavelength-selectable, energy-ratio variable Nd:YAG laser at mid-ultraviolet for chemical warfare agent detection. Curr. Opt. Photonics 2019, 3, 243–247. [Google Scholar]
- Melkonian, J.-M.; Armougom, J.; Raybaut, M.; Dherbecourt, J.B.; Gorju, G.; Cézard, N.; Godard, A.; Pašiškevičius, V.; Coetzee, R.; Kadlčák, J. Long-wave infrared multi-wavelength optical source for standoff detection of chemical warfare agents. Appl. Opt. 2020, 59, 11156–11166. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, B.-Y.; Liu, W.-W.; Fu, W.-X.; Kong, J.-L.; Li, J.-F. Rapidly Detection of Chemical Warfare Agent Simulants by Surface Enhanced Raman Spectroscopy. Guang Pu Xue Yu Guang Pu Fen Xi 2022, 42, 110–114. [Google Scholar]
- Ahrens, A.; Allers, M.; Bock, H.; Hitzemann, M.; Ficks, A.; Zimmermann, S. Detection of Chemical Warfare Agents with a Miniaturized High-Performance Drift Tube Ion Mobility Spectrometer Using High-Energetic Photons for Ionization. Anal. Chem. 2022, 94, 15440–15447. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, M.-Y.; Li, B.-Q. Rapid Detection of Chemical Warfare Agent Simulants Using Gold Nanoparticles Substrate-assisted Enhanced Low Temperature Plasma-Mass Spectrometry. Fēnxī Huà Xué 2020, 48, 1416–1421. [Google Scholar]
- Grabka, M.; Witkiewicz, Z.; Jasek, K.; Piwowarski, K. Acoustic Wave Sensors for Detection of Blister Chemical Warfare Agents and Their Simulants. Sensors 2022, 22, 5607. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Yang, L.; Cao, Y.S.; Yang, J.; Huang, Q.B. High-performance metal-oxide gas sensors based on hierarchical core–shell ZnFe2O4 microspheres for detecting 2-chloroethyl ethyl sulfide. Anal. Methods 2023, 15, 3084–3091. [Google Scholar] [CrossRef] [PubMed]
- Patil, L.A.; Deo, V.V.; Shinde, M.D.; Bari, A.R.; Patil, D.M.; Kaushik, M.P. Improved 2-CEES sensing performance of spray pyrolized Ru-CdSnO3 nanostructured thin films. Sens. Actuators B Chem. 2014, 191, 130–136. [Google Scholar] [CrossRef]
- Witkiewicz, Z.; Jasek, K.; Grabka, M. Semiconductor Gas Sensors for Detecting Chemical Warfare Agents and Their Simulants. Sensors 2023, 23, 3272. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Lim, K.; Jo, Y.-M.; Kim, S.; Yoon, J.W.; Jeong, S.Y.; Kim, J.S.; Choi, H.J.; Cho, Y.; Park, J.; Jeong, Y.W.; et al. Selective dual detection of hydrogen sulfide and methyl mercaptan using CuO/CuFe2O4 nanopattern chemiresistors. Sens. Actuators B Chem. 2021, 348, 130665. [Google Scholar] [CrossRef]
- Phuoc, P.H.; Van Hoang, N.; Hung, N.M.; Hoat, P.D.; Van Hieu, N. On-chip CuFe2O4 nanofiber for conductometric NO2 and H2S gas-sensors. Sens. Actuators B Chem. 2023, 380, 133306. [Google Scholar] [CrossRef]
- Wu, K.-D.; Xu, J.-Y.; Debliquy, M.; Zhang, C. Synthesis and NH3/TMA sensing properties of CuFe2O4 hollow microspheres at low working temperature. Rare Metals 2020, 40, 1768–1777. [Google Scholar] [CrossRef]
- Bai, S.; Chen, S.; Zhao, Y.; Guo, T.; Luo, R.; Li, D.; Chen, A. Gas sensing properties of Cd-doped ZnO nanofibers synthesized by the electrospinning method. J. Mater. Chem. A Mater. Energy Sustain. 2014, 2, 16697–16706. [Google Scholar] [CrossRef]
- Gao, H.; Yu, Q.; Zhang, S.; Wang, T.; Sun, P.; Lu, H.; Liu, F.; Yan, X.; Liu, F.; Liang, X.; et al. Nanosheet-assembled NiO microspheres modified by Sn2+ ions isovalent interstitial doping for xylene gas sensors. Sens. Actuators B Chem. 2018, 269, 210–222. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Yang, M.; Tang, L.; Wang, Q.; Sun, Y.; Xu, Y. Novel strategy to construct porous Sn-doped ZnO/ZnFe2O4 heterostructures for superior triethylamine detection. Mater. Sci. Semicond. Process. 2021, 125, 105643. [Google Scholar] [CrossRef]
- Yang, H.; Bai, X.; Hao, P.; Tian, J.; Bo, Y.; Wang, X.; Liu, H. A simple gas sensor based on zinc ferrite hollow spheres: Highly sensitivity, excellent selectivity and long-term stability. Sens. Actuators B 2019, 280, 34–40. [Google Scholar] [CrossRef]
- Tang, M.; Xia, F.; Gao, C.; Qiu, H. Preparation of magnetically recyclable CuFe2O4/RGO for catalytic hydrolysis of sodium borohydride. Int. J. Hydrog. Energy 2016, 41, 13058–13068. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, B.; Sun, H.; Wang, C.; Sun, P.; Li, X.; Hu, X.; Lu, G.Y. Template-free synthesis of hierarchical ZnFe2O4yolk–shell microspheres for high-sensitivity acetone sensors. Nanoscale 2016, 8, 5446–5453. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, X.; Huo, L.; Gao, S.; Guo, C.; Zhang, Y.; Major, Z.; Zhang, F.; Cheng, X.; Xu, Y.M. Controllable construction of ZnFe2O4-based micro-nano heterostructure for the rapid detection and degradation of VOCs. J. Hazard. Mater. 2022, 435, 129005. [Google Scholar] [CrossRef]
- Saji, K.J.; Subbaiah, Y.P.V.; Tian, K.; Tiwari, A. P-type SnO thin films and SnO/ZnO heterostructures for all-oxide electronic and optoelectronic device applications. Thin Solid films 2016, 605, 193–201. [Google Scholar] [CrossRef]
- Yi, Y.; Lyon, J.E.; Beerbom, M.M.; Schlaf, R. Characterization of indium tin oxide surfaces and interfaces using low intensity x-ray photoemission spectroscopy. J. Appl. Phys. 2006, 100, 93719. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Wang, L.; Liu, J.; Zhang, B.; Gao, Y.; Sun, P.; Sun, Y.; Zhang, T.; Lu, G.Y. Horseshoe-shaped SnO2 with annulus-like mesoporous for ethanol gas sensing application. Sens. Actuators B Chem. 2017, 240, 1321–1329. [Google Scholar] [CrossRef]
- Wieczorek, A.; Lai, H.; Pious, J.; Fu, F.; Siol, S. Resolving oxidation States and X-site composition of Sn preovskites through Auger parameter analysis in XPS. Adv. Mater. Interfaces 2023, 10, 2201828. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, H.; Han, D.; Gu, F. Electron compensation in p-type 3DOM NiO by Sn doping for enhanced formaldehyde sensing performance. J. Mater. Chem. C Mater. Opt. Electron. Devices 2017, 5, 3254–3263. [Google Scholar] [CrossRef]
- Wang, C.; Cui, X.; Liu, J. Design of Superior Ethanol Gas Sensor Based on Al-Doped NiO Nanorod-Flowers. ACS Sens. 2016, 1, 131–136. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, T.; Li, B.; Gao, R.; Zhang, X.; Cheng, X.; Huo, L.; Major, Z.; Xu, Y.M. Crosslinked WO3 nanonet for rapid detection of sulfur mustard gas simulant: Mechanism insights and sensing application. Sens. Actuators B Chem. 2023, 385, 133704. [Google Scholar] [CrossRef]
- Hübner, M.; Simion, C.E.; Tomescu-Stănoiu, A.; Pokhrel, S.; Bârsan, N.; Weimar, U. Influence of humidity on CO sensing with p-type CuO thick film gas sensors. Sens. Actuators B Chem. 2011, 153, 347–353. [Google Scholar] [CrossRef]
- Kim, J.; Hou, B.; Park, C. Effect of defects on reaction of NiO surface with Pb-contained solution. Sci. Rep. 2017, 7, 44805. [Google Scholar] [CrossRef] [PubMed]
- Kitakatsu, N.; Maurice, V.; Hinnen, C.; Marcus, P. Surface hydroxylation and local structure of NiO thin films formed on Ni(111). Surf. Sci. 1998, 407, 36–38. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Fan, J.; Zhu, B.; Yu, J. Triethylamine gas sensor based on Pt-functionalized hierarchical ZnO microspheres. Sens. Actuators B Chem. 2021, 331, 129425. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Cheng, P.; Xu, L.; Dang, F.; Wang, T.; Lei, Z. In-situ generated TiO2/α-Fe2O3 heterojunction arrays for batch manufacturing of conductometric acetone gas sensors. Sens. Actuators B Chem. 2021, 340, 129926. [Google Scholar] [CrossRef]
- Hermawan, A.; Zhang, B.; Taufik, A. CuO Nanoparticles/Ti3C2Tx MXene Hybrid Nanocomposites for Detection of Toluene Gas. ACS Appl. Nano Mater. 2020, 3, 4755. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.-S.; Kim, W. Chemiresistive Gas Sensors for Detection of Chemical Warfare Agent Simulants. Senseo Haghoeji 2019, 28, 139. [Google Scholar]
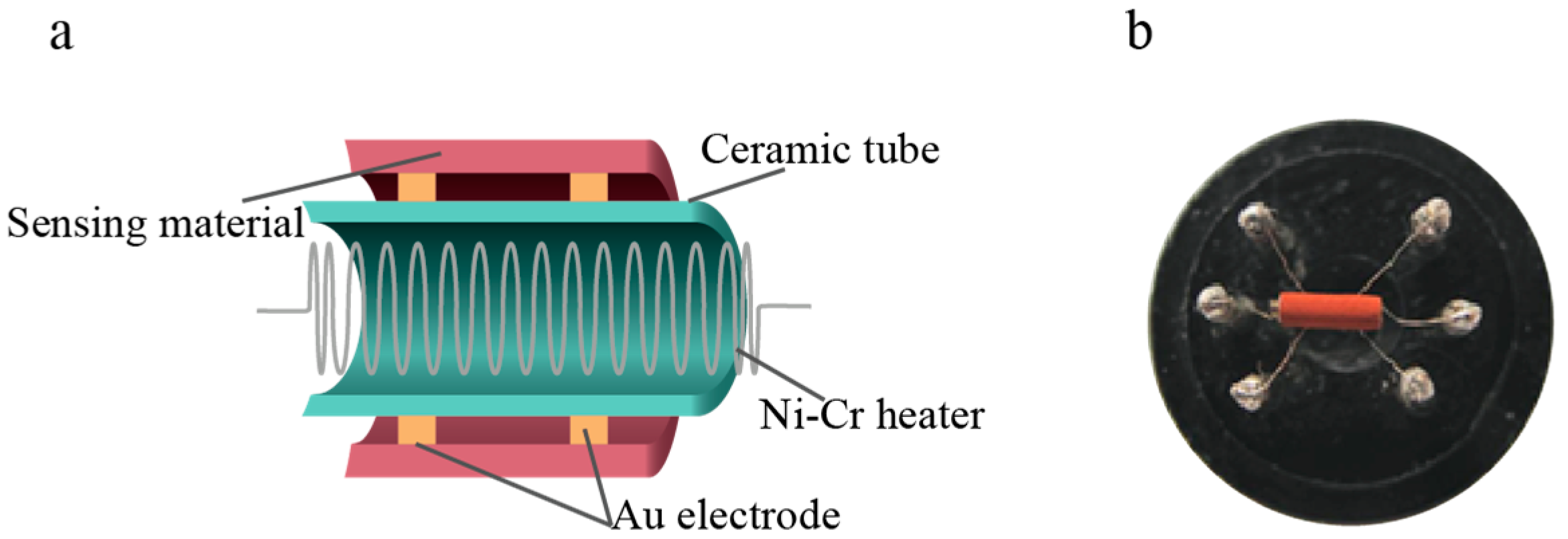

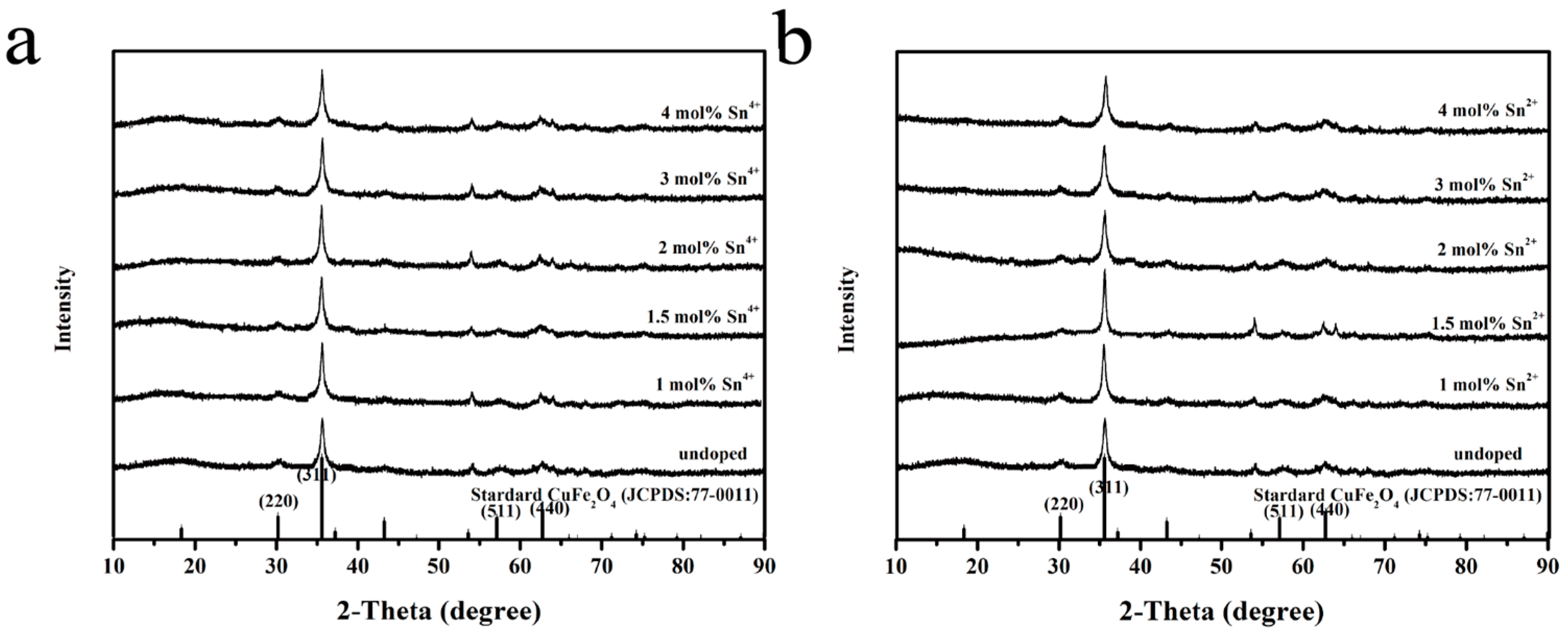
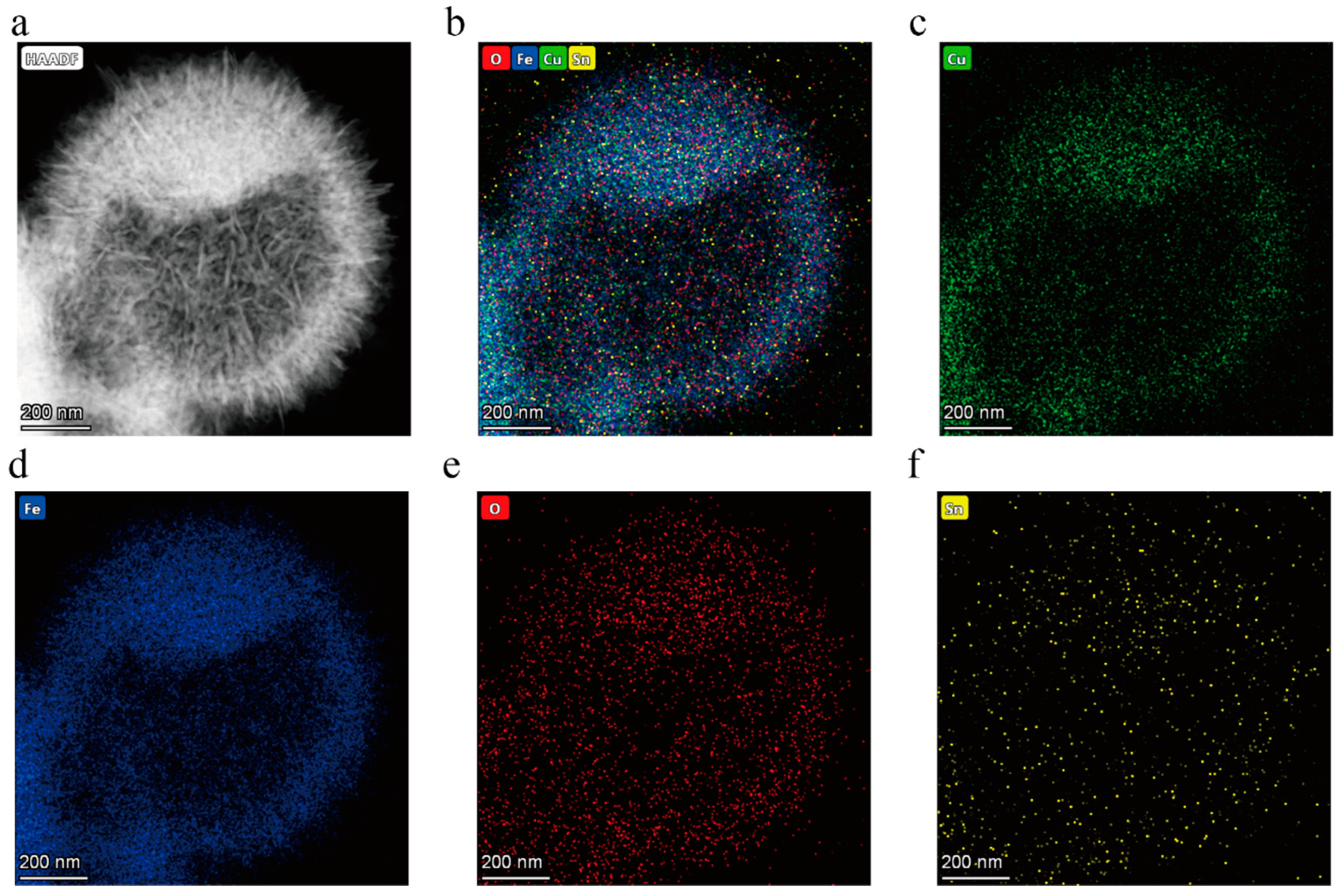

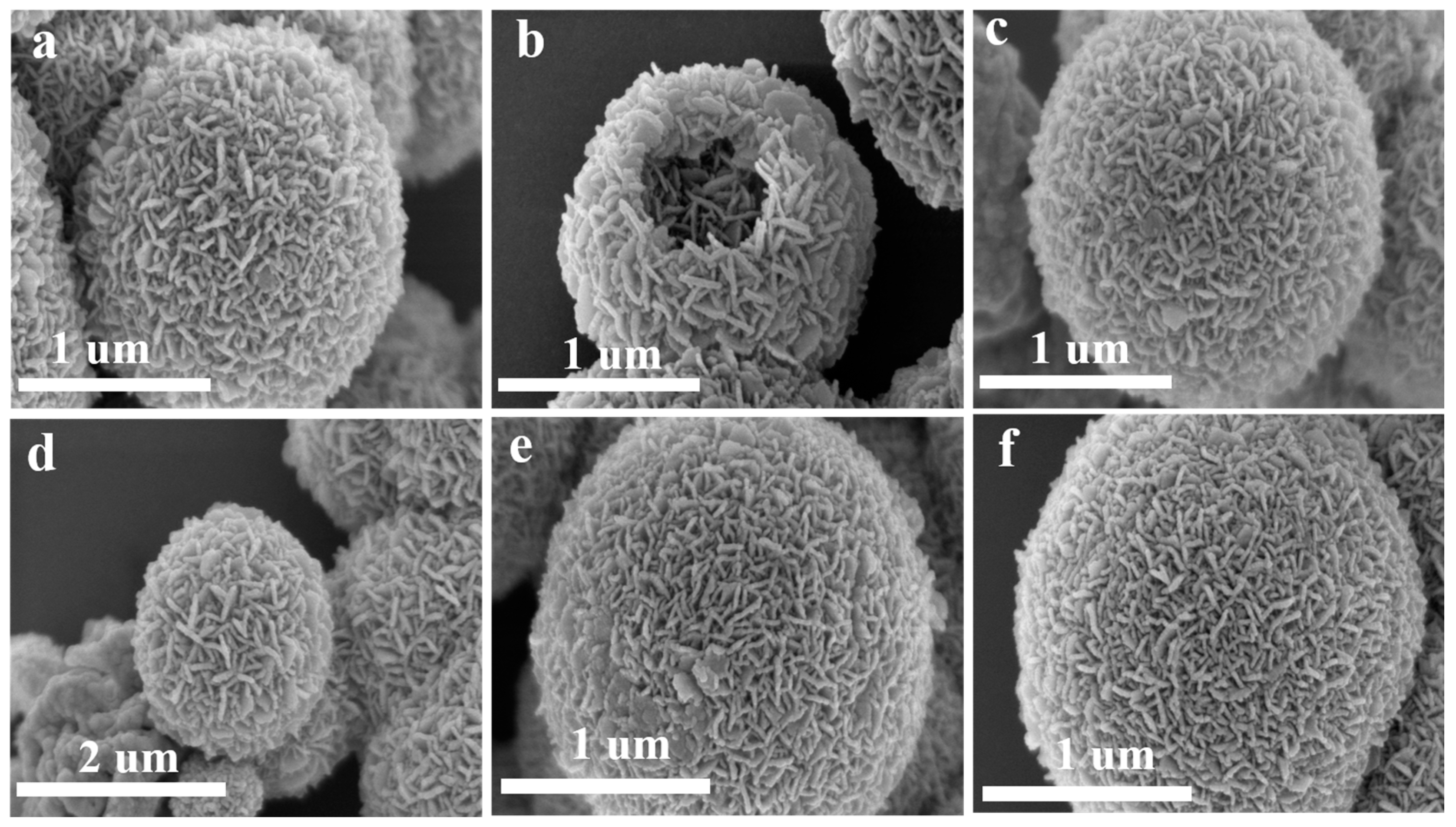
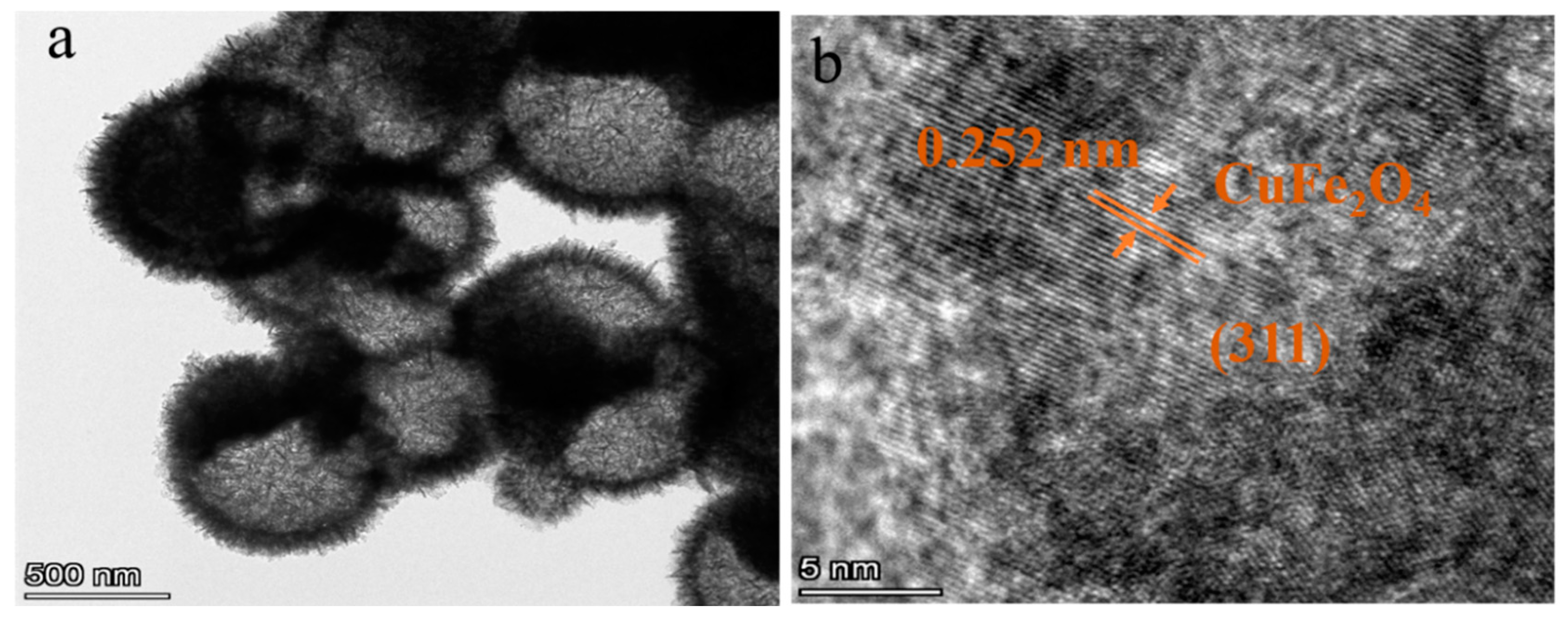
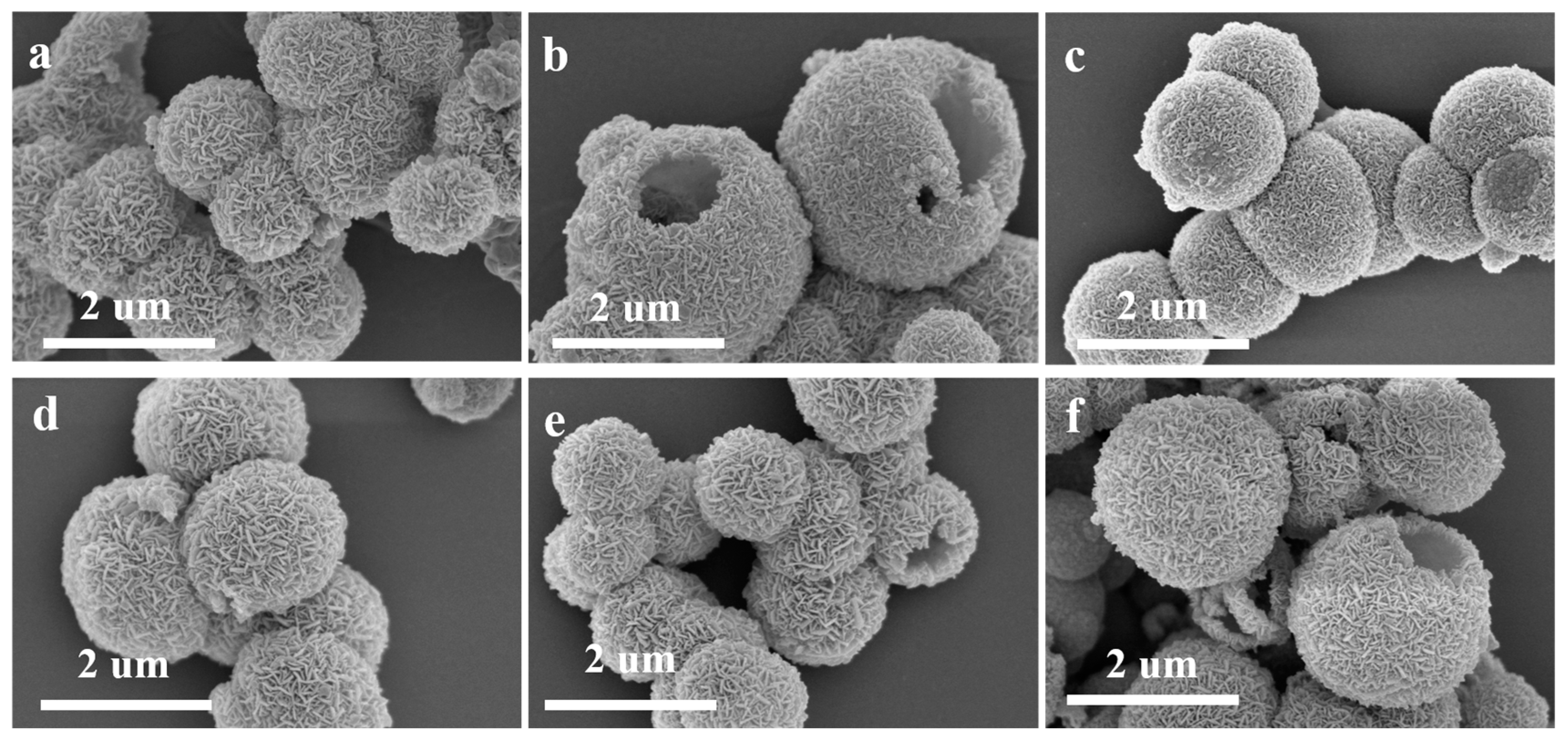
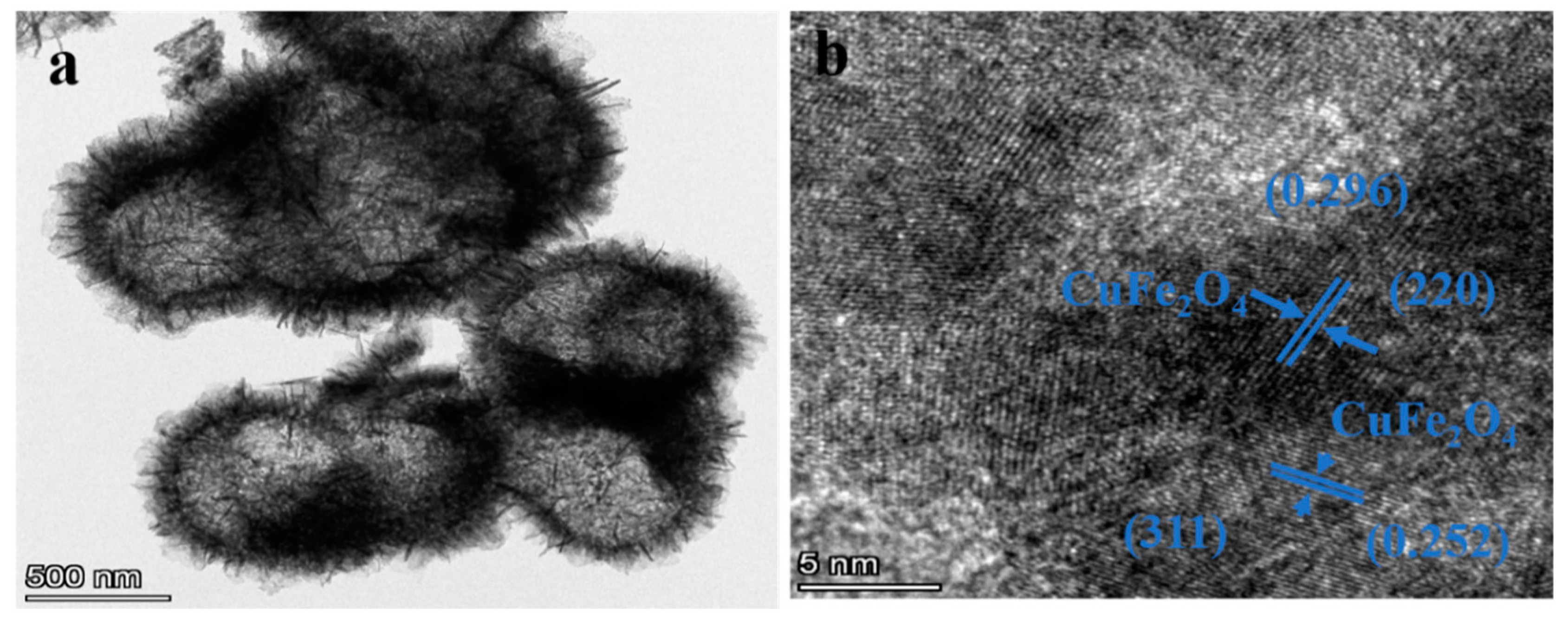
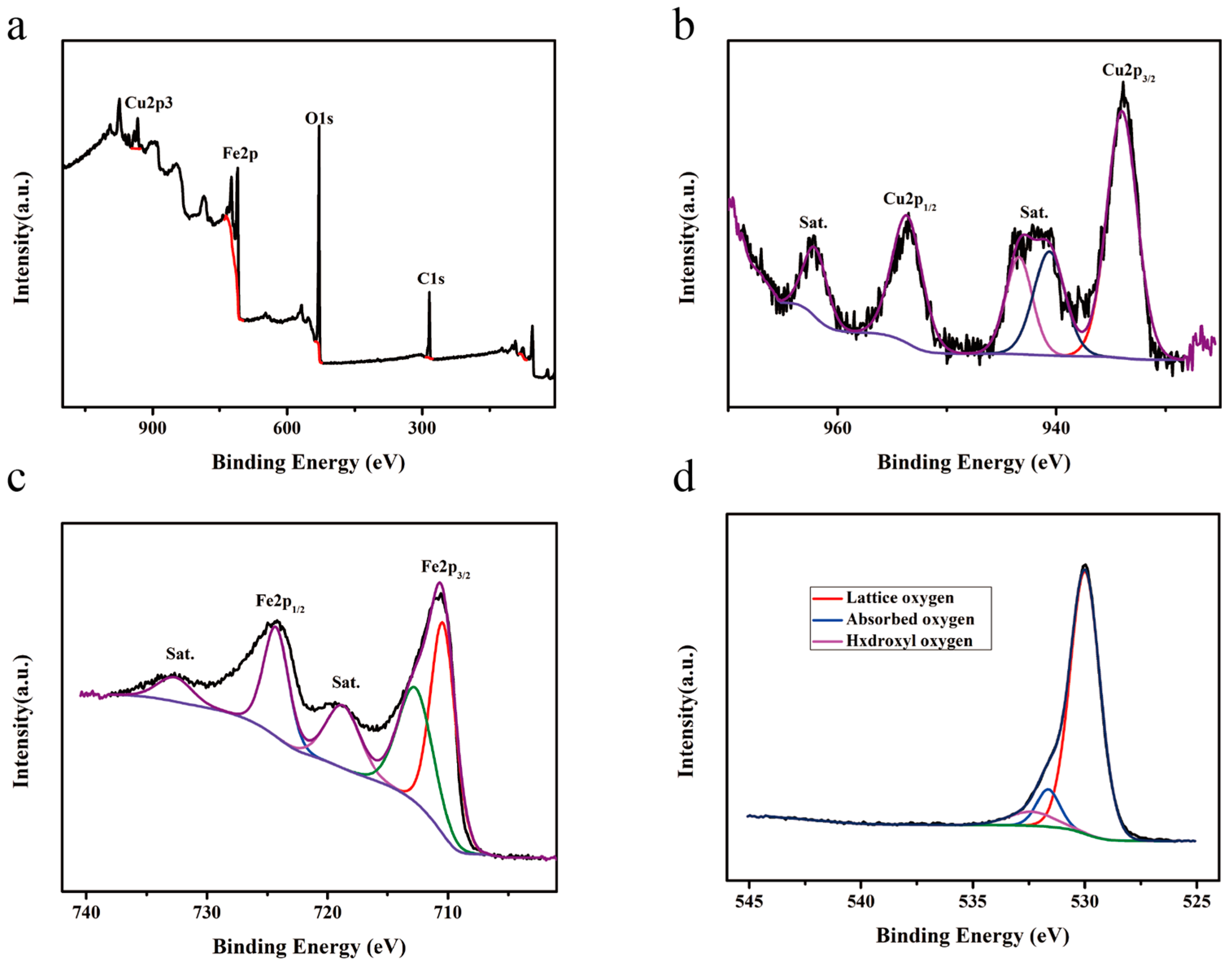
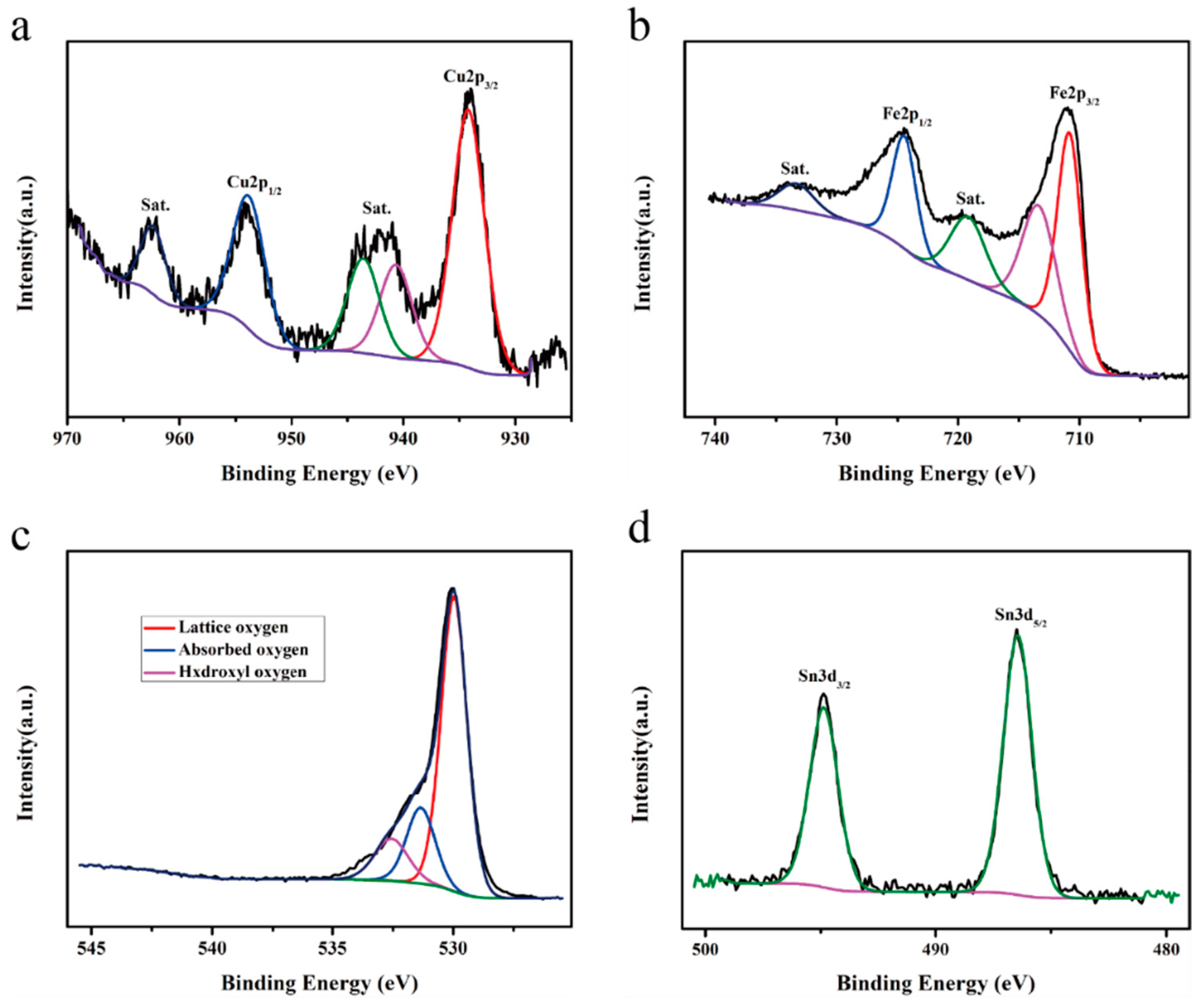
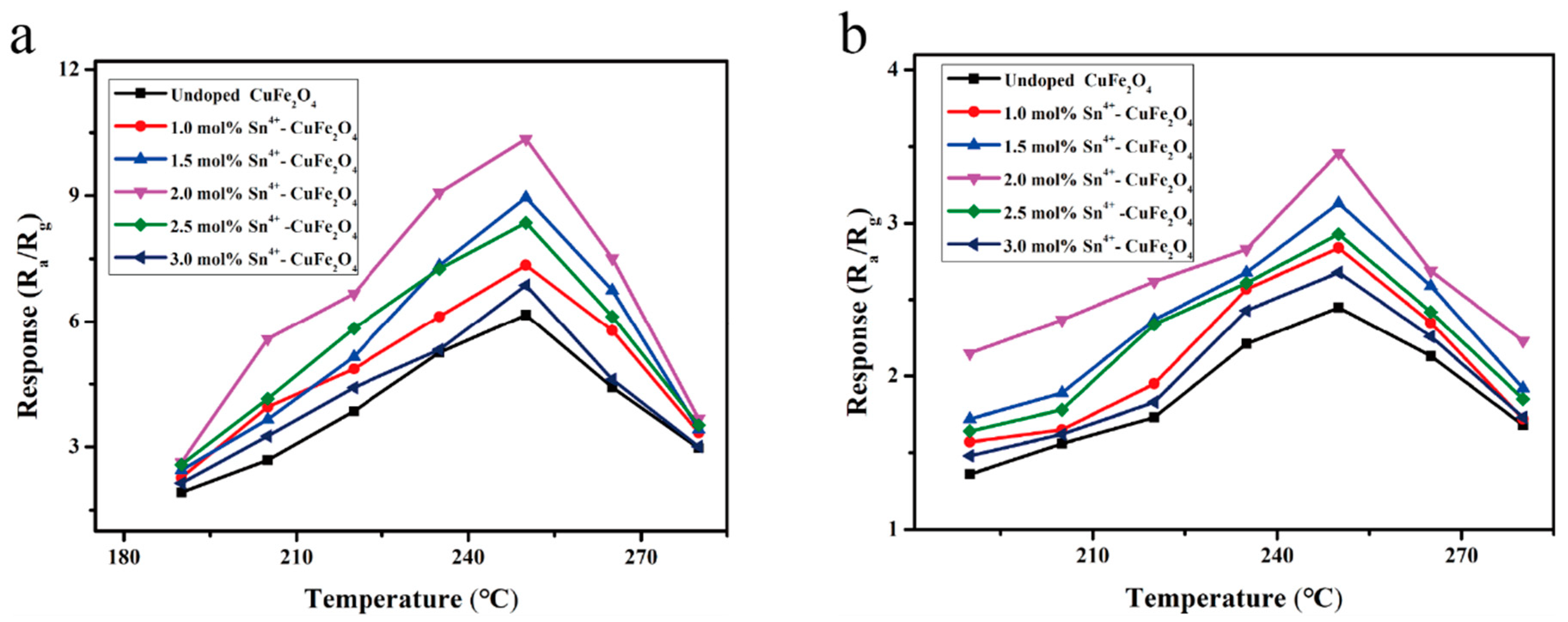

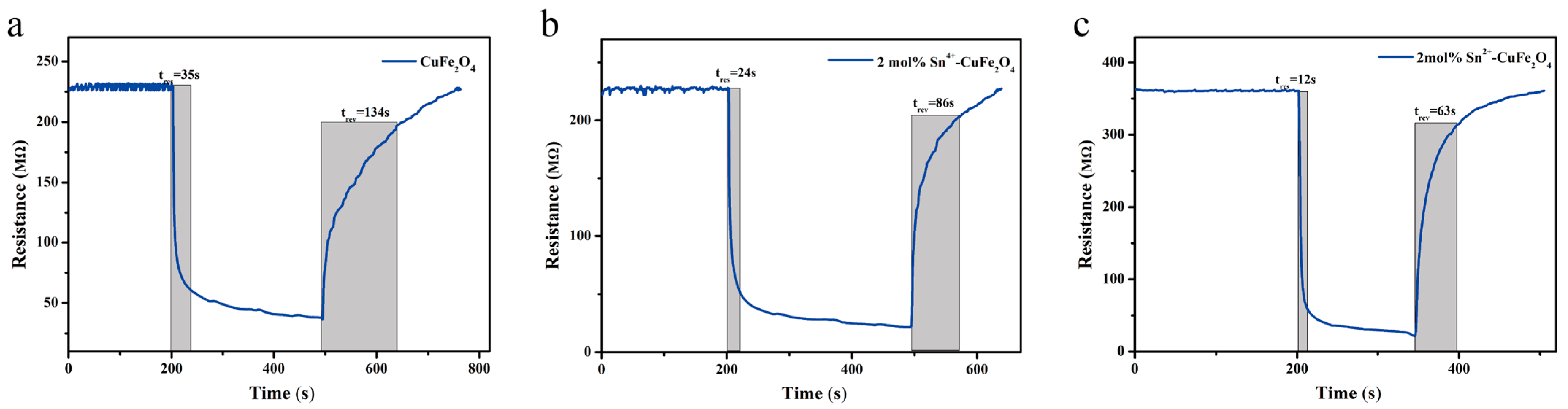

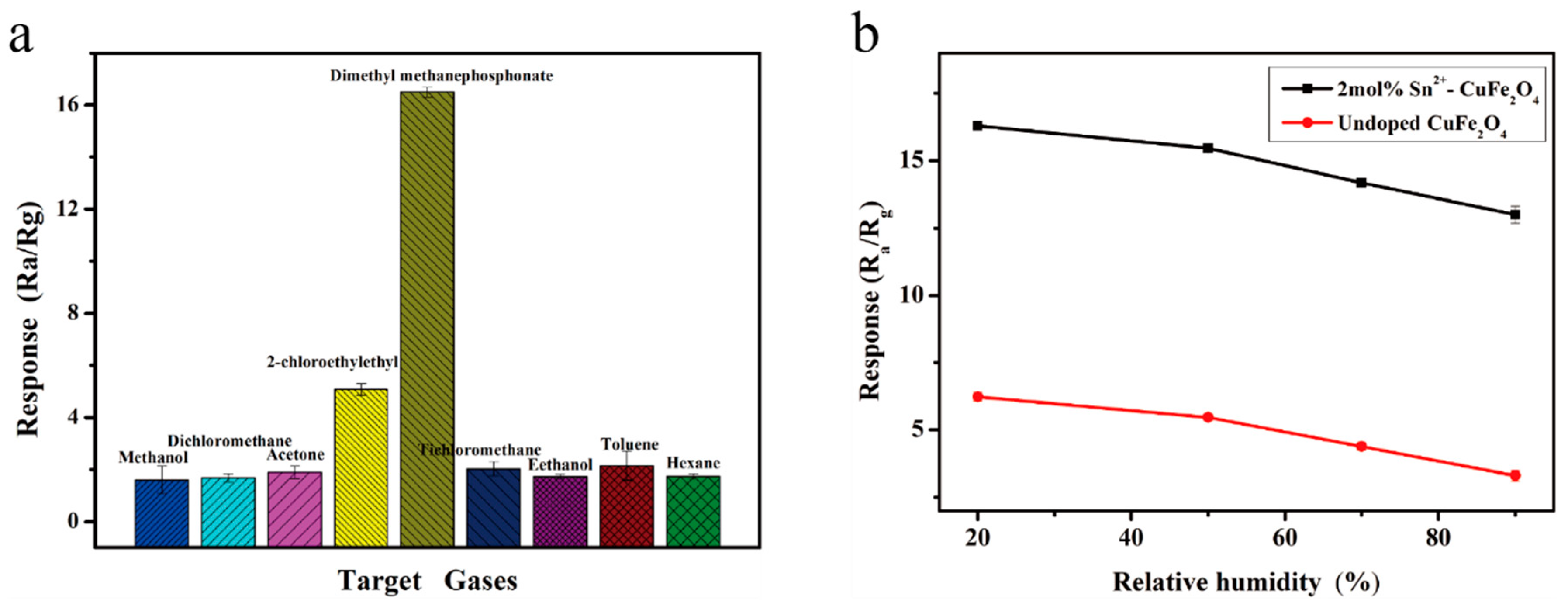
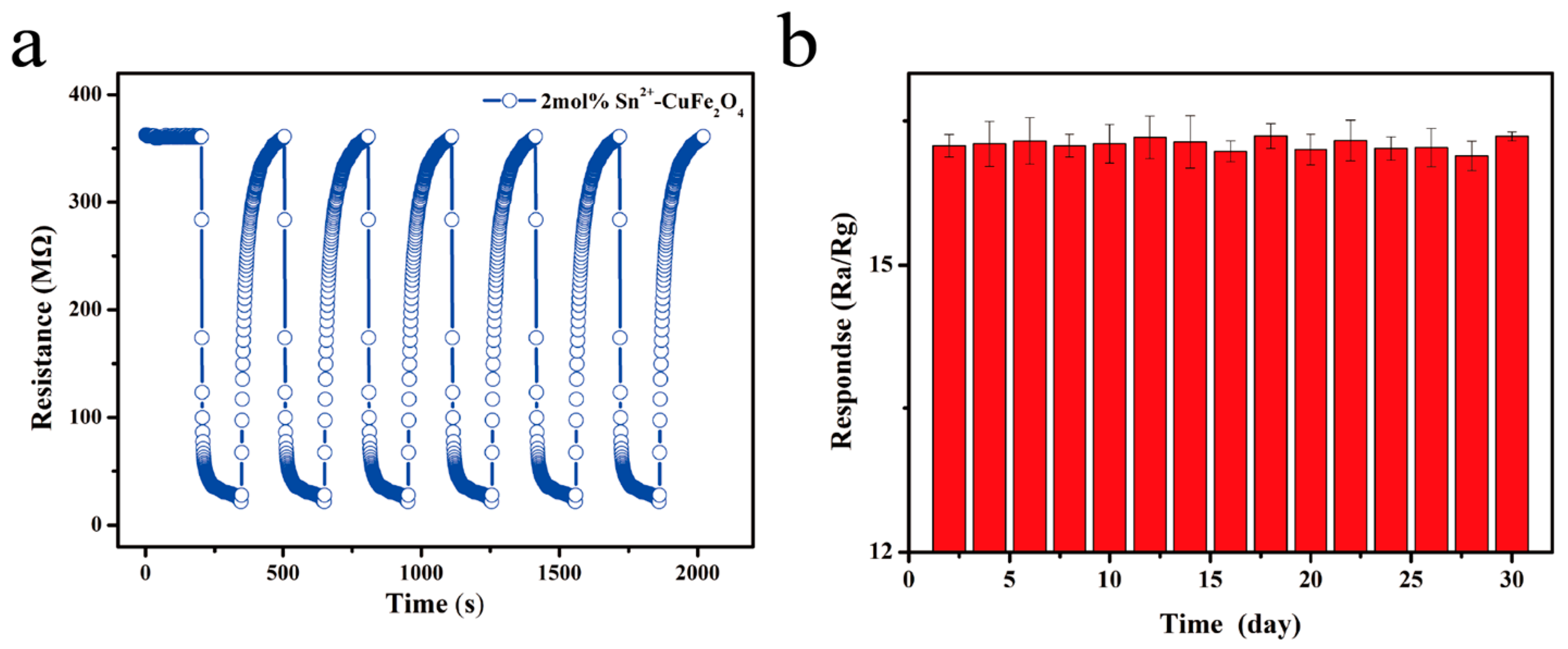
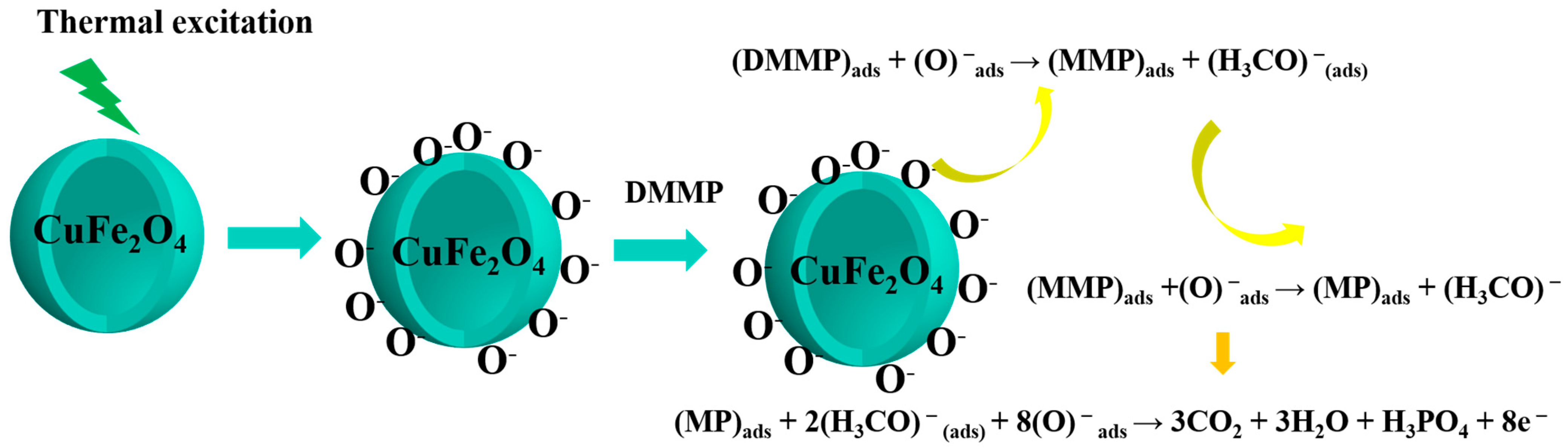
| Material | Cu | Fe | O | Sn |
|---|---|---|---|---|
| Pure-CuFe2O4 | 13.87% | 27.53% | 58.60% | 0 |
| 1 mol% Sn4+-CuFe2O4 | 13.60% | 26.89% | 59.40% | 0.11% |
| 1.5 mol% Sn4+-CuFe2O4 | 11.76% | 28.91% | 59.20% | 0.13% |
| 2 mol% Sn4+-CuFe2O4 | 12.72% | 28.21% | 58.90% | 0.17% |
| 3 mol% Sn4+-CuFe2O4 | 11.92% | 29.52% | 58.30% | 0.26% |
| 4 mol% Sn4+-CuFe2O4 | 12.15% | 29.32% | 58.20% | 0.33% |
| Material | Cu | Fe | O | Sn |
|---|---|---|---|---|
| Pure CuFe2O4 | 13.70% | 27.80% | 58.50% | 0 |
| 1 mol% Sn2+-CuFe2O4 | 12.74% | 32.80% | 54.38% | 0.08% |
| 1.5 mol% Sn2+-CuFe2O4 | 13.16% | 28.70% | 58.02% | 0.12% |
| 2 mol% Sn2+-CuFe2O4 | 12.10% | 29.60% | 58.12% | 0.18% |
| 3 mol% Sn2+-CuFe2O4 | 13.20% | 31.50% | 55.05% | 0.25% |
| 4 mol% Sn2+-CuFe2O4 | 12.90% | 29.20% | 57.56% | 0.34% |
| Appearance | Response Value | Response/Recovery Time |
|---|---|---|
| Undoped CuFe2O4 | 6.16 | 35 s/134 s |
| CuFe2O4 doped with 2 mol% Sn4+ | 10.35 | 24 s/86 s |
| CuFe2O4 doped with 2 mol% Sn2+ | 16.27 | 12 s/63 s |
| Appearance | Lattice Oxygen | Surface Adsorbed Oxygen | Hydroxyl Oxygen |
|---|---|---|---|
| Undoped CuFe2O4 | 79.34% | 11.23% | 9.43% |
| CuFe2O4 doped with 2 mol% Sn4+ | 73.49% | 15.37% | 11.14% |
| CuFe2O4 doped with 2 mol% Sn2+ | 69.44% | 18.85% | 12.51% |
| DMMP Concentration | 0.05 ppm | 0.1 ppm | 0.2 ppm | 0.5 ppm | 1 ppm | 1.8 ppm |
|---|---|---|---|---|---|---|
| Response value | 1.76 | 3.12 | 5.37 | 10.03 | 16.27 | 28.24 |
| Material | Operating Temperature | Concentration | Response Value | Limit of Detection | Literature |
|---|---|---|---|---|---|
| SnO2 | 350 °C | 0.5 ppm | 0.4 | 0.2 ppm | [2] |
| 4% ZnO-SnO2 | 300 °C | 1 ppm | 0.55 | 0.1 ppm | [4] |
| 2 mol% Sn2+-CuFe2O4 | 250 °C | 1 ppm | 16.27 | 0.05 ppm | This paper |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Yang, L.; Liang, T.; Zhang, L.; Wei, J.; Cao, S.; Huang, Q. High-Performance Sn2+-Doped CuFe2O4-Based Resistance Gas Sensor for the Detection of the Sarin Simulant DMMP. Sensors 2025, 25, 3042. https://doi.org/10.3390/s25103042
Yang J, Yang L, Liang T, Zhang L, Wei J, Cao S, Huang Q. High-Performance Sn2+-Doped CuFe2O4-Based Resistance Gas Sensor for the Detection of the Sarin Simulant DMMP. Sensors. 2025; 25(10):3042. https://doi.org/10.3390/s25103042
Chicago/Turabian StyleYang, Junchao, Liu Yang, Ting Liang, Ling Zhang, Jianan Wei, Shuya Cao, and Qibin Huang. 2025. "High-Performance Sn2+-Doped CuFe2O4-Based Resistance Gas Sensor for the Detection of the Sarin Simulant DMMP" Sensors 25, no. 10: 3042. https://doi.org/10.3390/s25103042
APA StyleYang, J., Yang, L., Liang, T., Zhang, L., Wei, J., Cao, S., & Huang, Q. (2025). High-Performance Sn2+-Doped CuFe2O4-Based Resistance Gas Sensor for the Detection of the Sarin Simulant DMMP. Sensors, 25(10), 3042. https://doi.org/10.3390/s25103042





