Greenhouse Gases Detection Exploiting a Multi-Wavelength Interband Cascade Laser Source in a Quartz-Enhanced Photoacoustic Sensor
Abstract
1. Introduction
2. Materials and Methods
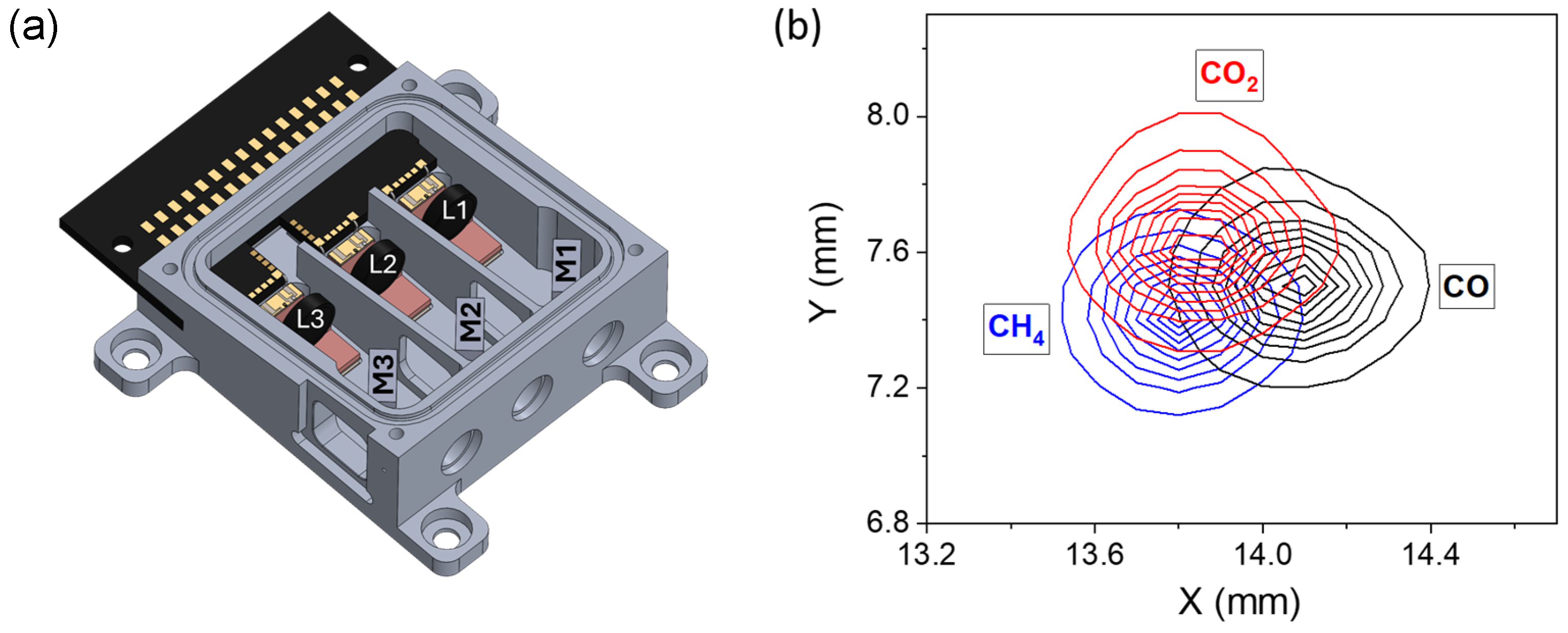
2.1. Three-Wavelength ICL Module
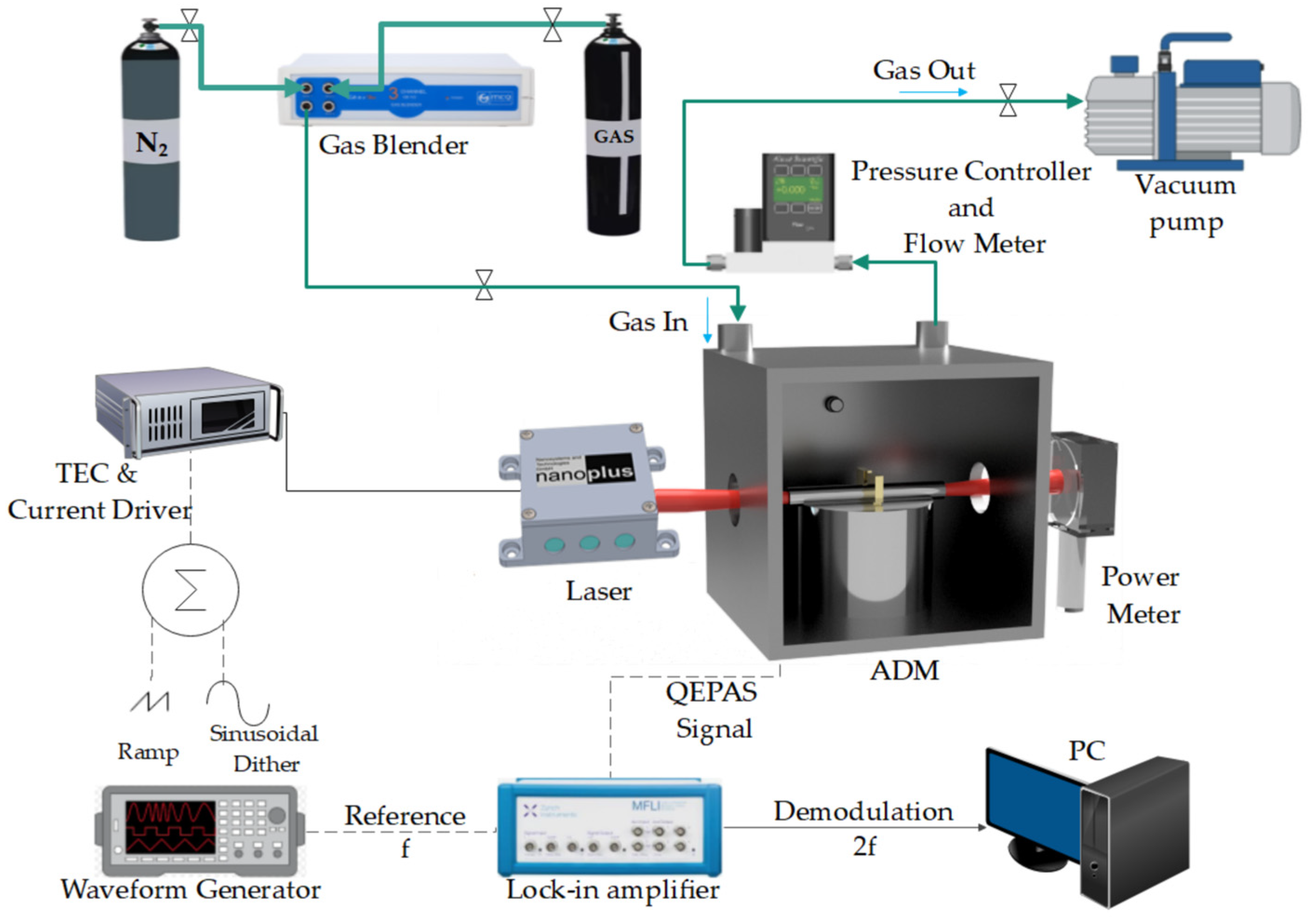
2.2. Experimental Setup for Multi-Gas QEPAS Sensor
3. Results
3.1. Direct Greenhouse Gases Detection
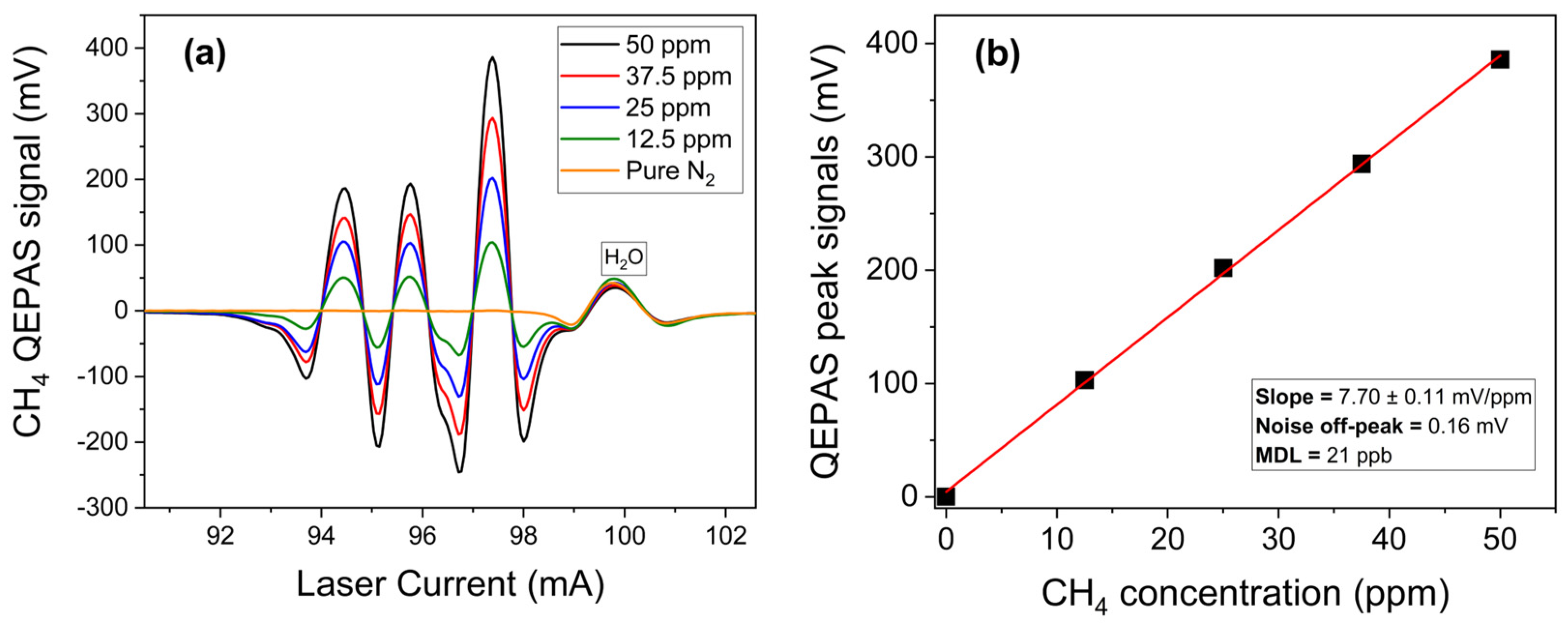
3.1.1. Methane Detection
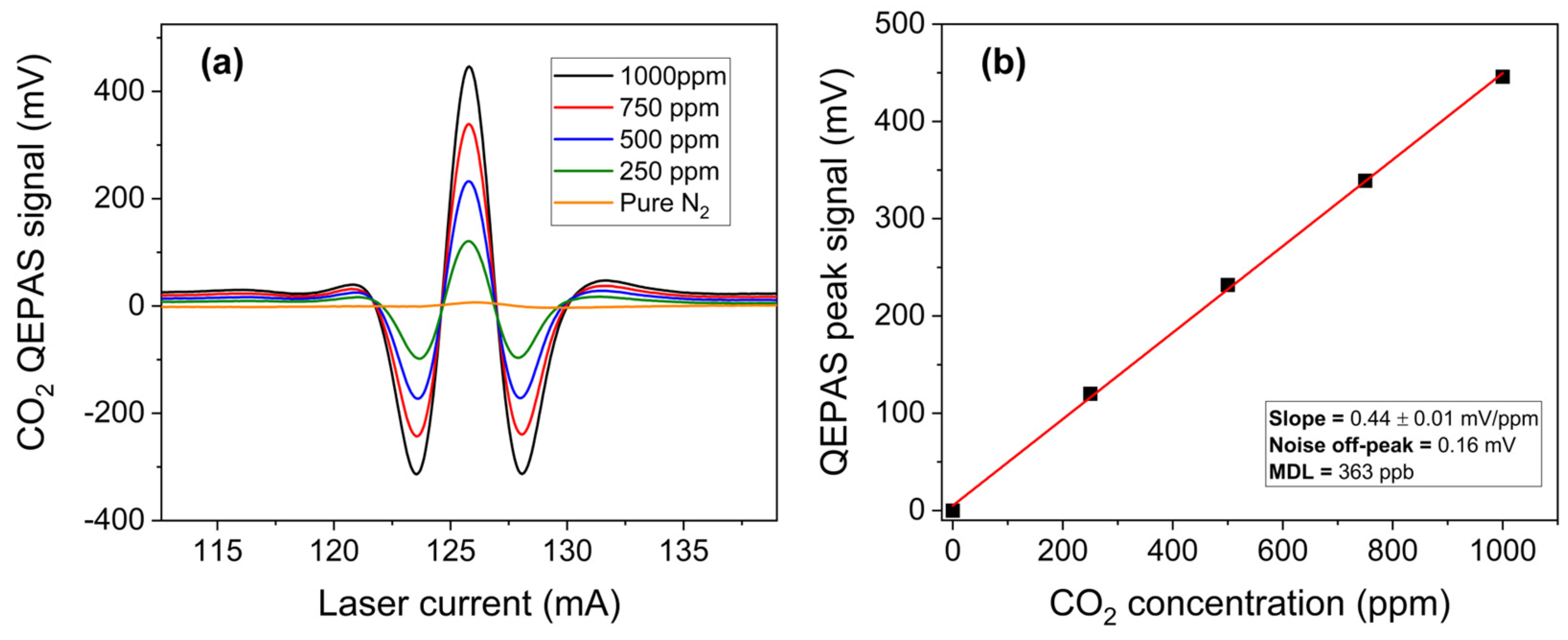
3.1.2. Carbon Dioxide Detection
3.2. Precursor Greenhouse Gas Detection: Carbon Monoxide
3.3. Long-Term Stability of the Sensor
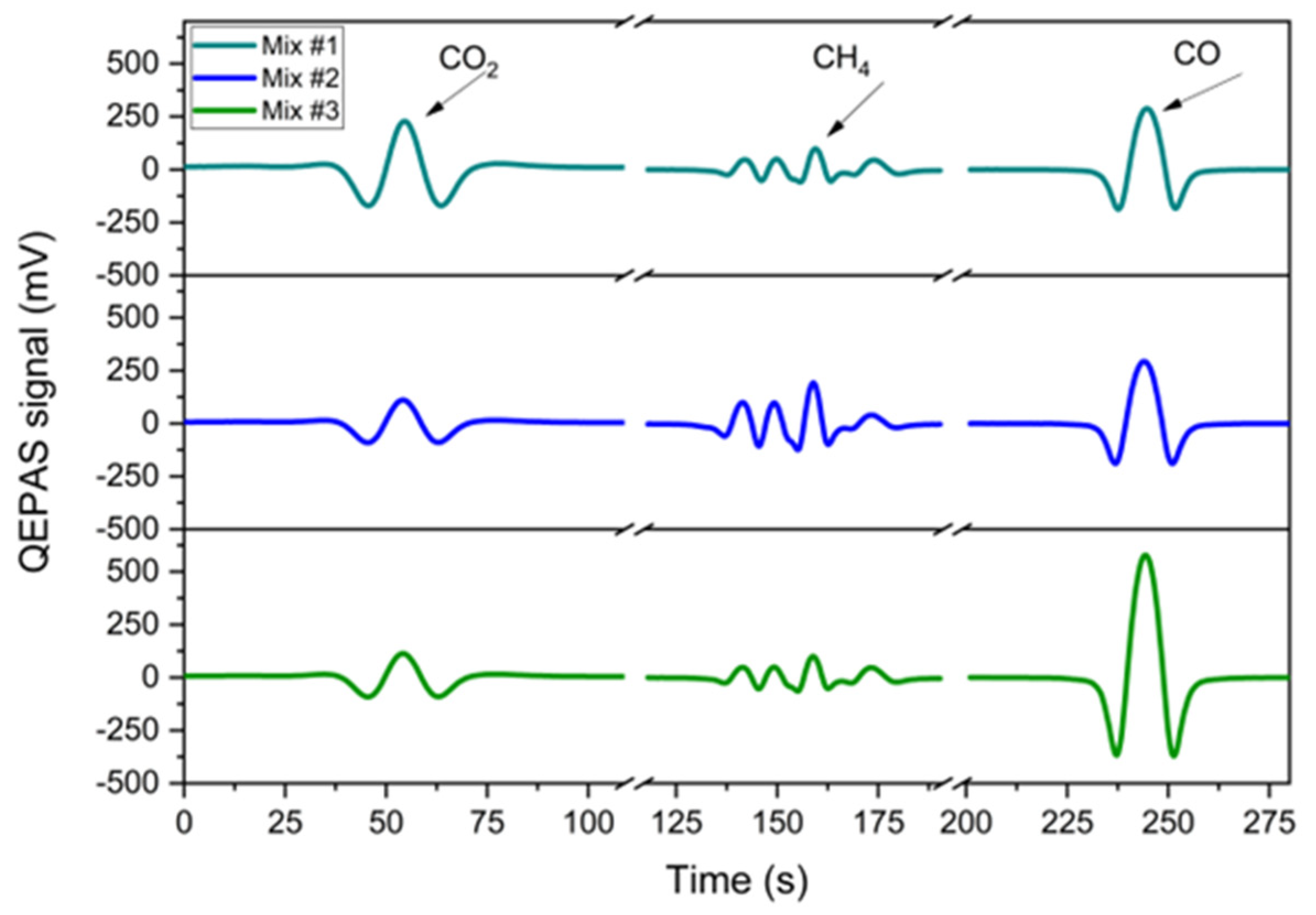
3.4. Gas Mixtures Detection and Analysis
- Mix #1: 500 ppm CO2, 12.5 ppm CH4, 250 ppm CO, in N2;
- Mix #2: 250 ppm CO2, 25 ppm CH4, 250 ppm CO, in N2;
- Mix #3: 250 ppm CO2, 12.5 ppm CH4, 500 ppm CO, in N2.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Climate Change Indicators Reached Record Levels in 2023: WMO. Available online: https://wmo.int/news/media-centre/climate-change-indicators-reached-record-levels-2023-wmo#:~:text=The%20WMO%20report%20confirmed%20that,across%20all%20major%20indicators. (accessed on 14 June 2024).
- Tuckett, R. Greenhouse Gases. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 362–372. [Google Scholar] [CrossRef]
- No Sign of Greenhouse Gases Increases Slowing in 2023—NOAA Research. Available online: https://research.noaa.gov/2024/04/05/no-sign-of-greenhouse-gases-increases-slowing-in-2023/ (accessed on 14 June 2024).
- Mar, K.A.; Unger, C.; Walderdorff, L.; Butler, T. Beyond CO2 Equivalence: The Impacts of Methane on Climate, Ecosystems, and Health. Environ. Sci. Policy 2022, 134, 127–136. [Google Scholar] [CrossRef]
- Nilrit, S.; Sampanpanish, P.; Bualert, S. Emission Factors of CH4 and CO2 Emitted from Vehicles. Am. J. Environ. Sci. 2013, 9, 38–44. [Google Scholar] [CrossRef]
- Evans, W.F.J.; Puckrin, E. An Observation of the Greenhouse Radiation Associated with Carbon Monoxide. Geophys. Res. Lett. 1995, 22, 925–928. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; WHO European Centre for Environment and Health: Bonn, Germany, 2021; ISBN 978-92-4-003422-8. [Google Scholar]
- Annual Report 2023|UNEP-UN Environment Programme. Available online: https://www.unep.org/resources/annual-report-2023 (accessed on 14 June 2024).
- Schneider, P.; Vogt, M.; Haugen, R.; Hassani, A.; Castell, N.; Dauge, F.R.; Bartonova, A. Deployment and Evaluation of a Network of Open Low-Cost Air Quality Sensor Systems. Atmosphere 2023, 14, 540. [Google Scholar] [CrossRef]
- Figaro Engineering Inc. TGS3870: Gas Sensors & Modules—Products. Available online: https://www.figarosensor.com/product/entry/tgs3870.html (accessed on 28 November 2024).
- Fu, L.; You, S.; Li, G.; Fan, Z. Enhancing Methane Sensing with NDIR Technology: Current Trends and Future Prospects. Rev. Anal. Chem. 2023, 42, 20230062. [Google Scholar] [CrossRef]
- Dubey, R.; Telles, A.; Nikkel, J.; Cao, C.; Gewirtzman, J.; Raymond, P.A.; Lee, X. Low-Cost CO2 NDIR Sensors: Performance Evaluation and Calibration Using Machine Learning Techniques. Sensors 2024, 24, 5675. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Tatam, R.P. Optical Gas Sensing: A Review. Meas. Sci. Technol. 2012, 24, 012004. [Google Scholar] [CrossRef]
- Mishra, V.; Rashmi, V.; Sukriti, K. Optical Gas Sensors. In Metal-Oxide Gas Sensors; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Genner, A.; Martín-mateos, P.; Moser, H.; Lendl, B. A Quantum Cascade Laser-Based Multi-Gas Sensor for Ambient Air Monitoring. Sensors 2020, 20, 1850. [Google Scholar] [CrossRef]
- Besson, J.P.; Schilt, S.; Thévenaz, L. Multi-Gas Sensing Based on Photoacoustic Spectroscopy Using Tunable Laser Diodes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004, 60, 3449–3456. [Google Scholar] [CrossRef]
- Gomez-Pelaez, A.J.; Ramos, R.; Cuevas, E.; Gomez-Trueba, V.; Reyes, E. Atmospheric CO2, CH4, and CO with the CRDS Technique at the Izaña Global GAW Station: Instrumental Tests, Developments, and First Measurement Results. Atmos. Meas. Tech. 2019, 12, 2043–2066. [Google Scholar] [CrossRef]
- G2301 Gas Concentration Analyzer|Picarro. Available online: https://www.picarro.com/environmental/products/g2301_gas_concentration_analyzer (accessed on 28 November 2024).
- De Palo, R.; Elefante, A.; Biagi, G.; Paciolla, F.; Weih, R.; Villada, V.; Zifarelli, A.; Giglio, M.; Sampaolo, A.; Spagnolo, V.; et al. Quartz-Enhanced Photoacoustic Sensors for Detection of Eight Air Pollutants. Adv. Photonics Res. 2023, 4, 2200353. [Google Scholar] [CrossRef]
- Ma, Y. Review of Recent Advances in QEPAS-Based Trace Gas Sensing. Appl. Sci. 2018, 8, 1822. [Google Scholar] [CrossRef]
- He, Y.; Ma, Y.; Tong, Y.; Yu, X.; Tittel, F.K. A Portable Gas Sensor for Sensitive CO Detection Based on Quartz-Enhanced Photoacoustic Spectroscopy. Opt. Laser Technol. 2019, 115, 129–133. [Google Scholar] [CrossRef]
- Sampaolo, A.; Patimisco, P.; Giglio, M.; Zifarelli, A.; Wu, H.; Dong, L.; Spagnolo, V. Quartz-Enhanced Photoacoustic Spectroscopy for Multi-Gas Detection: A Review. Anal. Chim. Acta 2022, 1202, 338894. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Laurent, O.; Schilt, S.; Ramonet, M.; Gao, X.; Dong, L.; Chen, W. Simultaneous Monitoring of Atmospheric CH4, N2O, and H2O Using a Single Gas Sensor Based on Mid-IR Quartz-Enhanced Photoacoustic Spectroscopy. Anal. Chem. 2022, 94, 17522–17532. [Google Scholar] [CrossRef]
- Contreras-Teran, M.A.; Berganza, A.; Lindner, F.; Bierlich, J.; Zubia, J.; Wondraczek, K.; Villatoro, J.; Villatoro, J. QEPAS Sensor for In-Situ Measurement of CO2 and Its Isotopic Fingerprint. In Proceedings of the Optica Sensing Congress 2024 (AIS, LACSEA, Sensors, QSM), Toulouse, France, 15–19 July 2024. Paper LM2E.2. [Google Scholar]
- Liu, H.; Chen, X.; Hu, M.; Wang, H.; Yao, L.; Xu, Z.; Ma, G.; Wang, Q.; Kan, R. In Situ High-Precision Measurement of Deep-Sea Dissolved Methane by Quartz-Enhanced Photoacoustic and Light-Induced Thermoelastic Spectroscopy. Anal. Chem. 2024, 96, 12846–12853. [Google Scholar] [CrossRef]
- Curl, R.F.; Kosterev, A.A.; Bakhirkin, Y.A.; Tittel, F.K. Quartz-Enhanced Photoacoustic Spectroscopy. Opt. Lett. 2002, 27, 1902–1904. [Google Scholar] [CrossRef]
- Patimisco, P.; Ardito, N.; De Toma, E.; Burghart, D.; Tigaev, V.; Belkin, M.A.; Spagnolo, V. Quartz-Enhanced Photoacoustic Sensor Based on a Multi-Laser Source for in-Sequence Detection of NO2, SO2, and NH3. Sensors 2023, 23, 9005. [Google Scholar] [CrossRef]
- Vurgaftman, I.; Weih, R.; Kamp, M.; Meyer, J.R.; Canedy, C.L.; Kim, C.S.; Kim, M.; Bewley, W.W.; Merritt, C.D.; Abell, J.; et al. Interband Cascade Lasers. J. Phys. D Appl. Phys. 2015, 48, 123001. [Google Scholar] [CrossRef]
- Vurgaftman, I.; Bewley, W.W.; Canedy, C.L.; Kim, C.S.; Kim, M.; Merritt, C.D.; Abell, J.; Meyer, J.R. Interband Cascade Lasers with Low Threshold Powers and High Output Powers. IEEE J. Sel. Top. Quantum Electron. 2013, 19, 1200210. [Google Scholar] [CrossRef]
- Vurgaftman, I.; Bewley, W.W.; Canedy, C.L.; Kim, C.S.; Kim, M.; Merritt, C.D.; Abell, J.; Lindle, J.R.; Meyer, J.R. Rebalancing of Internally Generated Carriers for Mid-Infrared Interband Cascade Lasers with Very Low Power Consumption. Nat. Commun. 2011, 2, 585. [Google Scholar] [CrossRef] [PubMed]
- Cantatore, A.F.P.; Menduni, G.; Zifarelli, A.; Patimisco, P.; Giglio, M.; Gonzalez, M.; Seren, H.R.; Luo, P.; Spagnolo, V.; Sampaolo, A. Methane, Ethane, and Propane Detection Using a Quartz-Enhanced Photoacoustic Sensor for Natural Gas Composition Analysis. Energy Fuels 2025, 39, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Tittel, F.K.; Sampaolo, A.; Mackowiak, V.; Cable, A.; Patimisco, P.; Spagnolo, V.; Dello Russo, S.; Rossmadl, H.; Giglio, M. Tuning Forks with Optimized Geometries for Quartz-Enhanced Photoacoustic Spectroscopy. Opt. Express 2019, 27, 1401–1415. [Google Scholar] [CrossRef]
- Stewart, G. Wavelength Modulation Spectroscopy with DFB Lasers. In Laser and Fiber Optic Gas Absorption Spectroscopy; Cambridge University Press: Cambridge, UK, 2021; pp. 53–84. [Google Scholar] [CrossRef]
- Gordon, I.E.; Rothman, L.S.; Hargreaves, R.J.; Hashemi, R.; Karlovets, E.V.; Skinner, F.M.; Conway, E.K.; Hill, C.; Kochanov, R.V.; Tan, Y.; et al. The HITRAN2020 Molecular Spectroscopic Database. J. Quant. Spectrosc. Radiat. Transf. 2022, 277, 107949. [Google Scholar] [CrossRef]





| Mix | Target Gas | Extracted Signal (mV) | Expected Signal (mV) | Expected Concentration (ppm) | Estimated Concentration (ppm) | Difference (ppm) | Relative Difference (%) |
|---|---|---|---|---|---|---|---|
| #1 | CO2 | 228.14 ± 0.17 | 220.0 | 500 ± 10 | 518.5 ± 11.8 | 18.5 | 3.7 |
| #1 | CH4 | 98.33 ± 0.16 | 96.3 | 12.5 ± 0.3 | 12.8 ± 0.2 | 0.3 | 2.2 |
| #1 | CO | 288.14 ± 0.17 | 287.5 | 250 ± 5 | 250.9 ± 10.9 | 0.9 | 0.4 |
| #2 | CO2 | 111.17 ± 0.17 | 110.0 | 250 ± 5 | 255.3 ± 11.1 | 5.3 | 2.1 |
| #2 | CH4 | 99.84 ± 0.16 | 96.3 | 25 ± 0.5 | 24.9 ± 0.4 | 0.1 | 0.2 |
| #2 | CO | 293.57 ± 0.17 | 287.5 | 250 ± 5 | 255.3 ± 11.1 | 5.3 | 2.1 |
| #3 | CO2 | 113.06 ± 0.17 | 110.0 | 250 ± 5.0 | 256.9 ± 5.9 | 6.9 | 2.7 |
| #3 | CH4 | 99.67 ± 0.16 | 96.3 | 12.5 ± 0.3 | 12.9 ± 0.2 | 0.4 | 3.6 |
| #3 | CO | 578.78 ± 0.17 | 575 | 500 ± 10 | 503.3 ± 21.9 | 3.3 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Palo, R.; Ardito, N.; Zifarelli, A.; Sampaolo, A.; Giglio, M.; Patimisco, P.; Ranieri, E.; Weih, R.; Nauschütz, J.; König, O.; et al. Greenhouse Gases Detection Exploiting a Multi-Wavelength Interband Cascade Laser Source in a Quartz-Enhanced Photoacoustic Sensor. Sensors 2025, 25, 2442. https://doi.org/10.3390/s25082442
De Palo R, Ardito N, Zifarelli A, Sampaolo A, Giglio M, Patimisco P, Ranieri E, Weih R, Nauschütz J, König O, et al. Greenhouse Gases Detection Exploiting a Multi-Wavelength Interband Cascade Laser Source in a Quartz-Enhanced Photoacoustic Sensor. Sensors. 2025; 25(8):2442. https://doi.org/10.3390/s25082442
Chicago/Turabian StyleDe Palo, Raffaele, Nicoletta Ardito, Andrea Zifarelli, Angelo Sampaolo, Marilena Giglio, Pietro Patimisco, Ezio Ranieri, Robert Weih, Josephine Nauschütz, Oliver König, and et al. 2025. "Greenhouse Gases Detection Exploiting a Multi-Wavelength Interband Cascade Laser Source in a Quartz-Enhanced Photoacoustic Sensor" Sensors 25, no. 8: 2442. https://doi.org/10.3390/s25082442
APA StyleDe Palo, R., Ardito, N., Zifarelli, A., Sampaolo, A., Giglio, M., Patimisco, P., Ranieri, E., Weih, R., Nauschütz, J., König, O., & Spagnolo, V. (2025). Greenhouse Gases Detection Exploiting a Multi-Wavelength Interband Cascade Laser Source in a Quartz-Enhanced Photoacoustic Sensor. Sensors, 25(8), 2442. https://doi.org/10.3390/s25082442










