Using Wearable Devices to Examine the Associations of Sedentary Behavior with Perceived and Performance Fatigability Among Older Adults: The Study of Muscle, Mobility and Aging (SOMMA)
Abstract
1. Introduction
2. Materials and Methods
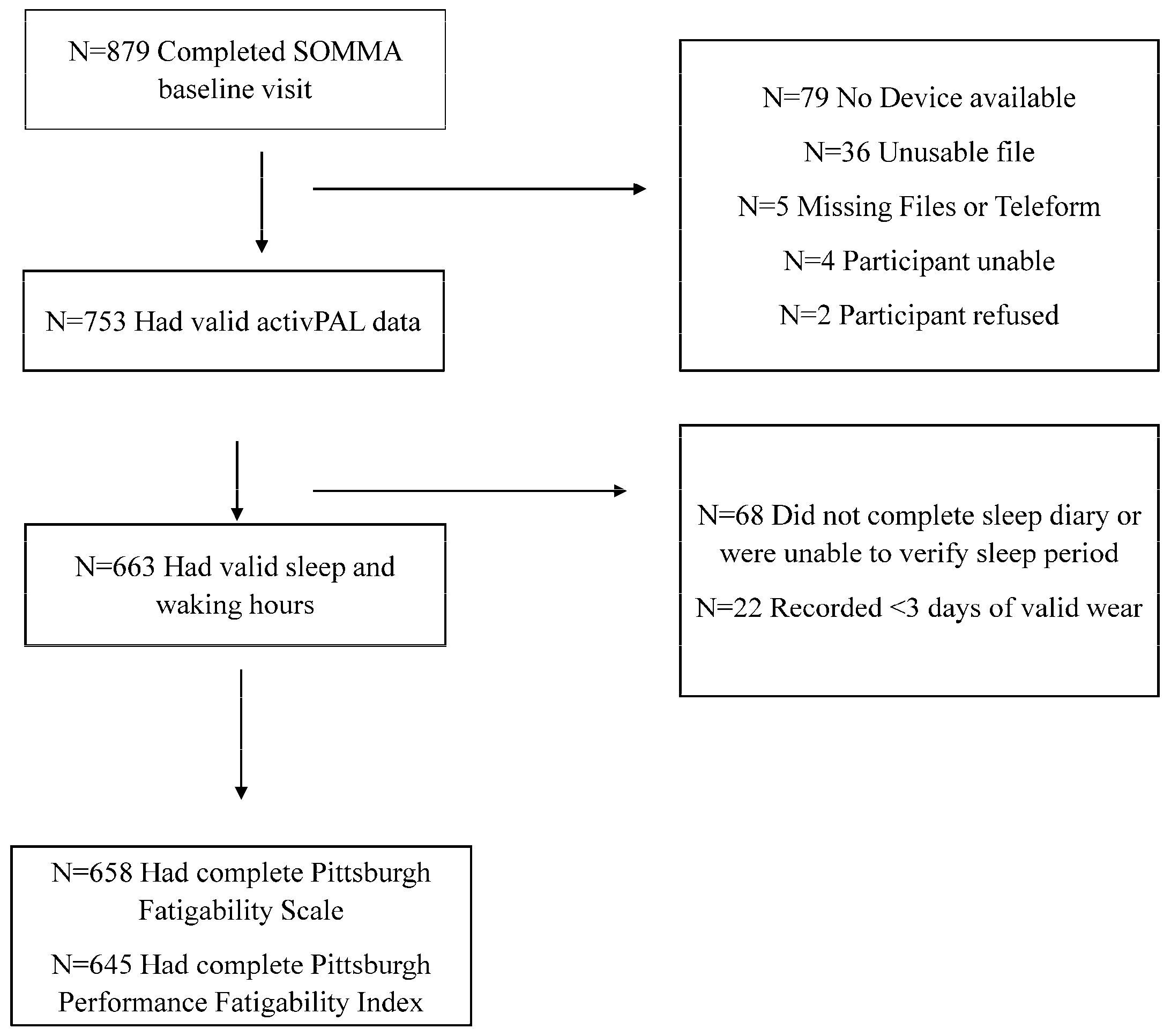
2.1. Study Population
2.2. Perceived and Performance Fatigability Measures
2.3. Sedentary Behavior Measurement
2.4. Confounders
2.5. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SOMMA | Study of Muscle, Mobility and Aging |
| PFS | Pittsburgh Fatigability Scale |
| PPFI | Pittsburgh Performance Fatigability Index |
References
- Glynn, N.W.; Qiao, Y.S. Measuring and understanding the health impact of greater fatigability in older adults: A call to action and opportunities. Fatigue 2023, 11, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Simonsick, E.M.; Schrack, J.A.; Santanasto, A.J.; Studenski, S.A.; Ferrucci, L.; Glynn, N.W. Pittsburgh Fatigability Scale: One-Page Predictor of Mobility Decline in Mobility-Intact Older Adults. J. Am. Geriatr. Soc. 2018, 66, 2092–2096. [Google Scholar] [CrossRef] [PubMed]
- Glynn, N.W.; Gmelin, T.; Renner, S.W.; Qiao, Y.; Boudreau, R.M.; Feitosa, M.F.; Wojczynski, M.K.; Cosentino, S.; Andersen, S.L.; Christensen, K.; et al. Perceived Physical Fatigability Predicts All-Cause Mortality in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 77, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Schrack, J.A.; Simonsick, E.M.; Glynn, N.W. Fatigability: A prognostic indicator of phenotypic aging. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, e63–e66. [Google Scholar] [CrossRef]
- Santanasto, A.J.; Glynn, N.W.; Jubrias, S.A.; Conley, K.E.; Boudreau, R.M.; Amati, F.; Mackey, D.C.; Simonsick, E.M.; Strotmeyer, E.S.; Coen, P.M.; et al. Skeletal muscle mitochondrial function and fatigability in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1379–1385. [Google Scholar] [CrossRef]
- Qiao, Y.; Santanasto, A.J.; Coen, P.M.; Cawthon, P.M.; Cummings, S.R.; Forman, D.E.; Goodpaster, B.H.; Harezlak, J.; Hawkins, M.; Kritchevsky, S.B.; et al. Associations between skeletal muscle energetics and accelerometry-based performance fatigability: Study of Muscle, Mobility and Aging. Aging Cell 2023, 23, e14015. [Google Scholar] [CrossRef]
- Schrack, J.A.; Wanigatunga, A.A.; Zipunnikov, V.; Kuo, P.-L.; Simonsick, E.M.; Ferrucci, L. Longitudinal Association Between Energy Regulation and Fatigability in Mid-to-Late Life. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, e74–e80. [Google Scholar] [CrossRef]
- Richardson, C.A.; Glynn, N.W.; Ferrucci, L.G.; Mackey, D.C. Walking energetics, fatigability, and fatigue in older adults: The study of energy and aging pilot. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 487–494. [Google Scholar] [CrossRef]
- Qiao, Y.; Gmelin, T.; Renner, S.W.; Boudreau, R.M.; Martin, S.; Wojczynski, M.K.; Christensen, K.; Andersen, S.L.; Cosentino, S.; Santanasto, A.J.; et al. Evaluation of the bidirectional relations of perceived physical fatigability and physical activity on slower gait speed. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, e237–e244. [Google Scholar] [CrossRef]
- Qiao, Y.; Moored, K.D.; Boudreau, R.M.; Roe, L.S.; Cawthon, P.M.; Stone, K.L.; Cauley, J.A.; Glynn, N.W. Changes in objectively measured physical activity are associated with perceived physical and mental fatigability in older men. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 2507–2516. [Google Scholar] [CrossRef]
- Graves, J.L.; Qiao, Y.; Moored, K.D.; Boudreau, R.M.; Venditti, E.M.; Krafty, R.T.; Shiroma, E.J.; Harezlak, J.; Glynn, N.W. Profiles of Accelerometry-Derived Physical Activity Are Related to Perceived Physical Fatigability in Older Adults. Sensors 2021, 21, 1718. [Google Scholar] [CrossRef] [PubMed]
- Wanigatunga, A.A.; Simonsick, E.M.; Zipunnikov, V.; Spira, A.P.; Studenski, S.; Ferrucci, L.; Schrack, J.A. Perceived Fatigability and Objective Physical Activity in Mid- to Late-Life. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 630–635. [Google Scholar] [CrossRef]
- Moored, K.D.; Qiao, Y.; Boudreau, R.M.; Roe, L.S.; Cawthon, P.M.; Cauley, J.A.; Glynn, N.W. Prospective associations between physical activity and perceived fatigability in older men: Differences by activity type and baseline marital status. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 2498–2506. [Google Scholar] [CrossRef]
- Qiao, Y.; Van Londen, G.J.; Brufsky, J.W.; Poppenberg, J.T.; Cohen, R.W.; Boudreau, R.M.; Glynn, N.W. Perceived physical fatigability improves after an exercise intervention among breast cancer survivors: A randomized clinical trial. Breast Cancer 2021, 29, 30–37. [Google Scholar] [CrossRef]
- Collins, K.J.; Schrack, J.A.; VanSwearingen, J.M.; Glynn, N.W.; Pospisil, M.C.; Gant, V.E.; Mackey, D.C. Randomized controlled trial of exercise to improve walking energetics in older adults. Innov. Aging 2018, 2, igy022. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.J.; Brintnall, R.A.; Given, B.A.; von Eye, A.; Jones, L.W.; Brown, J.K. Using Perceived Self-efficacy to Improve Fatigue and Fatigability in Postsurgical Lung Cancer Patients: A Pilot Randomized Controlled Trial. Cancer Nurs. 2017, 40, 1–12. [Google Scholar] [CrossRef]
- Schrack, J.A.; Kuo, P.L.; Wanigatunga, A.A.; Di, J.; Simonsick, E.M.; Spira, A.P.; Ferrucci, L.; Zipunnikov, V. Active-to-Sedentary Behavior Transitions, Fatigability, and Physical Functioning in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Palmberg, L.; Rantalainen, T.; Rantakokko, M.; Karavirta, L.; Siltanen, S.; Skantz, H.; Saajanaho, M.; Portegijs, E.; Rantanen, T. The Associations of Activity Fragmentation with Physical and Mental Fatigability Among Community-Dwelling 75-, 80-, and 85-Year-Old People. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, e103–e110. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Kozey-Keadle, S.; Libertine, A.; Lyden, K.; Staudenmayer, J.; Freedson, P.S. Validation of wearable monitors for assessing sedentary behavior. Med. Sci. Sports Exerc. 2011, 43, 1561–1567. [Google Scholar] [CrossRef]
- Montoye, A.H.; Pivarnik, J.M.; Mudd, L.M.; Biswas, S.; Pfeiffer, K.A. Validation and comparison of accelerometers worn on the hip, thigh, and wrists for measuring physical activity and sedentary behavior. AIMS Public Health 2016, 3, 298–312. [Google Scholar] [CrossRef]
- Suorsa, K.; Pulakka, A.; Leskinen, T.; Pentti, J.; Holtermann, A.; Heinonen, O.J.; Sunikka, J.; Vahtera, J.; Stenholm, S. Comparison of Sedentary Time Between Thigh-Worn and Wrist-Worn Accelerometers. J. Meas. Phys. Behav. 2020, 3, 234–243. [Google Scholar] [CrossRef]
- Qiao, Y.S.; Harezlak, J.; Moored, K.D.; Urbanek, J.K.; Boudreau, R.M.; Toto, P.; Hawkins, M.; Santanasto, A.J.; Schrack, J.A.; Simonsick, E.M.; et al. Development of a Novel Accelerometry-Based Performance Fatigability Measure for Older Adults. Med. Sci. Sports Exerc. 2022, 54, 1782–1793. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.T.; Powell, K.E.; Jakicic, J.M.; Troiano, R.P.; Piercy, K.; Tennant, B. Sedentary Behavior and Health: Update from the 2018 Physical Activity Guidelines Advisory Committee. Med. Sci. Sports Exerc. 2019, 51, 1227–1241. [Google Scholar] [CrossRef]
- Cummings, S.R.; Newman, A.B.; Coen, P.M.; Hepple, R.T.; Collins, R.; Kennedy, K.; Danielson, M.; Peters, K.; Blackwell, T.; Johnson, E.; et al. The Study of Muscle, Mobility and Aging (SOMMA). A Unique Cohort Study about the Cellular Biology of Aging and Age-related Loss of Mobility. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 2083–2093. [Google Scholar] [CrossRef]
- Glynn, N.W.; Santanasto, A.J.; Simonsick, E.M.; Boudreau, R.M.; Beach, S.R.; Schulz, R.; Newman, A.B. The Pittsburgh Fatigability Scale for older adults: Development and validation. J. Am. Geriatr. Soc. 2015, 63, 130–135. [Google Scholar] [CrossRef]
- Renner, S.W.; Bear, T.M.; Brown, P.J.; Andersen, S.L.; Cosentino, S.; Gmelin, T.; Boudreau, R.M.; Cauley, J.A.; Qiao, Y.; Simonsick, E.M.; et al. Validation of perceived mental fatigability using the Pittsburgh Fatigability Scale. J. Am. Geriatr. Soc. 2021, 69, 1343–1348. [Google Scholar] [CrossRef]
- Cooper, R.; Popham, M.; Santanasto, A.J.; Hardy, R.; Glynn, N.W.; Kuh, D. Are BMI and inflammatory markers independently associated with physical fatigability in old age? Int. J. Obes. 2019, 43, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.S.; Harezlak, J.; Cawthon, P.M.; Cummings, S.R.; Forman, D.E.; Goodpaster, B.H.; Hawkins, M.; Moored, K.D.; Nicklas, B.J.; Toledo, F.G.; et al. Validation of the Pittsburgh Performance Fatigability Index in the Study of Muscle, Mobility and Aging (SOMMA). J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 2387–2395. [Google Scholar] [CrossRef]
- Espeland, M.A.; Crimmins, E.M.; Grossardt, B.R.; Crandall, J.P.; Gelfond, J.A.; Harris, T.B.; Kritchevsky, S.B.; Manson, J.E.; Robinson, J.G.; Rocca, W.A.; et al. Clinical Trials Targeting Aging and Age-Related Multimorbidity. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 355–361. [Google Scholar] [CrossRef]
- Wolf, C.; Blackwell, T.L.; Johnson, E.; Glynn, N.W.; Nicklas, B.; Kritchevsky, S.B.; Carnero, E.A.; Cawthon, P.M.; Cummings, S.R.; Toledo, F.G.; et al. Cardiopulmonary exercise testing in a prospective multicenter cohort of older adults. Med. Sci. Sports Exerc. 2024, 56, 1574–1584. [Google Scholar] [CrossRef]
- Schrack, J.A.; Simonsick, E.M.; Ferrucci, L. The relationship of the energetic cost of slow walking and peak energy expenditure to gait speed in mid-to-late life. Am. J. Phys. Med. Rehabil. 2013, 92, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Johnson, W.D.; Katzmarzyk, P.T. Relationship between accelerometer-determined steps/day and other accelerometer outputs in US adults. J. Phys. Act. Health 2011, 8, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.M.; Duarte-Mendes, P.; Rusenhack, M.C.; Furmann, M.; Nobre, P.R.; Fachada, M.Â.; Soares, C.M.; Teixeira, A.; Ferreira, J.P. Objectively Measured Sedentary Behavior and Physical Fitness in Adults: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8660. [Google Scholar] [CrossRef] [PubMed]
- Van der Velde, J.H.; Koster, A.; Van der Berg, J.D.; Sep, S.J.; Van der Kallen, C.J.; Dagnelie, P.C.; Schram, M.T.; Henry, R.M.; Eussen, S.J.; Van Dongen, M.C.; et al. Sedentary Behavior, Physical Activity, and Fitness-The Maastricht Study. Med. Sci. Sports Exerc. 2017, 49, 1583–1591. [Google Scholar] [CrossRef]
- Prince, S.A.; Dempsey, P.C.; Reed, J.L.; Rubin, L.; Saunders, T.J.; Ta, J.; Tomkinson, G.R.; Merucci, K.; Lang, J.J. The Effect of Sedentary Behaviour on Cardiorespiratory Fitness: A Systematic Review and Meta-Analysis. Sports Med. 2024, 54, 997–1013. [Google Scholar] [CrossRef]
- Van der Velde, J.H.; Savelberg, H.H.; Van der Berg, J.D.; Sep, S.J.; Van der Kallen, C.J.; Dagnelie, P.C.; Schram, M.T.; Henry, R.M.; Reijven, P.L.; van Geel, T.A.; et al. Sedentary Behavior Is Only Marginally Associated with Physical Function in Adults Aged 40–75 Years-the Maastricht Study. Front. Physiol. 2017, 8, 242. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Schuna, J.M., Jr.; Barreira, T.V.; Mire, E.F.; Broyles, S.T.; Katzmarzyk, P.T.; Johnson, W.D. Normative steps/day values for older adults: NHANES 2005–2006. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1426–1432. [Google Scholar] [CrossRef]
- Dogra, S.; Stathokostas, L. Sedentary behavior and physical activity are independent predictors of successful aging in middle-aged and older adults. J. Aging Res. 2012, 2012, 190654. [Google Scholar] [CrossRef]
- Son, J.Y.; Zhou, W.; Webster-Dekker, K.E.; Marriott, D.J.; Larson, J.L. Association between accelerometry measured patterns of sedentary behaviors and functional status in older adults. Aging Clin. Exp. Res. 2024, 36, 11. [Google Scholar] [CrossRef]
- Nakagata, T.; Yamada, Y.; Hatamoto, Y.; Naito, H. Energy Expenditure of a Single Sit-to-Stand Movement with Slow Versus Normal Speed Using the Different Frequency Accumulation Method. Medicina 2019, 55, 77. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.W.; Wu, Y.; Petterson, J.L.; Bray, N.W.; Kimmerly, D.S. Validity of the ActivPAL monitor to distinguish postures: A systematic review. Gait Posture 2022, 94, 107–113. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Total N = 663 | Q1 (Low) [241.2, 547.6] n = 166 | Q2 (547.6, 617.1] n = 166 | Q3 (617.1, 691.6] n = 165 | Q4 (High) (691.6, 897.7] n = 166 | p-Trend |
|---|---|---|---|---|---|---|
| Age, years | 76.4 ± 5.1 | 76.2 ± 5.1 | 76.3 ± 4.7 | 76.7 ± 5.4 | 76.6 ± 5.0 | 0.8 |
| Sex, Women | 387 (58.4) | 118 (71.1) | 99 (59.6) | 97 (58.8) | 73 (44.0) | <0.001 |
| Race, Non-Hispanic White | 563 (84.9) | 141 (84.9) | 147 (88.6) | 137 (83.0) | 138 (83.1) | 0.5 |
| Multimorbidity Index † | 0.2 | |||||
| No conditions | 280 (42.8) | 67 (40.9) | 84 (50.9) | 70 (42.9) | 59 (36.4) | |
| One condition | 262 (40.1) | 71 (43.3) | 60 (36.4) | 62 (38.0) | 69 (42.6) | |
| Two or more conditions | 112 (17.1) | 26 (15.9) | 21 (12.7) | 31 (19.0) | 34 (21.0) | |
| Body Mass Index, kg/m2 | 27.7 ± 4.6 | 26.3 ± 4.8 | 27.1 ± 4.3 | 28.6 ± 4.8 | 28.7 ± 4.1 | <0.001 |
| Height, m | 1.7 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 | <0.001 |
| Weight, kg | 76.3 ± 15.5 | 69.2 ± 14.1 | 74.7 ± 15.1 | 79.1 ± 15.0 | 82.1 ± 14.7 | <0.001 |
| VO2peak, mL/kg/min | 20.0 ± 4.6 | 20.7 ± 4.7 | 20.8 ± 4.6 | 19.5 ± 4.8 | 19.0 ± 3.9 | <0.001 |
| VO2peak, mL/min | 1517.6 ± 426.0 | 1426.0 ± 400.7 | 1561.0 ± 447.2 | 1531.7 ± 445.5 | 1552.0 ± 397.3 | <0.001 |
| Average 400 m Walk Speed, m/s | 1.05 ± 0.18 | 1.06 ± 0.16 | 1.06 ± 0.17 | 1.05 ± 0.18 | 1.02 ± 0.19 | 0.2 |
| Daily Step Count, /day | 6928.3 ± 3120.0 | 8631.7 ± 3449.4 | 7483.1 ± 2951.6 | 6471.7 ± 2805.2 | 5123.9 ± 1977.3 | <0.001 |
| Total Sedentary Time, min/day | 614.8 ± 111.7 | 467.6 ± 64.6 | 585.5 ± 19.5 | 655.8 ± 22.4 | 750.7 ± 45.7 | <0.001 |
| Total Standing Time, min/day | 244.5 ± 90.7 | 350.2 ± 84.8 | 252.0 ± 54.0 | 203.6 ± 46.4 | 172.1 ± 50.2 | <0.001 |
| Sedentary Bout Length, min/day | 15.0 ± 5.5 | 11.2 ± 3.2 | 13.6 ± 3.9 | 16.1 ± 4.7 | 19.1 ± 6.2 | <0.001 |
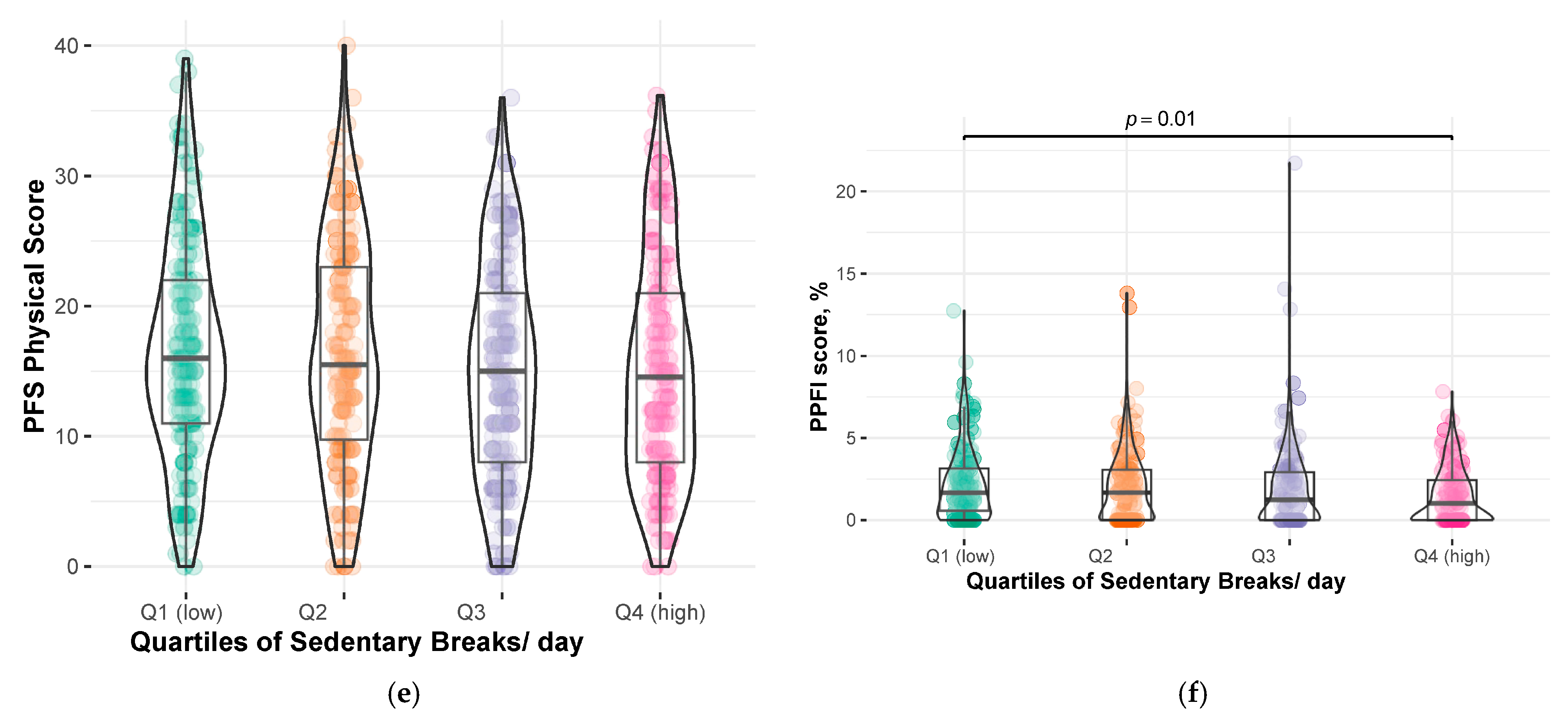
| Sedentary Breaks, /day | 46.1 ± 13.2 | 46.0 ± 13.3 | 47.8 ± 13.2 | 46.3 ± 13.6 | 44.3 ± 12.6 | 0.094 |
| PFS Physical score, 0–50 | 15.8 ± 8.5 | 14.1 ± 8.0 | 15.3 ± 9.0 | 15.7 ± 7.9 | 17.9 ± 8.8 | 0.001 |
| PPFI score *, % | 1.4 (0.0, 2.9) | 1.4 (0.0, 2.8) | 1.2 (0.0, 2.8) | 1.3 (0.0, 2.9) | 1.6 (0.0, 3.1) | 0.5 |
| 1 Standard Deviation | β (Standard Deviation) | 95% CI | |
|---|---|---|---|
| Total Sedentary Time, min/day | |||
| Continuous (per 1 standard deviation) † | 112 | 0.71 (0.34) * | 0.04, 1.38 |
| Q1 (lowest) # | Ref. | ||
| Q2 | 1.47 (0.88) | −0.26, 3.20 | |
| Q3 | 0.75 (0.90) | −1.01, 2.51 | |
| Q4 (highest) | 2.67 (0.94) * | 0.84, 4. 51 | |
| Mean Sedentary Bout Length, min/day | |||
| Continuous (per 1 standard deviation) † | 5.5 | 0.16 (0.33) | −0.49, 0.81 |
| Q1 (lowest) # | Ref. | ||
| Q2 | 0.49 (0.88) | −1.25, 2.22 | |
| Q3 | 1.19 (0.91) | −0.59, 2.97 | |
| Q4 (highest) | 0.98 (0.94) | −0.87, 2.82 | |
| Sedentary Breaks, /day | |||
| Continuous (per 1 standard deviation) † | 13.2 | 0.02 (0.32) | −0.60, 0.64 |
| 1 Standard Deviation | β (Standard Deviation) | 95% CI | |
|---|---|---|---|
| Total Sedentary Time, min/day | |||
| Continuous (per 1 standard deviation) † | 111.7 | −0.25 (0.14) | −0.54, 0.03 |
| Q1 (lowest) # | Ref. | ||
| Q2 | −0.49 (0.38) | −1.23, 0.25 | |
| Q3 | −1.12 (0.39) * | −1.88, −0.36 | |
| Q4 (highest) | −0.56 (0.40) | −1.34, 0.21 | |
| Mean Sedentary Bout Length, min/day | |||
| Continuous (per 1 standard deviation) † | 5.4 | −0.02 (0.14) | −0.29, 0.25 |
| Q1 (lowest) # | Ref. | ||
| Q2 | −0.54 (0.39) | −1.29, 0.22 | |
| Q3 | −0.16 (0.39) | −0.92, 0.60 | |
| Q4 (highest) | −0.28 (0.40) | −1.06, 0.50 | |
| Sedentary Breaks, /day | |||
| Continuous (per 1 standard deviation) † | 13.2 | −0.16 (0.14) | −0.43, 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, R.E.; Newman, A.B.; Johnson, E.; Qiao, Y.S.; Cawthon, P.M.; Nicklas, B.J.; Goodpaster, B.H.; Glynn, N.W. Using Wearable Devices to Examine the Associations of Sedentary Behavior with Perceived and Performance Fatigability Among Older Adults: The Study of Muscle, Mobility and Aging (SOMMA). Sensors 2025, 25, 2722. https://doi.org/10.3390/s25092722
Garcia RE, Newman AB, Johnson E, Qiao YS, Cawthon PM, Nicklas BJ, Goodpaster BH, Glynn NW. Using Wearable Devices to Examine the Associations of Sedentary Behavior with Perceived and Performance Fatigability Among Older Adults: The Study of Muscle, Mobility and Aging (SOMMA). Sensors. 2025; 25(9):2722. https://doi.org/10.3390/s25092722
Chicago/Turabian StyleGarcia, Reagan E., Anne B. Newman, Eileen Johnson, Yujia Susanna Qiao, Peggy M. Cawthon, Barbara J. Nicklas, Bret H. Goodpaster, and Nancy W. Glynn. 2025. "Using Wearable Devices to Examine the Associations of Sedentary Behavior with Perceived and Performance Fatigability Among Older Adults: The Study of Muscle, Mobility and Aging (SOMMA)" Sensors 25, no. 9: 2722. https://doi.org/10.3390/s25092722
APA StyleGarcia, R. E., Newman, A. B., Johnson, E., Qiao, Y. S., Cawthon, P. M., Nicklas, B. J., Goodpaster, B. H., & Glynn, N. W. (2025). Using Wearable Devices to Examine the Associations of Sedentary Behavior with Perceived and Performance Fatigability Among Older Adults: The Study of Muscle, Mobility and Aging (SOMMA). Sensors, 25(9), 2722. https://doi.org/10.3390/s25092722








