Fluorometric and Colorimetric Biosensors for the Assay of Cholinesterase Inhibitors
Abstract
1. Introduction
2. Common Principles in Fluorometric and Colorimetric Biosensors
3. Cholinesterases
4. Inhibitors of AChE and BChE
5. Cholinesterase Activity Assays and Cholinesterase Biosensors
6. Recently Developed Colorimetric and Fluorometric Cholinesterase Biosensors
7. Future Trends
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrov, K.A.; Proskurina, S.E.; Krejci, E. Cholinesterases in Tripartite Neuromuscular Synapse. Front. Mol. Neurosci. 2021, 14, 811220. [Google Scholar] [CrossRef]
- Gok, M.; Cicek, C.; Bodur, E. Butyrylcholinesterase in lipid metabolism: A new outlook. J. Neurochem. 2024, 168, 381–385. [Google Scholar] [CrossRef]
- Ha, Z.Y.; Mathew, S.; Yeong, K.Y. Butyrylcholinesterase: A Multifaceted Pharmacological Target and Tool. Curr. Protein Pept. Sci. 2020, 21, 99–109. [Google Scholar] [CrossRef]
- Bagrowska, W.; Karasewicz, A.; Góra, A. Comprehensive analysis of acetylcholinesterase inhibitor and reactivator complexes: Implications for drug design and antidote development. Drug Discov. Today 2024, 29, 104217. [Google Scholar] [CrossRef]
- Jaqua, E.E.; Tran, M.N.; Hanna, M. Alzheimer Disease: Treatment of Cognitive and Functional Symptoms. Am. Fam. Physician 2024, 110, 281–293. [Google Scholar]
- Kaur, S.; Chowdhary, S.; Kumar, D.; Bhattacharyya, R.; Banerjee, D. Organophosphorus and carbamate pesticides: Molecular toxicology and laboratory testing. Clin. Chim. Acta 2023, 551, 117584. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Nian, B.; Yu, C.; Maimaiti, D.; Chai, M.; Yang, X.; Zang, X.; Xu, D. Mechanisms of Neurotoxicity of Organophosphate Pesticides and Their Relation to Neurological Disorders. Neuropsychiatr. Dis. Treat. 2024, 20, 2237–2254. [Google Scholar] [CrossRef]
- Shentema, M.G.; Kumie, A.; Bråtveit, M.; Deressa, W.; Ngowi, A.V.; Moen, B.E. Pesticide Use and Serum Acetylcholinesterase Levels among Flower Farm Workers in Ethiopia-A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 964. [Google Scholar] [CrossRef]
- Voros, C.; Dias, J.; Timperley, C.M.; Nachon, F.; Brown, R.C.D.; Baati, R. The risk associated with organophosphorus nerve agents: From their discovery to their unavoidable threat, current medical countermeasures and perspectives. Chem. Biol. Interact. 2024, 395, 110973. [Google Scholar] [CrossRef]
- Shimada, H.; Kiyozumi, Y.; Koga, Y.; Ogata, Y.; Katsuda, Y.; Kitamura, Y.; Iwatsuki, M.; Nishiyama, K.; Baba, H.; Ihara, T. A novel cholinesterase assay for the evaluation of neurotoxin poisoning based on the electron-transfer promotion effect of thiocholine on an Au electrode. Sens. Actuator B-Chem. 2019, 298, 126893. [Google Scholar] [CrossRef]
- Lokar, N.; Kononenko, V.; Drobne, D.; Vrtacnik, D. Electrochemical acetylcholinesterase biosensor for detection of cholinesterase inhibitors: Study with eserine. Inf. Midem-J. Microelectron. Electron. Compon. Mater. 2018, 48, 235–240. [Google Scholar] [CrossRef]
- Ciriello, R.; Lo Magro, S.; Guerrieri, A. Assay of serum cholinesterase activity by an amperometric biosensor based on a co-crosslinked choline oxidase/overoxidized polypyrrole bilayer. Analyst 2018, 143, 920–929. [Google Scholar] [CrossRef]
- Dimcheva, N.; Horozova, E.; Ivanov, Y.; Godjevargova, T. Self-assembly of acetylcholinesterase on gold nanoparticles electrodeposited on graphite. Cent. Eur. J. Chem. 2013, 11, 1740–1748. [Google Scholar] [CrossRef]
- Teng, Y.Q.; Fu, Y.; Xu, L.L.; Lin, B.; Wang, Z.C.; Xu, Z.A.; Jin, L.T.; Zhang, W. Three-Dimensional Ordered Macroporous (3DOM) Composite for Electrochemical Study on Acetylcholinesterase Inhibition Induced by Endogenous Neurotoxin. J. Phys. Chem. B 2012, 116, 11180–11186. [Google Scholar] [CrossRef]
- Li, Q.L.; Li, J.T.; Yang, D.Z.; Xiang, C.; Yang, Y.L. Dual-mode colorimetric-fluorescence biosensor for endotoxin detection based on CS@Fe,Cu/CDs-MnO2 nanomaterials. Talanta 2025, 285, 127330. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, J.W.; Kim, T.H.; Lee, K.W.; Lee, T.S. Synthesis of NAD-functionalized organic semiconducting polymer dots for fluorometric γ-aminobutyric acid sensing. Macromol. Res. 2024, 1–9. [Google Scholar] [CrossRef]
- Govindaraj, P.; Alungal, N.; Kannan, S. Silver conjugated nickel oxide nanoparticle dependent microfluid non-enzymatic colorimetric paper-based biosensor for uric acid detection. Biochem. Eng. J. 2025, 215, 109622. [Google Scholar] [CrossRef]
- Liu, S.; Chao, H.L.; He, D.J.; Wang, Y.; Yang, Y. Biomimetic co-immobilization of (3-glucosidase, glucose oxidase, and horseradish peroxidase to construct a multi-enzyme biosensor for determination of amygdalin. Int. J. Biol. Macromol. 2025, 297, 139868. [Google Scholar] [CrossRef]
- Cheng, S.; Luo, L.X.; Bao, M.Y.; Bao, T.; Gao, Y.; Wu, Z.; Zhang, X.H.; Wang, S.F.; Wen, W. A dual-mode colorimetric/photothermal lateral flow biosensor based on Au/Ti3C2TX for HIV-DNA detection. Analytica Chimica Acta 2025, 1338, 343588. [Google Scholar] [CrossRef]
- Nejadmansouri, M.; Majdinasab, M.; Nunes, G.S.; Marty, J.L. An Overview of Optical and Electrochemical Sensors and Biosensors for Analysis of Antioxidants in Food during the Last 5 Years. Sensors 2021, 21, 1176. [Google Scholar] [CrossRef]
- Oushyani Roudsari, Z.; Karami, Y.; Khoramrooz, S.S.; Rouhi, S.; Ghasem, H.; Khatami, S.H.; Alizadeh, M.; Ahmad Khosravi, N.; Mansoriyan, A.; Ghasemi, E.; et al. Electrochemical and optical biosensors for the detection of E. Coli. Clin. Chim. Acta 2025, 565, 119984. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhong, S.; Hu, X.; Koh, K.; Chen, H. Perspectives and trends in advanced optical and electrochemical biosensors based on engineered peptides. Mikrochim. Acta 2023, 190, 327. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.A.; Müller, T.D. (Patho)Physiology of Glycosylphosphatidylinositol-Anchored Proteins I: Localization at Plasma Membranes and Extracellular Compartments. Biomolecules 2023, 13, 855. [Google Scholar] [CrossRef]
- Ordentlich, A.; Barak, D.; Kronman, C.; Flashner, Y.; Leitner, M.; Segall, Y.; Ariel, N.; Cohen, S.; Velan, B.; Shafferman, A. Dissection of the human acetylcholinesterase active center determinants of substrate specificity. Identification of residues constituting the anionic site, the hydrophobic site, and the acyl pocket. J. Biol. Chem. 1993, 268, 17083–17095. [Google Scholar] [CrossRef]
- Shafferman, A.; Kronman, C.; Flashner, Y.; Leitner, M.; Grosfeld, H.; Ordentlich, A.; Gozes, Y.; Cohen, S.; Ariel, N.; Barak, D.; et al. Mutagenesis of human acetylcholinesterase. Identification of residues involved in catalytic activity and in polypeptide folding. J. Biol. Chem. 1992, 267, 17640–17648. [Google Scholar] [CrossRef]
- Johnson, G.; Moore, S.W. The peripheral anionic site of acetylcholinesterase: Structure, functions and potential role in rational drug design. Curr. Pharm. Des. 2006, 12, 217–225. [Google Scholar] [CrossRef]
- Koellner, G.; Kryger, G.; Millard, C.B.; Silman, I.; Sussman, J.L.; Steiner, T. Active-site gorge and buried water molecules in crystal structures of acetylcholinesterase from Torpedo californica. J. Mol. Biol. 2000, 296, 713–735. [Google Scholar] [CrossRef]
- Saxena, A.; Redman, A.M.G.; Jiang, X.L.; Lockridge, O.; Doctor, B.P. Differences in active-site gorge dimensions of cholinesterases revealed by binding of inhibitors to human butyrylcholinesterase. Chem.-Biol. Interact. 1999, 119, 61–69. [Google Scholar] [CrossRef]
- Chiou, S.Y.; Huang, C.F.; Hwang, M.T.; Lin, G. Comparison of Active Sites of Butyrylcholinesterase and Acetylcholinesterase Based on Inhibition by Geometric Isomers of Benzene-di-N-Substituted Carbamates. J. Biochem. Mol. Toxicol. 2009, 23, 303–308. [Google Scholar] [CrossRef]
- Macdonald, I.R.; Martin, E.; Rosenberry, T.L.; Darvesh, S. Probing the peripheral site of human butyrylcholinesterase. Biochemistry 2012, 51, 7046–7053. [Google Scholar] [CrossRef]
- Osawa, S.; Kariyone, K.; Ichihara, F.; Arai, K.; Takagasa, N.; Ito, H. Development and application of serum cholinesterase activity measurement using benzoylthiocholine iodide. Clinica Chimica Acta 2005, 351, 65–72. [Google Scholar] [CrossRef]
- Sine, H.; El Grafel, K.; Alkhammal, S.; Achbani, A.; Filali, K. Serum cholinesterase biomarker study in farmers—Souss Massa region-, Morocco: Case-control study. Biomarkers 2019, 24, 771–775. [Google Scholar] [CrossRef]
- Naik, R.S.; Liu, W.Y.; Saxena, A. Development and validation of a simple assay for the determination of cholinesterase activity in whole blood of laboratory animals. J. Appl. Toxicol. 2013, 33, 290–300. [Google Scholar] [CrossRef]
- Zhan, C.G.; Zheng, F.; Landry, D.W. Fundamental reaction mechanism for cocaine hydrolysis in human butyrylcholinesterase. J. Am. Chem. Soc. 2003, 125, 2462–2474. [Google Scholar] [CrossRef]
- Gao, D.Q.; Zhan, C.G. Modeling evolution of hydrogen bonding and stabilization of transition states in the process of cocaine hydrolysis catalyzed by human butyrylcholinesterase. Proteins 2006, 62, 99–110. [Google Scholar] [CrossRef]
- Zheng, F.; Hou, S.R.; Xue, L.; Yang, W.C.; Zhan, C.G. Human Butyrylcholinesterase Mutants for (-)-Cocaine Hydrolysis: A Correlation Relationship between Catalytic Efficiency and Total Hydrogen Bonding Energy with an Oxyanion Hole. J. Phys. Chem. B 2023, 127, 10723–10729. [Google Scholar] [CrossRef]
- Aman, S.; Paul, S.; Chowdhury, F.R. Management of Organophosphorus Poisoning: Standard Treatment and Beyond. Crit. Care Clin. 2021, 37, 673–686. [Google Scholar] [CrossRef]
- Zoofaghari, S.; Maghami-Mehr, A.; Abdolrazaghnejad, A. Organophosphate Poisoning: Review of Prognosis and Management. Adv. Biomed. Res. 2024, 13, 82. [Google Scholar] [CrossRef]
- Vale, A.; Lotti, M. Organophosphorus and carbamate insecticide poisoning. Handb. Clin. Neurol. 2015, 131, 149–168. [Google Scholar]
- Moralev, S.N.; Tikhonov, D.B. Investigation of structure-activity relationships in organophosphates-cholinesterase interaction using docking analysis. Chem.-Biol. Interact. 2010, 187, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Perra, M.T.; Serra, A.; Sirigu, P.; Turno, F. Histochemical demonstration of acetylcholinesterase activity in human Meibomian glands. Eur. J. Histochem. 1996, 40, 39–44. [Google Scholar] [PubMed]
- Darvesh, S.; Darvesh, K.V.; McDonald, R.S.; Mataija, D.; Walsh, R.; Mothana, S.; Lockridge, O.; Martin, E. Carbamates with differential mechanism of inhibition toward acetylcholinesterase and butyrylcholinesterase. J. Med. Chem. 2008, 51, 4200–4212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Ma, C.; Li, Y.B.; Li, M.Z.; Cui, T.; Zhao, X.Q.; Li, Z.L.; Jia, H.W.; Wang, H.X.; Xiu, X.M.; et al. Design, synthesis and biological evaluation of carbamate derivatives incorporating multifunctional carrier scaffolds as pseudo-irreversible cholinesterase inhibitors for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2024, 265, 116071. [Google Scholar] [CrossRef]
- Meden, A.; Knez, D.; Brazzolotto, X.; Nachon, F.; Dias, J.; Svete, J.; Stojan, J.; Groselj, U.; Gobec, S. From tryptophan-based amides to tertiary amines: Optimization of a butyrylcholinesterase inhibitor series. Eur. J. Med. Chem. 2022, 234, 114248. [Google Scholar] [CrossRef]
- Wilkinson, D.G. The pharmacology of donepezil: A new treatment of Alzheimer’s disease. Expert. Opin. Pharmacother. 1999, 1, 121–135. [Google Scholar] [CrossRef]
- Pohanka, M.; Dobes, P. Caffeine inhibits acetylcholinesterase, but not butyrylcholinesterase. Int. J. Mol. Sci. 2013, 14, 9873–9882. [Google Scholar] [CrossRef]
- Fu, Q.; Tang, J.; Cui, M.; Zheng, Z.; Liu, Z.; Liu, S. Development of ESI-MS-based continuous enzymatic assay for real-time monitoring of enzymatic reactions of acetylcholinesterase. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 990, 169–173. [Google Scholar] [CrossRef]
- Xu, Z.; Yao, S.; Wei, Y.; Zhou, J.; Zhang, L.; Wang, C.; Guo, Y. Monitoring enzyme reaction and screening of inhibitors of acetylcholinesterase by quantitative matrix-assisted laser desorption/ionization Fourier transform mass spectrometry. J. Am. Soc. Mass. Spectrom. 2008, 19, 1849–1855. [Google Scholar] [CrossRef]
- Lilienfeld, S. Galantamine—A novel cholinergic drug with a unique dual mode of action for the treatment of patients with Alzheimer’s disease. CNS Drug. Rev. 2002, 8, 159–176. [Google Scholar] [CrossRef]
- Loy, C.; Schneider, L. Galantamine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2004, 4, Cd001747. [Google Scholar] [CrossRef]
- Bucur, M.P.; Bucur, B.; Radu, G.L. Critical evaluation of acetylcholine iodide and acetylthiocholine chloride as substrates for amperometric biosensors based on acetylcholinesterase. Sensors 2013, 13, 1603–1613. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, A.; Sanjaya, A.R.; Putri, Y.; Gunlazuardi, J.; Ivandini, T.A. An acetylcholinesterase-based biosensor for isoprocarb using a gold nanoparticles-polyaniline modified graphite pencil electrode. Anal. Sci. 2023, 39, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Akdag, A.; Isik, M.; Göktas, H. Conducting polymer-based electrochemical biosensor for the detection of acetylthiocholine and pesticide via acetylcholinesterase. Biotechnol. Appl. Biochem. 2021, 68, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Bai, Y.F.; Han, G.Y.; Li, M.Y. Porous-reduced graphene oxide for fabricating an amperometric acetylcholinesterase biosensor. Sens. Actuator B-Chem. 2013, 185, 706–712. [Google Scholar] [CrossRef]
- Arduini, F.; Forchielli, M.; Amine, A.; Neagu, D.; Cacciotti, I.; Nanni, F.; Moscone, D.; Palleschi, G. Screen-printed biosensor modified with carbon black nanoparticles for the determination of paraoxon based on the inhibition of butyrylcholinesterase. Microchim. Acta 2015, 182, 643–651. [Google Scholar] [CrossRef]
- Kok, F.N.; Hasirci, V. Determination of binary pesticide mixtures by an acetylcholinesterase-choline oxidase biosensor. Biosens. Bioelectron. 2004, 19, 661–665. [Google Scholar] [CrossRef]
- Sousa, S.C.A.; Rebelo, M.J.F. Acetylcholinesterase–Choline Oxidase Biosensor for Pirimicarb Determination. Port. Electrochim. Acta 2008, 26, 65–75. [Google Scholar] [CrossRef]
- Fennouh, S.; Casimiri, V.; Burstein, C. Increased paraoxon detection with solvents using acetylcholinesterase inactivation measured with a choline oxidase biosensor. Biosens. Bioelectron. 1997, 12, 97–104. [Google Scholar] [CrossRef]
- Kok, F.N.; Bozoglu, F.; Hasirci, V. Construction of an acetylcholinesterase-choline oxidase biosensor for aldicarb determination. Biosens. Bioelectron. 2002, 17, 531–539. [Google Scholar] [CrossRef]
- Saito, H.; Suzuki, Y.; Gessei, T.; Miyajima, K.; Arakawa, T.; Mitsubayashi, K. Bioelectronic Sniffer (Biosniffer) Based on Enzyme Inhibition of Butyrylcholinesterase for Toluene Detection. Sens. Mater. 2014, 26, 121–129. [Google Scholar]
- Pohanka, M. Diagnoses of Pathological States Based on Acetylcholinesterase and Butyrylcholinesterase. Curr. Med. Chem. 2020, 27, 2994–3011. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Rathish, D.; Senavirathna, I.; Jayasumana, C.; Agampodi, S. Red blood cell acetylcholinesterase activity among healthy dwellers of an agrarian region in Sri Lanka: A descriptive cross-sectional study. Environ. Health Prev. Med. 2018, 23, 25. [Google Scholar] [CrossRef]
- Sanz, P.; Rodriguez-Vicente, M.C.; Diaz, D.; Repetto, J.; Repetto, M. Red blood cell and total blood acetylcholinesterase and plasma pseudocholinesterase in humans: Observed variances. J. Toxicol. Clin. Toxicol. 1991, 29, 81–90. [Google Scholar] [CrossRef]
- Kolf-Clauw, M.; Jez, S.; Ponsart, C.; Delamanche, I.S. Acetyl- and pseudo-cholinesterase activities of plasma, erythrocytes, and whole blood in male beagle dogs using Ellman’s assay. Vet. Hum. Toxicol. 2000, 42, 216–219. [Google Scholar]
- Thiphom, S.; Prapamontol, T.; Chantara, S.; Mangklabruks, A.; Suphavilai, C. A method for measuring cholinesterase activity in human saliva and its application to farmers and consumers. Anal. Methods 2013, 5, 4687–4693. [Google Scholar] [CrossRef]
- Haigh, J.R.; Lefkowitz, L.J.; Capacio, B.R.; Doctor, B.P.; Gordon, R.K. Advantages of the WRAIR whole blood cholinesterase assay: Comparative analysis to the micro-Ellman, Test-mate ChE™ and Michel (ΔpH) assays. Chem.-Biol. Interact. 2008, 175, 417–420. [Google Scholar] [CrossRef]
- Yu, Q.Y.; Guo, Q.; Zhou, J.R.; Yuan, X.; Huang, K.; Chen, P.P. Filter-assisted smartphone colorimetry/ICP-MS dual-mode biosensor of butyrylcholinesterase in clinical samples. Sens. Actuator B-Chem. 2022, 370, 132472. [Google Scholar] [CrossRef]
- Matejovsky, L.; Pitschmann, V. A Strip Biosensor with Guinea Green B and Fuchsin Basic Color Indicators on a Glass Nanofiber Carrier for the Cholinesterase Detection of Nerve Agents. ACS Omega 2019, 4, 20978–20986. [Google Scholar] [CrossRef]
- Matejovsky, L.; Pitschmann, V. New Carrier Made from Glass Nanofibres for the Colorimetric Biosensor of Cholinesterase Inhibitors. Biosensors 2018, 8, 51. [Google Scholar] [CrossRef]
- Cavalcante, S.F.A.; Kitagawa, D.A.S.; Rodrigues, R.B.; Silva, T.C.; Bernardo, L.B.; Correa, A.B.A.; Simas, A.B.C. One-Pot Synthesis of NEMP, a VX Surrogate, and Reactivation of NEMP-Inhibited Electrophorus Eel Acetylcholinesterase by Current Antidotes. J. Braz. Chem. Soc. 2019, 30, 1095–1102. [Google Scholar] [CrossRef]
- Villatte, F.; Bachman, T.T.; Hussein, A.S.; Schmid, R.D. Acetylcholinesterase assay for rapid expression screening in liquid and solid media. Biotechniques 2001, 30, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Ramallo, I.A.; García, P.; Furlan, R.L.E. A reversed-phase compatible thin-layer chromatography autography for the detection of acetylcholinesterase inhibitors. J. Sep. Sci. 2015, 38, 3788–3794. [Google Scholar] [CrossRef]
- Du, T.F.; Zhou, S.G.; Tang, M.S. A new micro-detection tube for cholinesterase inhibitors in water. Environ. Pollut. 1989, 57, 217–222. [Google Scholar] [CrossRef]
- Li, S.Z.; Huang, R.L.; Solomon, S.; Liu, Y.T.; Zhao, B.; Santillo, M.F.; Xia, M.H. Identification of acetylcholinesterase inhibitors using homogenous cell-based assays in quantitative high-throughput screening platforms. Biotechnol. J. 2017, 12, 1600715. [Google Scholar] [CrossRef]
- Santillo, M.F.; Liu, Y.T. A fluorescence assay for measuring acetylcholinesterase activity in rat blood and a human neuroblastoma cell line (SH-SY5Y). J. Pharmacol. Toxicol. Methods 2015, 76, 15–22. [Google Scholar] [CrossRef]
- Cui, K.; Chen, Z.L.; Wang, Z.; Zhang, G.X.; Zhang, D.Q. A naked-eye visible and fluorescence “turn-on” probe for acetyl-cholinesterase assay and thiols as well as imaging of living cells. Analyst 2011, 136, 191–195. [Google Scholar] [CrossRef]
- Dhull, V.; Gahlaut, A.; Hooda, V. Nanomaterials based biosensors for the detection of organophosphate compounds: A review. Int. J. Environ. Anal. Chem. 2023, 103, 4200–4224. [Google Scholar] [CrossRef]
- Stepánková, S.; Vorcáková, K. Cholinesterase-based biosensors. J. Enzym. Inhib. Med. Chem. 2016, 31, 180–193. [Google Scholar] [CrossRef]
- Sabullah, M.K.; Khalidi, S.A.M.; Abdullah, R.; Sani, S.A.; Gansau, J.A.; Ahmad, S.A.; Shukor, M.Y. Cholinesterase-based biosensor for preliminary detection of toxic heavy metals in the environment and agricultural-based products. Int. Food Res. J. 2020, 27, 597–609. [Google Scholar]
- Xu, Y.L.; Li, F.Y.; Ndikuryayo, F.; Yang, W.C.; Wang, H.M. Cholinesterases and Engineered Mutants for the Detection of Organophosphorus Pesticide Residues. Sensors 2018, 18, 4281. [Google Scholar] [CrossRef] [PubMed]
- Pundir, C.S.; Malik, A.; Preety. Bio-sensing of organophosphorus pesticides: A review. Biosens. Bioelectron. 2019, 140, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Brízová, A.; Pitschmann, V. Simple Chemical and Cholinesterase Methods for the Detection of Nerve Agents Using Optical Evaluation. Biosensors 2023, 13, 995. [Google Scholar] [CrossRef]
- Karadurmus, L.; Kaya, S.I.; Ozkan, S.A. Recent advances of enzyme biosensors for pesticide detection in foods. J. Food Meas. Charact. 2021, 15, 4582–4595. [Google Scholar] [CrossRef]
- Ivanov, A.; Shamagsumova, R.; Larina, M.; Evtugyn, G. Electrochemical Acetylcholinesterase Sensors for Anti-Alzheimer’s Disease Drug Determination. Biosensors 2024, 14, 93. [Google Scholar] [CrossRef]
- Bucur, B.; Munteanu, F.D.; Marty, J.L.; Vasilescu, A. Advances in Enzyme-Based Biosensors for Pesticide Detection. Biosensors 2018, 8, 27. [Google Scholar] [CrossRef]
- Soldatkin, O.O.; Pyeshkova, V.M.; Kucherenko, I.S.; Velychko, T.P.; Bakhmat, V.A.; Arkhypova, V.M.; Soldatkin, A.P.; Dzyadevych, S.V. Application of butyrylcholinesterase-based biosensor for simultaneous determination of different toxicants using inhibition and reactivation steps. Electroanalysis 2024, 36, e202300400. [Google Scholar] [CrossRef]
- Mouawad, L.; Istamboulie, G.; Catanante, G.; Noguer, T. Enhancing Biocide Safety of Milk Using Biosensors Based on Cholinesterase Inhibition. Biosensors 2025, 15, 26. [Google Scholar] [CrossRef]
- Wongta, A.; Anand, P.; Aning, N.A.A.; Sawarng, N.; Hongsibsong, S. Advancing micro-electrometric techniques for the detection of organophosphate and carbamate residues using cricket cholinesterase. PLoS ONE 2024, 19, e0308112. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhu, J.; Yang, B.; Hao, H.; Lou, S. A green photocatalytic-biosensor for colorimetric detection of pesticide (carbaryl) based on inhibition of acetylcholinesterase. Talanta 2022, 246, 123525. [Google Scholar] [CrossRef]
- Zheng, M.E.; Liu, M.X.; Song, Z.C.; Ma, F.; Zhu, H.D.; Guo, H.L.; Sun, H.M. High-precision colorimetric-fluorescent dual-mode biosensor for detecting acetylcholinesterase based on a trimetallic nanozyme for efficient peroxidase-mimicking. J. Mater. Sci. Technol. 2024, 191, 168–180. [Google Scholar] [CrossRef]
- Hermanto, D.; Ismillayli, N.; Hamdiani, S.; Kamali, S.R.; Wirawan, R.; Muliasari, H.; Sanjaya, R.K. Inhibitive determination of organophosphate pesticides using acetylcholinesterase and silver nanoparticle as colorimetric. Environ. Eng. Res. 2024, 29, 230503. [Google Scholar] [CrossRef]
- Shah, M.M.; Ren, W.; Irudayaraj, J.; Sajini, A.A.; Ali, M.I.; Ahmad, B. Colorimetric Detection of Organophosphate Pesticides Based on Acetylcholinesterase and Cysteamine Capped Gold Nanoparticles as Nanozyme. Sensors 2021, 21, 8050. [Google Scholar] [CrossRef]
- Lu, L.L.; Hu, X.H.; Zeng, R.J.; Lin, Q.Y.; Huang, X.; Li, M.J.; Tang, D.P. Dual-mode colorimetric-photothermal sensing platform of acetylcholinesterase activity based on the peroxidase-like activity of Fe-N-C nanozyme. Anal. Chim. Acta 2022, 1229, 340383. [Google Scholar] [CrossRef]
- Guan, J.P.; Wang, M.; Ma, R.Z.; Liu, Q.; Sun, X.T.; Xiong, Y.; Chen, X.Q. Single-atom Rh nanozyme: An efficient catalyst for highly sensitive colorimetric detection of acetylcholinesterase activity and adrenaline. Sens. Actuator B-Chem. 2023, 375, 375. [Google Scholar] [CrossRef]
- Li, D.; Li, J.Y.; Wu, C.; Liu, H.Q.; Zhao, M.X.; Shi, H.Y.; Zhang, Y.; Wang, T. Smartphone-assisted colorimetric biosensor for the determination of organophosphorus pesticides on the peel of fruits. Food Chem. 2024, 443, 138459. [Google Scholar] [CrossRef]
- Wu, P.X.; Xia, H.; Wu, Y.Y.; Wang, M.H.; Li, N.; Liu, F.; Gong, H.Y.; Yang, Q.L.; Tan, X.F. Breaking the pH limitation and boosting peroxidase-like activity of Au aerogels via amalgam strategy for sensitive colorimetric bioassay. Microchem. J. 2025, 208, 112550. [Google Scholar] [CrossRef]
- Cha, B.S.; Lee, E.S.; Kim, S.; Kim, J.M.; Hwang, S.H.; Oh, S.S.; Park, K.S. Simple colorimetric detection of organophosphorus pesticides using naturally occurring extracellular vesicles. Microchem. J. 2020, 158, 105130. [Google Scholar] [CrossRef]


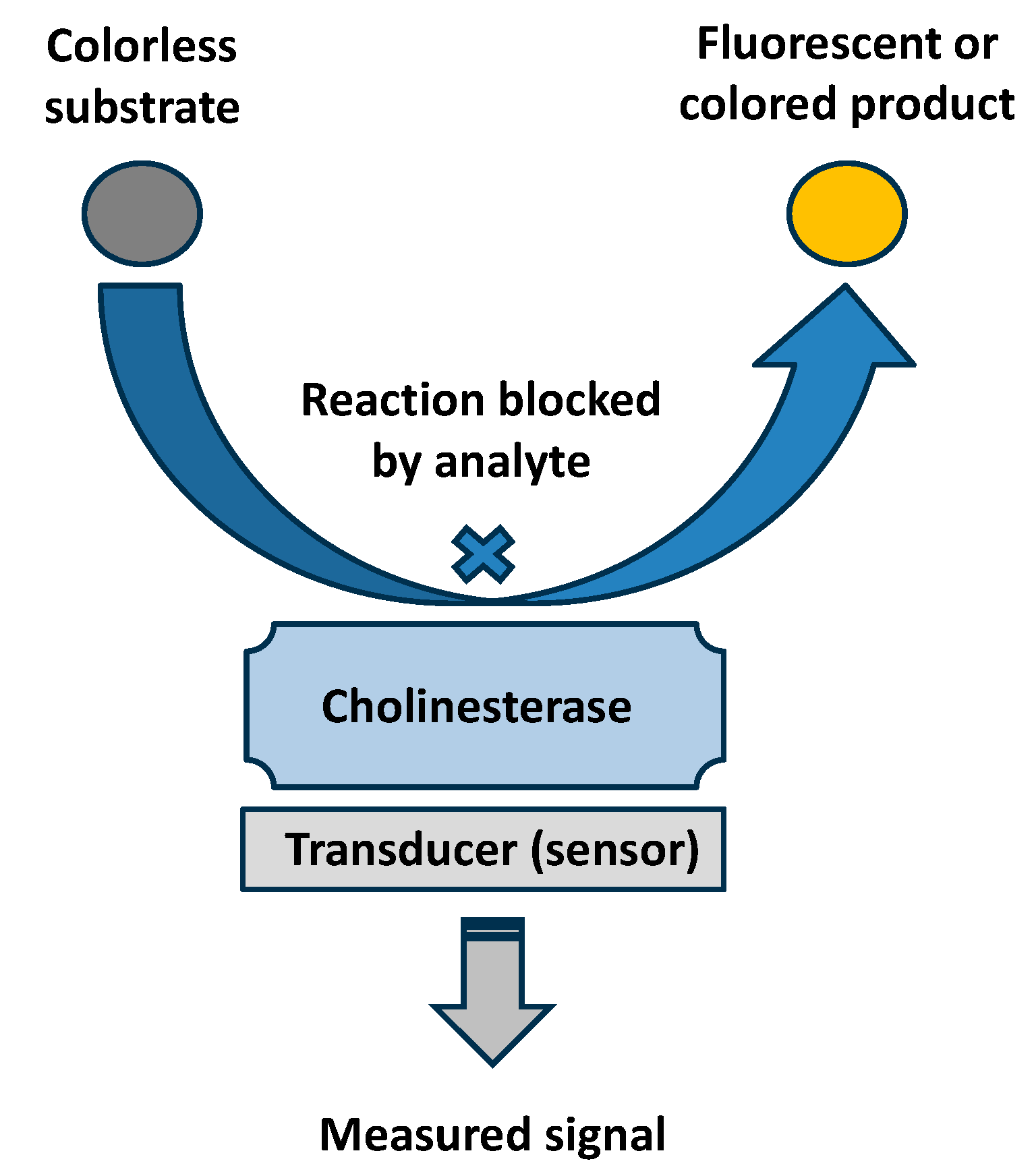
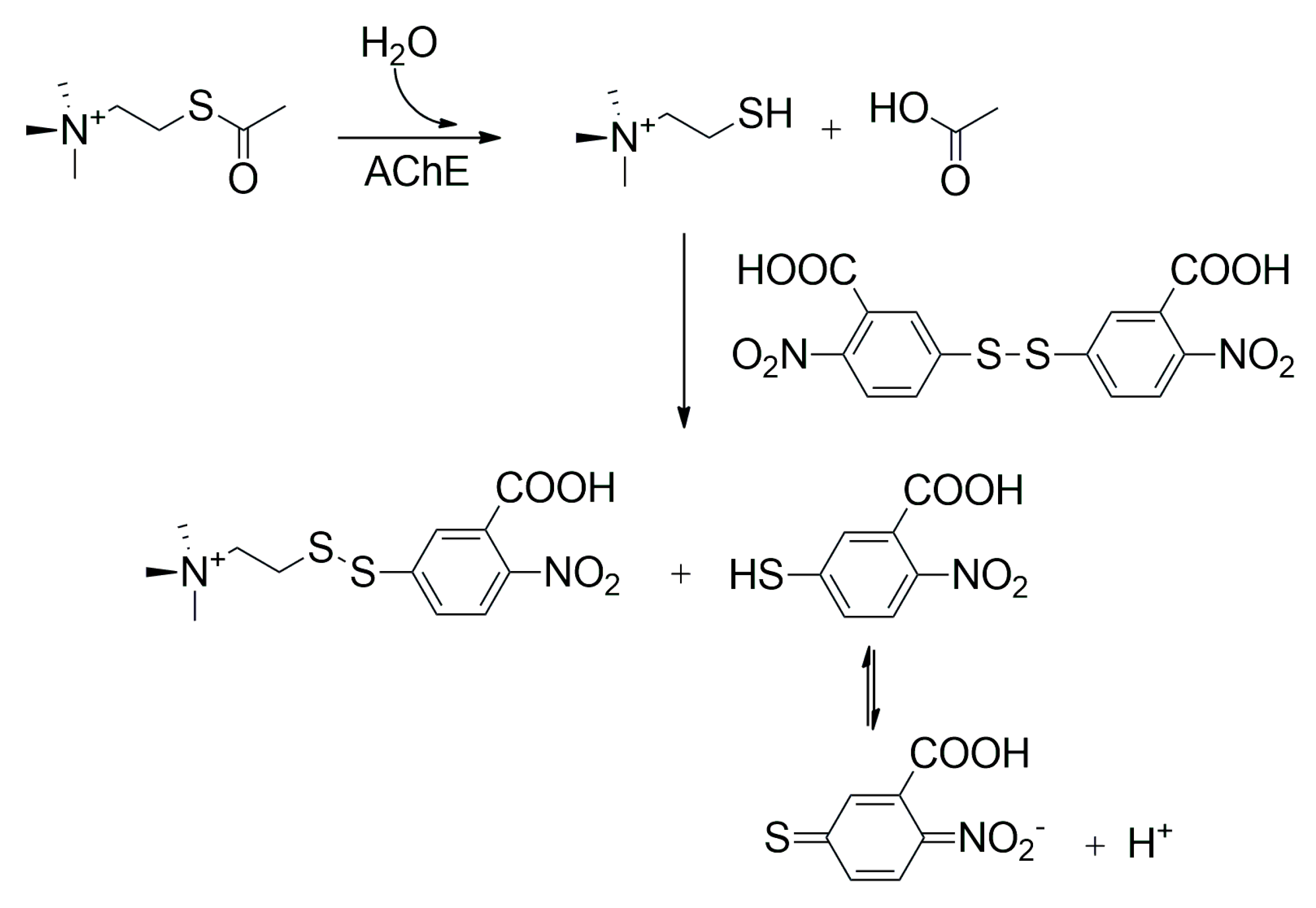
| Type of Inhibitor | Chemical Compounds and Examples | Site of Action | Targeted Cholinesterase |
|---|---|---|---|
| Irreversible | Organophosphate nerve agents like sarin or soman and former pesticides like paraoxon and malaoxon | Serine of catalytic triad | AChE and BChE |
| Pseudoirreversible | Carbamate inhibitors like pesticide carbofuran and drugs rivastigmine or pyridostigmine | Serine of catalytic triad | AChE and BChE |
| Noncompetitive | The drugs donepezil and tacrine, as well as natural compounds like caffeine | Alpha anionic site, active-site gorge, and peripheral anionic site | Mainly AChE |
| Mixed noncompetitive and competitive | Huperzine A | Alpha anionic site, active-site gorge, and peripheral anionic site | AChE |
| Competitive | Galantamine | Mainly alpha anionic site | AChE |
| Type of Biosensor | Analyte | Analytical Specifications | References |
|---|---|---|---|
| AChE biosensor based on conversion of acetylthiocholine to thiocholine followed by multiple steps with including redox reaction of 3,3′,5,5′-tetramethylbenzidine | Carbaryl | Limit of detection: 0.008 ng/mL; linear response: 0.01 to 0.25 ng/mL | [90] |
| Colorimetric-fluorescent dual-mode biosensor with a trimetallic nanozyme with peroxidase-mimicking properties | AChE | Limit of detection: 0.13 μU/mL for colorimetric detection and 0.04 μU/mL for fluorescence detection | [91] |
| A colorimetric biosensor with AChE and silver nanoparticles immobilized on an alginate–chitosan film | Profenofos | Limit of detection of 0.04 mg/L and a linear detection range from 0.05 to 6.00 mg/L | [92] |
| A colorimetric biosensor with AChE and cysteamine-capped gold nanoparticles as a nanozyme | Parathion ethyl | Limit of detection of 5.8 ng/mL and a linear detection range from 11.6 to 92.8 ng/mL | [93] |
| Colorimetric-photothermal biosensor containing Fe–N–C nanozyme with peroxidase-like activity | Paraoxon ethyl, AChE | Limits of detection of 1.9 mU/mL (colorimetric) and 2.2 mU/mL (photothermal) for AChE, and 0.012 μg/mL (colorimetric) and 0.013 μg/mL (photothermal) for Paraoxon ethyl | [94] |
| Rhodium nanozyme for highly sensitive colorimetric detection of AChE activity | AChE | limit of detection of 3.2 × 10−4 mU/mL for AChE | [95] |
| Smartphone-assisted colorimetric biosensor utilizing PtCu3 alloy nanocrystals with peroxidase-like activity | AChE and pesticide dipterex | Limit of detection of 0.05 mU/mL for AChE and 0.5 ng/mL for dipterex | [96] |
| A colorimetric biosensor using AuHg aerogels exerting peroxidase-like activity | AChE and paraoxon ethyl | 0.02 mU/mL for AChE and 0.86 ng/mL for paraoxon ethyl | [97] |
| A colorimetric biosensor using AChE encapsulated in naturally occurring extracellular vesicles | Paraoxon ethyl | Limit of detection of 53.8 pmol/L | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pohanka, M. Fluorometric and Colorimetric Biosensors for the Assay of Cholinesterase Inhibitors. Sensors 2025, 25, 2674. https://doi.org/10.3390/s25092674
Pohanka M. Fluorometric and Colorimetric Biosensors for the Assay of Cholinesterase Inhibitors. Sensors. 2025; 25(9):2674. https://doi.org/10.3390/s25092674
Chicago/Turabian StylePohanka, Miroslav. 2025. "Fluorometric and Colorimetric Biosensors for the Assay of Cholinesterase Inhibitors" Sensors 25, no. 9: 2674. https://doi.org/10.3390/s25092674
APA StylePohanka, M. (2025). Fluorometric and Colorimetric Biosensors for the Assay of Cholinesterase Inhibitors. Sensors, 25(9), 2674. https://doi.org/10.3390/s25092674






