Na-Ag Ion-Exchanged Glass Substrates for Plasmon-Enhanced Fluorescence Imaging of Neutrophils
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Fabrication
2.2. Substrate Characterization
2.3. Neutrophil Isolation
2.4. Neutrophil Staining Procedure
2.5. Fluorescence Microscopy
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, Y.; Yu, Y.-J.; Jiang, Q.-Y.; Sun, Y.; Mao, Z.; Wang, J.; Chen, F. The Advance of Plasmonic-Electric Nanopipette Sensing in Single Cells. Curr. Pharmacol. Rep. 2021, 7, 55–66. [Google Scholar] [CrossRef]
- Lee, S.; Sun, Y.; Cao, Y.; Kang, S.H. Plasmonic Nanostructure-Based Bioimaging and Detection Techniques at the Single-Cell Level. TrAC Trends Anal. Chem. 2019, 117, 58–68. [Google Scholar] [CrossRef]
- Barshutina, M.; Doroshina, N.; Baizhumanov, A.; Nikelshparg, E.; Fedotova, A.; Popov, A.; Semyanov, A.; Yakubovsky, D.; Tselikov, G.; Luneva, O.; et al. SERS Substrates Based on Rose Petal Replicas for the Oxidative Stress Detection. Appl. Surf. Sci. 2023, 626, 157281. [Google Scholar] [CrossRef]
- Liedberg, B.; Nylander, C.; Lundström, I. Biosensing with Surface Plasmon Resonance–How It All Started. Biosens. Bioelectron. 1995, 10, i–ix. [Google Scholar] [CrossRef]
- Huang, S.; Chen, J.; Zhang, T.; Dai, X.; Wang, X.; Zhou, J.; Kong, W.; Liu, Q.; Qu, J.; Shao, Y. Recent Advances in Surface Plasmon Resonance Microscopy. Chemosensors 2022, 10, 509. [Google Scholar] [CrossRef]
- Cao, Z.; Gong, F.-C.; Huang, X.-X.; Tan, S.-Z.; Takei, H.; Pipper, J. Biosensing with Localized Surface Plasmon Resonance Based on Cap-Shaped Gold Nano-Particles. In Proceedings of the 2008 2nd International Conference on Bioinformatics and Biomedical Engineering, Shanghai, China, 16–18 May 2008. [Google Scholar]
- Peterson, A.W.; Halter, M.; Tona, A.; Bhadriraju, K.; Plant, A.L. Surface Plasmon Resonance Imaging of Cells and Surface-Associated Fibronectin. BMC Cell Biol. 2009, 10, 16. [Google Scholar] [CrossRef]
- Dorion-Thibaudeau, J.; Raymond, C.; Lattová, E.; Perreault, H.; Durocher, Y.; De Crescenzo, G. Towards the Development of a Surface Plasmon Resonance Assay to Evaluate the Glycosylation Pattern of Monoclonal Antibodies Using the Extracellular Domains of CD16a and CD64. J. Immunol. Methods 2014, 408, 24–34. [Google Scholar] [CrossRef]
- Peterson, A.W.; Halter, M.; Tona, A.; Plant, A.L. High Resolution Surface Plasmon Resonance Imaging for Single Cells. BMC Cell Biol. 2014, 15, 35. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Lv, Z.-T.; Lv, W.-L.; Liu, X.-W. Plasmonic Probing of the Adhesion Strength of Single Microbial Cells. Proc. Natl. Acad. Sci. USA 2020, 117, 27148–27153. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Liu, Q.; Wu, J.; Tao, N. Mapping Single-Cell–Substrate Interactions by Surface Plasmon Resonance Microscopy. Langmuir 2012, 28, 13373–13379. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, J.; Wang, X.; Cai, Z.; Shao, Y. Wavelength-Scanning Surface Plasmon Resonance Microscopy: A Novel Tool for Real Time Sensing of Cell-Substrate Interactions. Biosens. Bioelectron. 2019, 145, 111717. [Google Scholar] [CrossRef]
- Yanase, Y.; Hiragun, T.; Yanase, T.; Kawaguchi, T.; Ishii, K.; Hide, M. Evaluation of Peripheral Blood Basophil Activation by Means of Surface Plasmon Resonance Imaging. Biosens. Bioelectron. 2012, 32, 62–68. [Google Scholar] [CrossRef]
- Yanase, Y.; Hiragun, T.; Ishii, K.; Kawaguchi, T.; Yanase, T.; Kawai, M.; Sakamoto, K.; Hide, M. Surface Plasmon Resonance for Cell-Based Clinical Diagnosis. Sensors 2014, 14, 4948–4959. [Google Scholar] [CrossRef] [PubMed]
- Yanase, Y.; Hiragun, T.; Kaneko, S.; Gould, H.J.; Greaves, M.W.; Hide, M. Detection of Refractive Index Changes in Individual Living Cells by Means of Surface Plasmon Resonance Imaging. Biosens. Bioelectron. 2010, 26, 674–681. [Google Scholar] [CrossRef]
- Zhang, Y.; Aslan, K.; Previte’, M.J.R.; Geddes, C.D. Metal-Enhanced Excimer (P-Type) Fluorescence. Chem. Phys. Lett. 2008, 458, 147–151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gabudean, A.M.; Focsan, M.; Astilean, S. Gold Nanorods Performing as Dual-Modal Nanoprobes via Metal-Enhanced Fluorescence (MEF) and Surface-Enhanced Raman Scattering (SERS). J. Phys. Chem. C 2012, 116, 12240–12249. [Google Scholar] [CrossRef]
- El-Bashir, S.M.; Barakat, F.M.; AlSalhi, M.S. Metal-Enhanced Fluorescence of Mixed Coumarin Dyes by Silver and Gold Nanoparticles: Towards Plasmonic Thin-Film Luminescent Solar Concentrator. J. Lumin. 2013, 143, 43–49. [Google Scholar] [CrossRef]
- Seth, A.; Mittal, E.; Luan, J.; Kolla, S.; Mazer, M.B.; Joshi, H.; Gupta, R.; Rathi, P.; Wang, Z.; Morrissey, J.J.; et al. High-Resolution Imaging of Protein Secretion at the Single-Cell Level Using Plasmon-Enhanced FluoroDOT Assay. Cell Rep. Methods 2022, 2, 100267. [Google Scholar] [CrossRef]
- Kennedy, B.J.; Spaeth, S.; Dickey, M.; Carron, K.T. Determination of the Distance Dependence and Experimental Effects for Modified SERS Substrates Based on Self-Assembled Monolayers Formed Using Alkanethiols. J. Phys. Chem. B 1999, 103, 3640–3646. [Google Scholar] [CrossRef]
- Dasgupta, S.; Ray, K. Plasmon-Enhanced Fluorescence for Biophotonics and Bio-Analytical Applications. Front. Chem. 2024, 12, 1407561. [Google Scholar] [CrossRef]
- Lee, S.T.; Kuboki, T.; Kidoaki, S.; Aida, Y.; Arima, Y.; Tamada, K. A Plasmonic Metasurface Reveals Differential Motility of Breast Cancer Cell Lines at Initial Phase of Adhesion. Colloids Surf. B Biointerfaces 2024, 238, 113876. [Google Scholar] [CrossRef]
- Klantsataya, E.; François, A.; Ebendorff-Heidepriem, H.; Sciacca, B.; Zuber, A.; Monro, T.M. Effect of Surface Roughness on Metal Enhanced Fluorescence in Planar Substrates and Optical Fibers. Opt. Mater. Express OME 2016, 6, 2128–2138. [Google Scholar] [CrossRef]
- Berneschi, S.; Righini, G.C.; Pelli, S. Towards a Glass New World: The Role of Ion-Exchange in Modern Technology. Appl. Sci. 2021, 11, 4610. [Google Scholar] [CrossRef]
- West, B.R. Ion-Exchanged Glass Waveguide Technology: A Review. Opt. Eng. 2011, 50, 071107. [Google Scholar] [CrossRef]
- Karvonen, L.; Chen, Y.; Säynätjoki, A.; Taiviola, K.; Tervonen, A.; Honkanen, S. SERS-Active Silver Nanoparticle Aggregates Produced in High-Iron Float Glass by Ion Exchange Process. Opt. Mater. 2011, 34, 1–5. [Google Scholar] [CrossRef]
- Zhurikhina, V.V.; Brunkov, P.N.; Melehin, V.G.; Kaplas, T.; Svirko, Y.; Rutckaia, V.V.; Lipovskii, A.A. Self-Assembled Silver Nanoislands Formed on Glass Surface via out-Diffusion for Multiple Usages in SERS Applications. Nanoscale Res. Lett. 2012, 7, 676. [Google Scholar] [CrossRef] [PubMed]
- Chervinskii, S.; Sevriuk, V.; Reduto, I.; Lipovskii, A. Formation and 2D-Patterning of Silver Nanoisland Film Using Thermal Poling and out-Diffusion from Glass. J. Appl. Phys. 2013, 114, 224301. [Google Scholar] [CrossRef]
- Sgibnev, Y.; Nikonorov, N.; Ignatiev, A. Governing Functionality of Silver Ion-Exchanged Photo-Thermo-Refractive Glass Matrix by Small Additives. Appl. Sci. 2021, 11, 3891. [Google Scholar] [CrossRef]
- Elangovan, G.; Mello-Neto, J.M.; Tadakamadla, S.K.; Reher, P.; Figueredo, C.M.S. A Systematic Review on Neutrophils Interactions with Titanium and Zirconia Surfaces: Evidence from in Vitro Studies. Clin. Exp. Dental Res. 2022, 8, 950–958. [Google Scholar] [CrossRef]
- Pleskova, S.N.; Kriukov, R.N.; Gorshkova, E.N.; Boryakov, A.V. Characteristics of Quantum Dots Phagocytosis by Neutrophil Granulocytes. Heliyon 2019, 5, e01439. [Google Scholar] [CrossRef]
- Galkina, S.I.; Molotkovsky, J.G.; Ullrich, V.; Sud’ina, G.F. Scanning Electron Microscopy Study of Neutrophil Membrane Tubulovesicular Extensions (Cytonemes) and Their Role in Anchoring, Aggregation and Phagocytosis. The Effect of Nitric Oxide. Exp. Cell Res. 2005, 304, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Shvedov, M.; Sherstyukova, E.; Kandrashina, S.; Inozemtsev, V.; Sergunova, V. Atomic Force Microscopy and Scanning Ion-Conductance Microscopy for Investigation of Biomechanical Characteristics of Neutrophils. Cells 2024, 13, 1757. [Google Scholar] [CrossRef] [PubMed]
- Obraztsov, I.V.; Godkov, M.A.; Kulabukhov, V.V.; Vladimirova, G.A.; Izmailov, D.Y.; Proskurnina, E.V. Functional Activity of Neutrophils in Burn Sepsis. Gen. Reanimatol. 2017, 13, 40–51. [Google Scholar] [CrossRef][Green Version]
- Sergunova, V.; Inozemtsev, V.; Vorobjeva, N.; Kozlova, E.; Sherstyukova, E.; Lyapunova, S.; Chernysh, A. Morphology of Neutrophils during Their Activation and NETosis: Atomic Force Microscopy Study. Cells 2023, 12, 2199. [Google Scholar] [CrossRef]
- Inozemtsev, V.; Sergunova, V.; Vorobjeva, N.; Kozlova, E.; Sherstyukova, E.; Lyapunova, S.; Chernysh, A. Stages of NETosis Development upon Stimulation of Neutrophils with Activators of Different Types. Int. J. Mol. Sci. 2023, 24, 12355. [Google Scholar] [CrossRef] [PubMed]
- Grebenchikov, O.A.; Kasatkina, I.S.; Kadantseva, K.K.; Meshkov, M.A.; Bayeva, A.A. The Effect of Lithium Chloride on Neutrophil Activation on Exposure to Serum of Patients with Septic Shock. Gen. Reanimatol. 2020, 16, 45–55. [Google Scholar] [CrossRef]
- Pleskova, S.N.; Bezrukov, N.A.; Gorshkova, E.N.; Bobyk, S.Z.; Lazarenko, E.V. Exploring the Process of Neutrophil Transendothelial Migration Using Scanning Ion-Conductance Microscopy. Cells 2023, 12, 1806. [Google Scholar] [CrossRef]
- Vorobjeva, N.V. Neutrophil Extracellular Traps: New Aspects. Mosc. Univ. Biol. Sci. Bull. 2020, 75, 173–188. [Google Scholar] [CrossRef]
- Sgibnev, Y.; Tananaev, P.; Shelaev, A.; Yankovskii, G.; Baryshev, A. Relative Humidity Optical Sensor Based on Self-Assembled Gold Nanoparticles Covered with Nafion. Photonics 2023, 10, 975. [Google Scholar] [CrossRef]
- Sgibnev, Y.; Asamoah, B.; Nikonorov, N.; Honkanen, S. Tunable Photoluminescence of Silver Molecular Clusters Formed in Na+-Ag+ Ion-Exchanged Antimony-Doped Photo-Thermo-Refractive Glass Matrix. J. Lumin. 2020, 226, 117411. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, A.; Skowronski, L.; Trzcinski, M.; Szoplik, T. Controlling the Optical Parameters of Self-Assembled Silver Films with Wetting Layers and Annealing. Appl. Surf. Sci. 2017, 421, 349–356. [Google Scholar] [CrossRef]
- Christensen, N.E. The Band Structure of Silver and Optical Interband Transitions. Phys. Status Solidi 1972, 54, 551–563. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface Plasmon Resonance in Gold Nanoparticles: A Review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Ciesielski, A.; Skowronski, L.; Trzcinski, M.; Górecka, E.; Trautman, P.; Szoplik, T. Evidence of Germanium Segregation in Gold Thin Films. Surf. Sci. 2018, 674, 73–78. [Google Scholar] [CrossRef]
- Rioux, D.; Vallières, S.; Besner, S.; Muñoz, P.; Mazur, E.; Meunier, M. An Analytic Model for the Dielectric Function of Au, Ag, and Their Alloys. Adv. Opt. Mater. 2014, 2, 176–182. [Google Scholar] [CrossRef]
- Efimov, A.M.; Ignatiev, A.I.; Nikonorov, N.V.; Postnikov, E.S. Photo-Thermo-Refractive Glasses: Effects of Dopants on Their Ultraviolet Absorption Spectra. Int. J. Appl. Glass Sci. 2015, 6, 109–127. [Google Scholar] [CrossRef]
- Sgibnev, E.M.; Ignatiev, A.I.; Nikonorov, N.V.; Efimov, A.M.; Postnikov, E.S. Effects of Silver Ion Exchange and Subsequent Treatments on the UV–VIS Spectra of Silicate Glasses. I. Undoped, CeO2-Doped, and (CeO2 + Sb2O3)-Codoped Photo-Thermo-Refractive Matrix Glasses. J. Non-Cryst. Solids 2013, 378, 213–226. [Google Scholar] [CrossRef]
- Hlaing, M.; Gebear-Eigzabher, B.; Roa, A.; Marcano, A.; Radu, D.; Lai, C.-Y. Absorption and Scattering Cross-Section Extinction Values of Silver Nanoparticles. Opt. Mater. 2016, 58, 439–444. [Google Scholar] [CrossRef]
- Kumbhar, A.S.; Kinnan, M.K.; Chumanov, G. Multipole Plasmon Resonances of Submicron Silver Particles. J. Am. Chem. Soc. 2005, 127, 12444–12445. [Google Scholar] [CrossRef]
- Kinnan, M.K.; Chumanov, G. Plasmon Coupling in Two-Dimensional Arrays of Silver Nanoparticles: II. Effect of the Particle Size and Interparticle Distance. J. Phys. Chem. C 2010, 114, 7496–7501. [Google Scholar] [CrossRef]
- Kinnan, M.K.; Kachan, S.; Simmons, C.K.; Chumanov, G. Plasmon Coupling in Two-Dimensional Arrays of Silver Nanoparticles: I. Effect of the Dielectric Medium. J. Phys. Chem. C 2009, 113, 7079–7084. [Google Scholar] [CrossRef]
- Horimoto, N.N.; Imura, K.; Okamoto, H. Dye Fluorescence Enhancement and Quenching by Gold Nanoparticles: Direct near-Field Microscopic Observation of Shape Dependence. Chem. Phys. Lett. 2008, 467, 105–109. [Google Scholar] [CrossRef]
- Hong, C.-W. Extracellular Vesicles of Neutrophils. Immune Netw. 2018, 18, e43. [Google Scholar] [CrossRef]
- Li, J.; Krasavin, A.V.; Webster, L.; Segovia, P.; Zayats, A.V.; Richards, D. Spectral Variation of Fluorescence Lifetime near Single Metal Nanoparticles. Sci. Rep. 2016, 6, 21349. [Google Scholar] [CrossRef]
- Kostyuk, A.I.; Rapota, D.D.; Morozova, K.I.; Fedotova, A.A.; Jappy, D.; Semyanov, A.V.; Belousov, V.V.; Brazhe, N.A.; Bilan, D.S. Modern Optical Approaches in Redox Biology: Genetically Encoded Sensors and Raman Spectroscopy. Free Radic. Biol. Med. 2024, 217, 68–115. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, Y.; Chen, J.; Lu, M.; Wang, X.; Zhang, Z.; Xiong, H.; Choo, J.; Chen, L. Plasmonic Nanomaterial-Enhanced Fluorescence and Raman Sensors: Multifunctional Platforms and Applications. Coord. Chem. Rev. 2024, 507, 215768. [Google Scholar] [CrossRef]
- Ming, T.; Chen, H.; Jiang, R.; Li, Q.; Wang, J. Plasmon-Controlled Fluorescence: Beyond the Intensity Enhancement. J. Phys. Chem. Lett. 2012, 3, 191–202. [Google Scholar] [CrossRef]
- Bauch, M.; Toma, K.; Toma, M.; Zhang, Q.; Dostalek, J. Plasmon-Enhanced Fluorescence Biosensors: A Review. Plasmonics 2014, 9, 781–799. [Google Scholar] [CrossRef]
- Wheat Germ Agglutinin (WGA). Available online: https://www.thermofisher.com/order/catalog/product/W11262 (accessed on 19 December 2024).
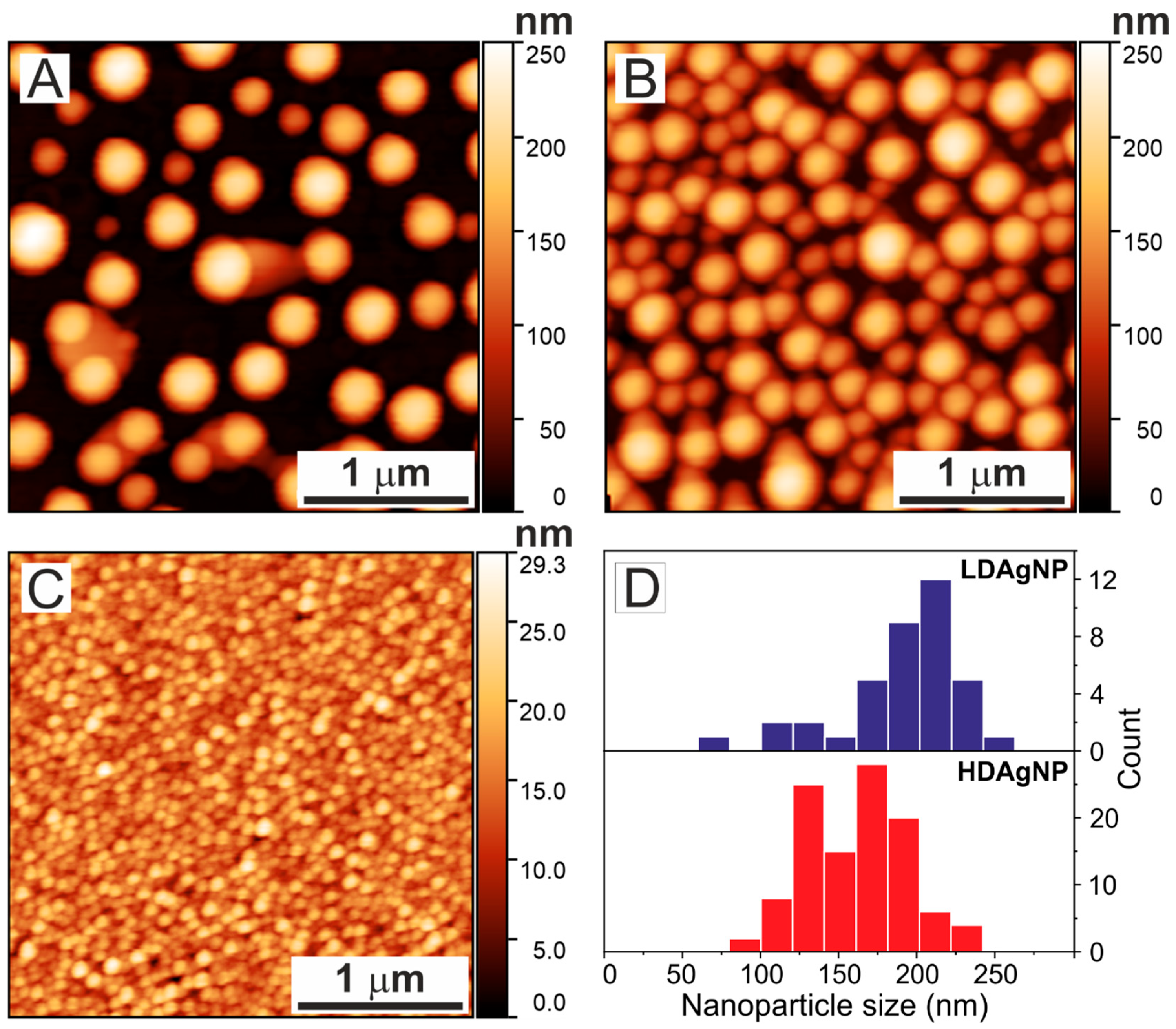
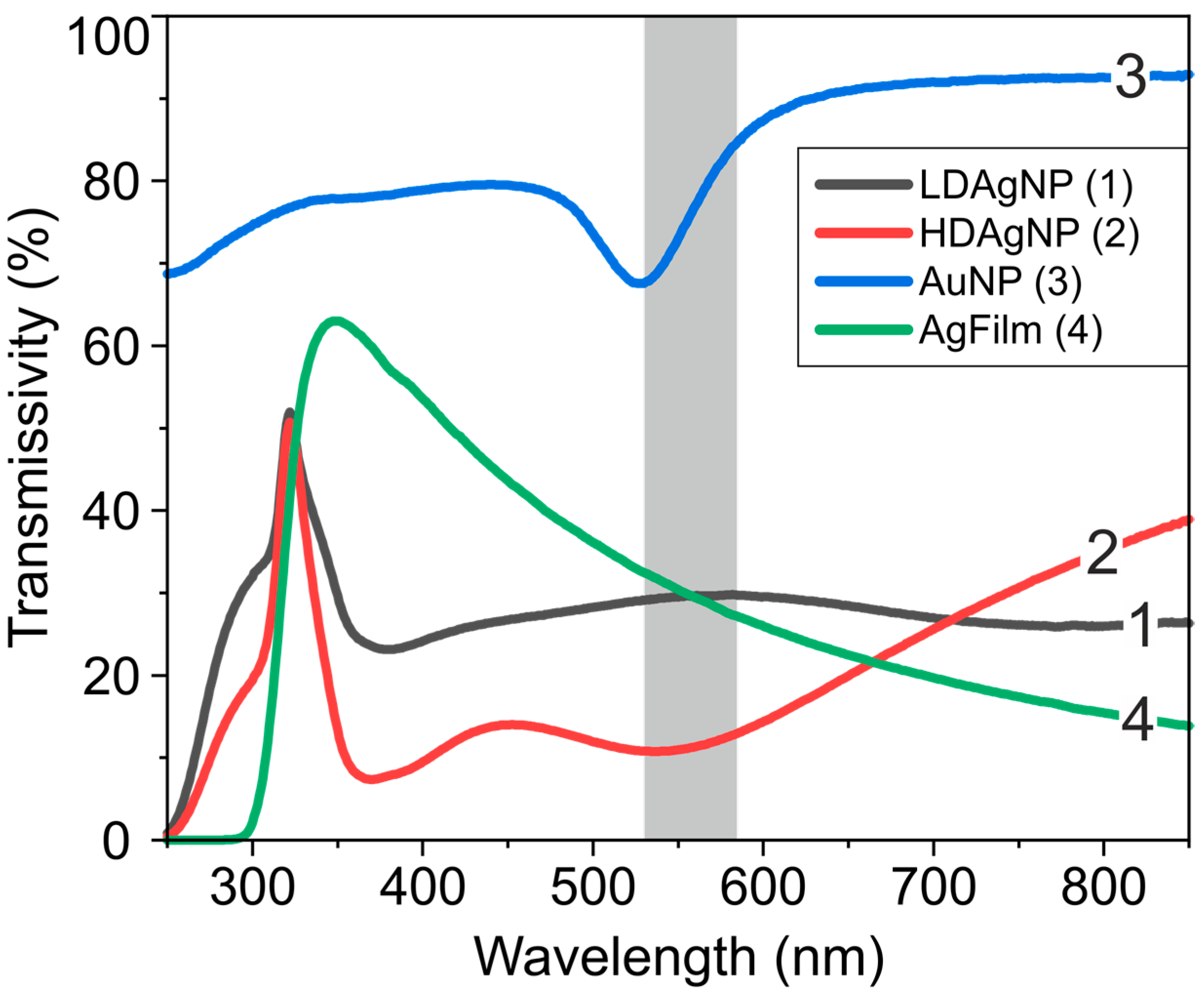

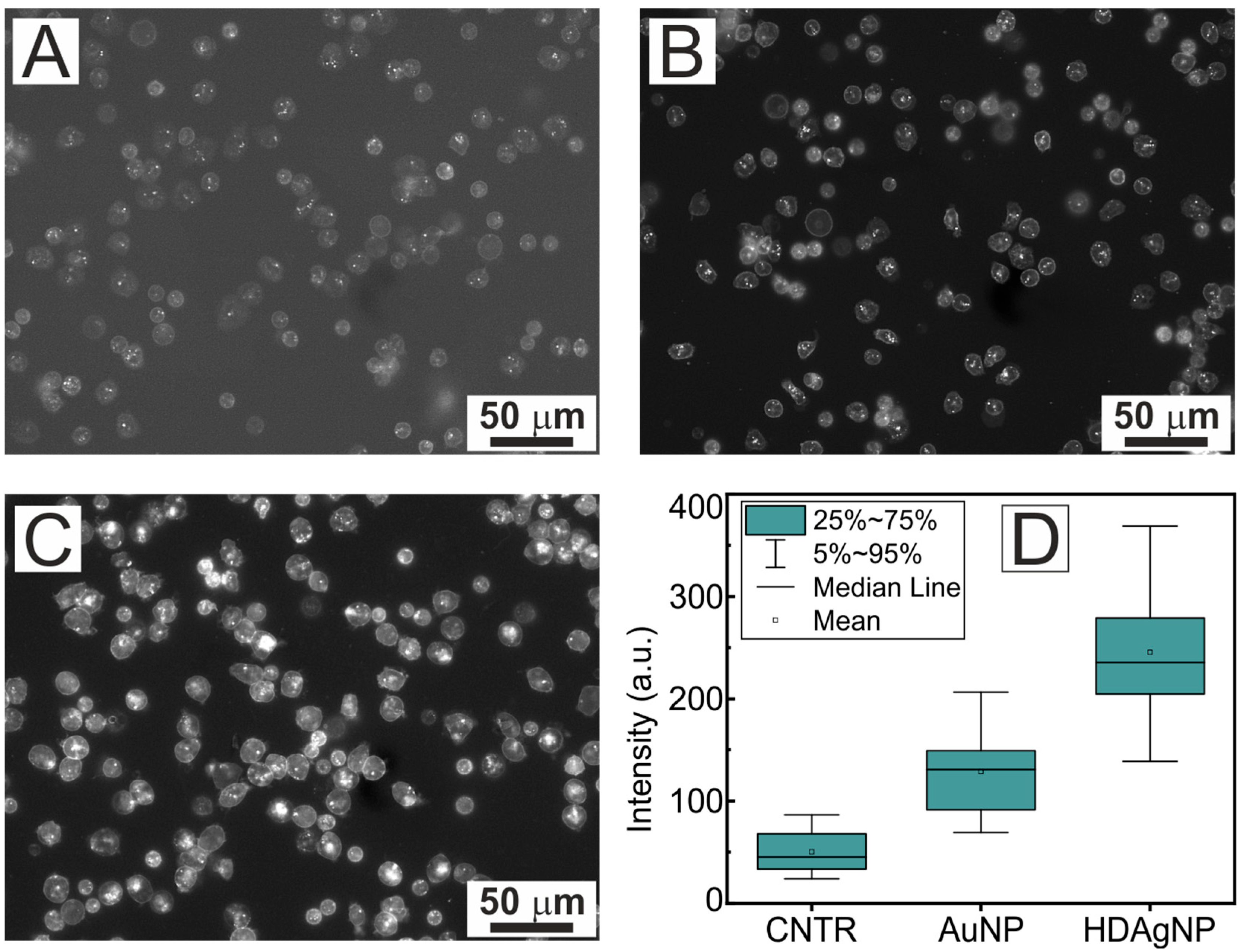
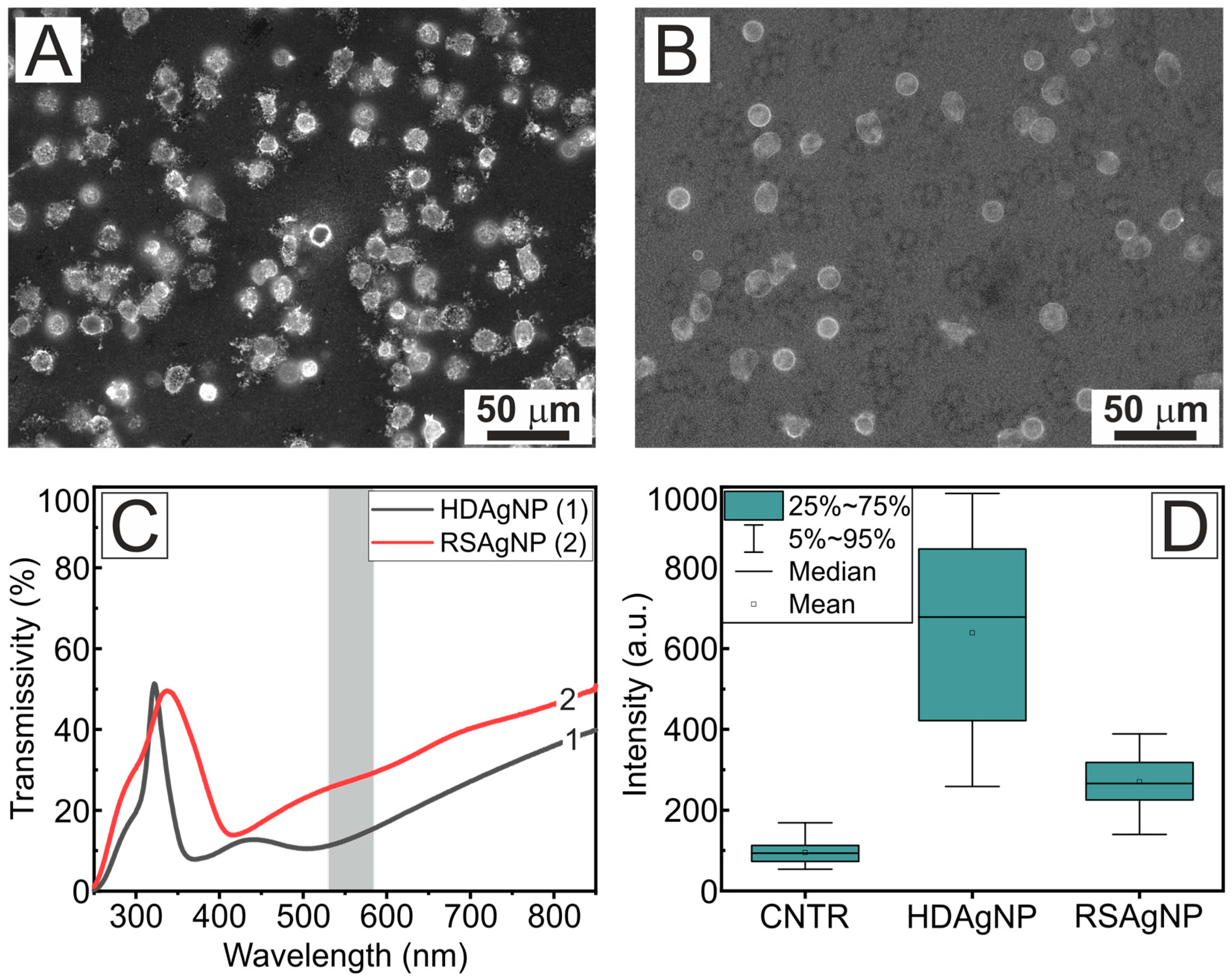
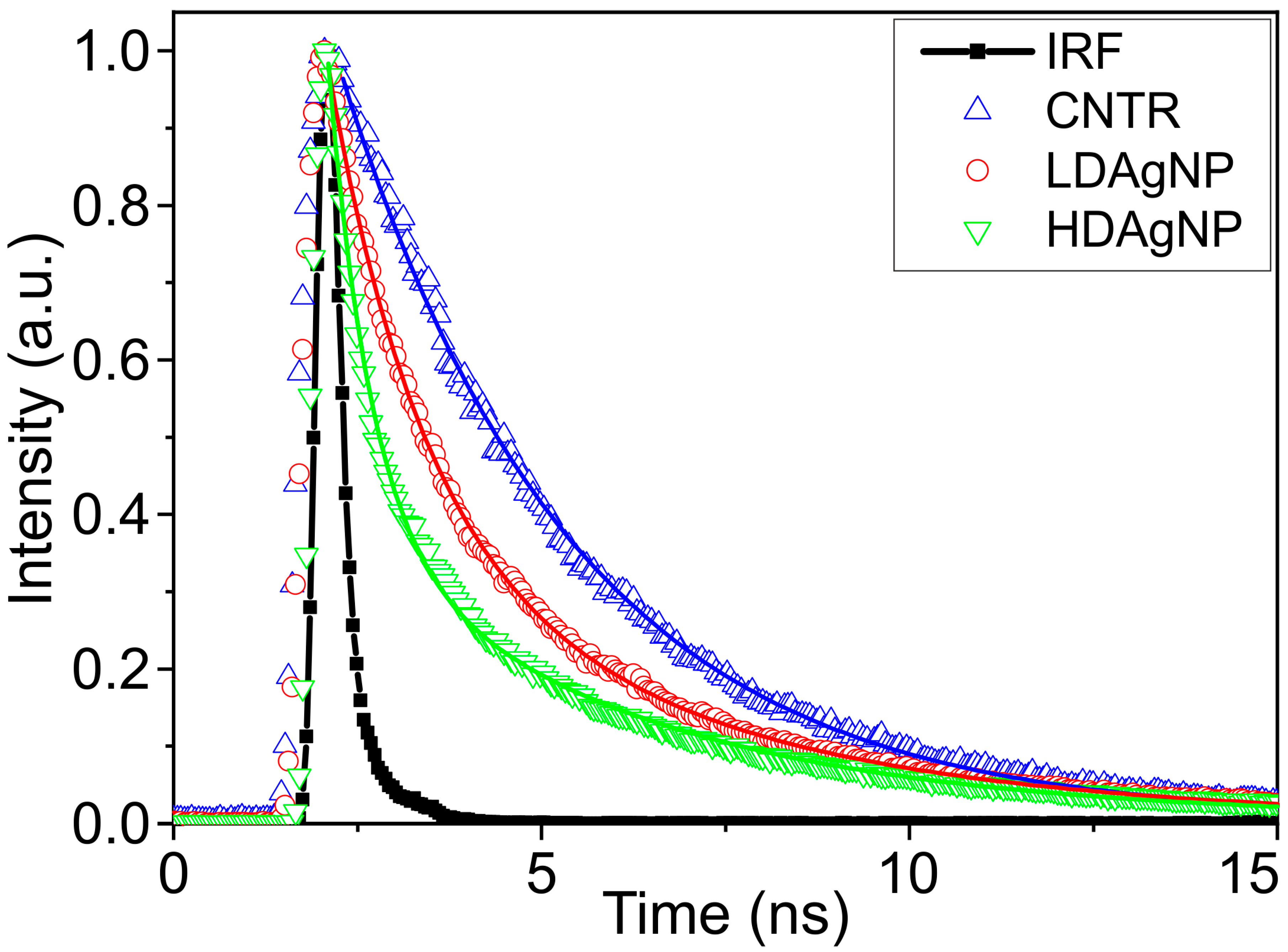
| Sample | A1 | τ1, ns | A2 | τ2, ns | τAV, ns |
|---|---|---|---|---|---|
| CNTR | 1 | 3.171 | - | - | - |
| LDAgNP | 0.833 | 1.253 | 0.167 | 4.448 | 2.581 |
| HDAgNP | 0.974 | 0.579 | 0.026 | 4.098 | 1.138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inozemtsev, V.A.; Dokukin, M.E.; Sgibnev, Y.M.; Sherstyukova, E.A.; Kandrashina, S.S.; Shvedov, M.A.; Shelaev, A.V.; Nikonorov, N.V.; Sergunova, V.A.; Baryshev, A.V. Na-Ag Ion-Exchanged Glass Substrates for Plasmon-Enhanced Fluorescence Imaging of Neutrophils. Sensors 2025, 25, 2278. https://doi.org/10.3390/s25072278
Inozemtsev VA, Dokukin ME, Sgibnev YM, Sherstyukova EA, Kandrashina SS, Shvedov MA, Shelaev AV, Nikonorov NV, Sergunova VA, Baryshev AV. Na-Ag Ion-Exchanged Glass Substrates for Plasmon-Enhanced Fluorescence Imaging of Neutrophils. Sensors. 2025; 25(7):2278. https://doi.org/10.3390/s25072278
Chicago/Turabian StyleInozemtsev, Vladimir A., Maxim E. Dokukin, Yevgeniy M. Sgibnev, Ekaterina A. Sherstyukova, Snezhanna S. Kandrashina, Mikhail A. Shvedov, Artem V. Shelaev, Nikolay V. Nikonorov, Viktoria A. Sergunova, and Alexander V. Baryshev. 2025. "Na-Ag Ion-Exchanged Glass Substrates for Plasmon-Enhanced Fluorescence Imaging of Neutrophils" Sensors 25, no. 7: 2278. https://doi.org/10.3390/s25072278
APA StyleInozemtsev, V. A., Dokukin, M. E., Sgibnev, Y. M., Sherstyukova, E. A., Kandrashina, S. S., Shvedov, M. A., Shelaev, A. V., Nikonorov, N. V., Sergunova, V. A., & Baryshev, A. V. (2025). Na-Ag Ion-Exchanged Glass Substrates for Plasmon-Enhanced Fluorescence Imaging of Neutrophils. Sensors, 25(7), 2278. https://doi.org/10.3390/s25072278








