Nanomaterial-Based Sensing Systems to Detect Neuropharmaceutical Compounds and Neurotransmitters
Abstract
1. Introduction
2. Sensor Systems in Neuroscience
2.1. Nanomaterial-Based Electrochemical Sensors
2.1.1. Noble Metal Nanomaterials
2.1.2. Metal Oxide Nanomaterials
2.1.3. Carbon-Based Nanomaterials
2.1.4. Polymeric Nanomaterials
2.1.5. Bionanomaterials
2.1.6. Two-Dimensional Nanomaterials for Neurosensing
2.2. Nanomaterial-Based Optical Sensors
3. Polymers Used for the Synthesis of Nanosensors
3.1. Molecularly Imprinted Polymers
3.2. Hybrid Polymers and Polymeric Nanocomposites
3.3. Acrylic Polymers
3.4. Conductive Polymers
3.5. Polymers with Chiral Motifs
4. Application of Sensor Systems in Neuroscience and Neurotransmitters
4.1. Sensors in Neuroscience
4.2. Sensors for Neurological Drugs
4.3. Sensors for Neurotransmitters
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nano-materials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Shabani, L.; Abbasi, M.; Azarnew, Z.; Amani, A.M.; Vaez, A. Neuro-nanotechnology: Diagnostic and therapeutic nano-based strategies in applied neuroscience. Biomed. Eng. OnLine 2023, 22, 1–41. [Google Scholar] [CrossRef]
- Gilmore, J.L.; Yi, X.; Quan, L.; Kabanov, A.V. Novel nanomaterials for clinical neuroscience. J. NeuroImmune Pharmacol. 2008, 3, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Paulchamy, B.; Arthi, G.; Lignesh, B.D. A simple approach to stepwise synthesis of graphene oxide nanomaterial. J. Nanomed. Nanotechnol. 2015, 6, 1. [Google Scholar]
- Qian, L.; Durairaj, S.; Prins, S.; Chen, A. Nanomaterial-based electrochemical sensors and biosensors for the detection of pharmaceutical compounds. Biosens. Bioelectron. 2021, 175, 112836. [Google Scholar] [CrossRef]
- Polo, E.; Kruss, S. Nanosensors for neurotransmitters. Anal. Bioanal. Chem. 2016, 408, 2727–2741. [Google Scholar] [CrossRef] [PubMed]
- Maysinger, D.; Ji, J.; Hutter, E.; Cooper, E. Nanoparticle-based and bioengineered probes and sensors to detect physiological and pathological biomarkers in neural cells. Front. Neurosci. 2015, 9, 480. [Google Scholar] [CrossRef]
- Eccles, J.C. The synapse: From electrical to chemical transmission. Annu. Rev. Neurosci. 1982, 5, 325–339. [Google Scholar] [CrossRef]
- Kim, E.H.; Chin, G.; Rong, G.; Poskanzer, K.E.; Clark, H.A. Optical probes for neurobiological sensing and imaging. Acc. Chem. Res. 2018, 51, 1023–1032. [Google Scholar] [CrossRef]
- Perry, M.; Li, Q.; Kennedy, R.T. Review of recent advances in analytical techniques for the determination of neurotransmitters. Anal. Chim. Acta 2009, 653, 1–22. [Google Scholar] [CrossRef]
- Lai, M.; Slaughter, G. Label-free MicroRNA optical biosensors. Nanomaterials 2019, 9, 1573. [Google Scholar] [CrossRef] [PubMed]
- Bucher, E.S.; Wightman, R.M. Electrochemical analysis of neurotransmitters. Annu. Rev. Anal. Chem. 2015, 8, 239–261. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Wilhelm, T.; Furst, A.L. Perspective—Electrochemical sensors for neurotransmitters and psychiatrics: Steps toward physiological mental health monitoring. J. Electrochem. Soc. 2022, 169, 047513. [Google Scholar] [CrossRef]
- Ozkan, S.A.; Dogan, B.; Uslu, B. Voltammetric analysis of the novel atypical antipsychotic drug quetiapine in human serum and urine. Microchim. Acta 2006, 153, 27–35. [Google Scholar] [CrossRef]
- Özkan, S.A.; Uslu, B.; Sentürk, Z. Electroanalytical characteristics of amisulpride and voltammetric determination of the drug in pharmaceuticals and biological media. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2004, 16, 231–237. [Google Scholar] [CrossRef]
- Ozkan, S.A.; Uslu, B.; Aboul-Enein, H.Y. Analysis of pharmaceuticals and biological fluids using modern electroanalytical techniques. Crit. Rev. Anal. Chem. 2003, 33, 155–181. [Google Scholar] [CrossRef]
- Soleymani, J. Advanced materials for optical sensing and biosensing of neurotransmitters. TrAC Trends Anal. Chem. 2015, 72, 27–44. [Google Scholar] [CrossRef]
- Mac, K.D.; Su, J. Optical biosensors for diagnosing neurodegenerative diseases. Npj Biosens. 2025, 2, 20. [Google Scholar]
- Kaur, B.; Kumar, S.; Nedoma, J.; Martinek, R.; Marques, C. Advancements in optical biosensing techniques: From fundamentals to future prospects. APL Photonics 2024, 9, 091102. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Wolfbeis, O.S.; Hirsch, T.; Swami, N.S. Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Microchim. Acta 2015, 182, 1–41. [Google Scholar] [CrossRef]
- Yonzon, C.R.; Stuart, D.A.; Zhang, X.; McFarland, A.D.; Haynes, C.L.; Van Duyne, R.P. Towards advanced chemical and biological nanosensors—An overview. Talanta 2005, 67, 438–448. [Google Scholar] [CrossRef]
- Parmin, N.A.; Hashim, U.; Gopinath, S.C.; Uda, M.N.A. Biosensor recognizes the receptor molecules. In Nanobiosensors for Biomolecular Targeting; Elsevier: Amsterdam, The Netherlands, 2019; pp. 195–210. [Google Scholar]
- Zhang, X.; Yin, J.; Yoon, J. Recent advances in development of chiral fluorescent and colorimetric sensors. Chem. Rev. 2014, 114, 4918–4959. [Google Scholar] [CrossRef]
- Feynman, R. There’s plenty of room at the bottom. In Feynman and Computation; CRC Press: Boca Raton, FL, USA, 2018; pp. 63–76. [Google Scholar]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured mate-rials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Ho, H.A.; Leclerc, M. Optical sensors based on hybrid aptamer/conjugated polymer complexes. J. Am. Chem. Soc. 2004, 126, 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- Masitas, R.A.; Allen, S.L.; Zamborini, F.P. Size-dependent electrophoretic deposition of catalytic gold nanoparticles. J. Am. Chem. Soc. 2016, 138, 15295–15298. [Google Scholar] [CrossRef]
- Gupta, A.; Moyano, D.F.; Parnsubsakul, A.; Papadopoulos, A.; Wang, L.S.; Landis, R.F.; Das, R.; Rotello, V.M. Ultrastable and biofunctionalizable gold nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 14096–14101. [Google Scholar] [CrossRef]
- Chang, Z.; Zhou, Y.; Hao, L.; Hao, Y.; Zhu, X.; Xu, M. Simultaneous determination of dopamine and ascorbic acid using β-cyclodextrin/Au nanoparticles/graphene-modified electrodes. Anal. Methods 2017, 9, 664–671. [Google Scholar] [CrossRef]
- Liu, Z.; Forsyth, H.; Khaper, N.; Chen, A. Sensitive electrochemical detection of nitric oxide based on AuPt and reduced graphene oxide nanocomposites. Analyst 2016, 141, 4074–4083. [Google Scholar] [CrossRef]
- Chen, A.; Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013, 42, 5425–5438. [Google Scholar] [CrossRef]
- Dang, X.; Hu, H.; Wang, S.; Hu, S. Nanomaterials-based electrochemical sensors for nitric oxide. Microchim. Acta 2015, 182, 455–467. [Google Scholar] [CrossRef]
- Bracamonte, M.V.; Melchionna, M.; Giuliani, A.; Nasi, L.; Tavagnacco, C.; Prato, M.; Fornasiero, P. H2O2 sensing enhancement by mutual integration of single walled carbon nanohorns with metal oxide catalysts: The CeO2 case. Sens. Actuators B Chem. 2017, 239, 923–932. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Wang, D.; Qiao, Z.; Qu, L. Synthesis of nanoneedle-like copper oxide on N-doped reduced graphene oxide: A three-dimensional hybrid for nonenzymatic glucose sensor. Sens. Actuators B Chem. 2017, 238, 588–595. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, P.; Su, Z. Fabrication technologies and sensing applications of graphene-based composite films: Advances and challenges. Biosens. Bioelectron. 2017, 89, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, B.J.; Mobin, S.M.; Mathur, P.; Lahiri, G.K.; Srivastava, A.K. Biomimetic sensor for certain catecholamines employing copper (II) complex and silver nanoparticle modified glassy carbon paste electrode. Biosens. Bioelectron. 2013, 39, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.J.; Hoshino, K. Chapter 5—Optical Transducers: Optical Molecular Sensors and Optical Spectroscopy. In Molecular Sensors and Nanodevices; CRC Press: Boca Raton, FL, USA, 2014; pp. 233–320. [Google Scholar]
- Wu, K.; Wang, H.; Chen, F.; Hu, S. Electrochemistry and voltammetry of procaine using a carbon nanotube film coated electrode. Bioelectrochemistry 2006, 68, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Kachoosangi, R.T.; Wildgoose, G.G.; Compton, R.G. Carbon nanotube-based electrochemical sensors for quantifying the ‘heat’of chilli peppers: The adsorptive stripping voltammetric determination of capsaicin. Analyst 2008, 133, 888–895. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Srivastava, A.K. Simultaneous voltammetric determination of acetaminophen, aspirin and caffeine using an in situ surfactant-modified multiwalled carbon nanotube paste electrode. Electrochim. Acta 2010, 55, 8638–8648. [Google Scholar] [CrossRef]
- Hudari, F.F.; Duarte, E.H.; Pereira, A.C.; Dall‘Antonia, L.H.; Kubota, L.T.; Tarley, C.R.T. Voltammetric method optimized by multi-response assays for the simultaneous measurements of uric acid and acetaminophen in urine in the presence of surfactant using MWCNT paste electrode. J. Electroanal. Chem. 2013, 696, 52–58. [Google Scholar] [CrossRef]
- Ghadimi, H.; Tehrani, R.M.; Ali, A.S.M.; Mohamed, N.; Ab Ghani, S. Sensitive voltammetric determination of paracetamol by poly (4-vinylpyridine)/multiwalled carbon nanotubes modified glassy carbon electrode. Anal. Chim. Acta 2013, 765, 70–76. [Google Scholar] [CrossRef]
- Dalmasso, P.R.; Pedano, M.L.; Rivas, G.A. Electrochemical determination of ascorbic acid and paracetamol in pharmaceutical formulations using a glassy carbon electrode modified with multi-wall carbon nanotubes dispersed in polyhistidine. Sens. Actuators B Chem. 2012, 173, 732–736. [Google Scholar] [CrossRef]
- Arvand, M.; Gholizadeh, T.M. Simultaneous voltammetric determination of tyrosine and paracetamol using a carbon nano-tube-graphene nanosheet nanocomposite modified electrode in human blood serum and pharmaceuticals. Colloids Surf. B Biointerfaces 2013, 103, 84–93. [Google Scholar] [CrossRef]
- Li, Y.; Feng, S.; Li, S.; Zhang, Y.; Zhong, Y. A high effect polymer-free covalent layer by layer self-assemble carboxylated MWCNTs films modified GCE for the detection of paracetamol. Sens. Actuators B Chem. 2014, 190, 999–1005. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.H.; Lu, H.T.; Zhang, Q. Electrochemical behavior and voltammetric determination of paracetamol on Nafion/TiO2–graphene modified glassy carbon electrode. Colloids Surf. B Biointerfaces 2011, 85, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Xu, Y.; Luo, L.; Ding, Y.; Wang, Y.; Liu, X. LaNi0.5Ti0.5O3/CoFe2O4-based sensor for sensitive determination of paracetamol. J. Solid State Electrochem. 2012, 16, 1635–1642. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, Y.; Wang, L.; Lin, S.; Wang, C.; Chen, X. Sensitive detection of acetaminophen based on Fe3O4 nanoparti-cles-coated poly (diallyldimethylammonium chloride)-functionalized graphene nanocomposite film. Talanta 2012, 88, 181–186. [Google Scholar] [CrossRef]
- Houshmand, M.; Jabbari, A.; Heli, H.; Hajjizadeh, M.; Moosavi-Movahedi, A.A. Electrocatalytic oxidation of aspirin and acetaminophen on a cobalt hydroxide nanoparticles modified glassy carbon electrode. J. Solid State Electrochem. 2008, 12, 1117–1128. [Google Scholar] [CrossRef]
- Sartori, E.R.; Medeiros, R.A.; Rocha-Filho, R.C.; Fatibello-Filho, O. Square-wave voltammetric determination of acetylsalicylic acid in pharmaceutical formulations using a boron-doped diamond electrode without the need of previous alkaline hydrolysis step. J. Braz. Chem. Soc. 2009, 20, 360–366. [Google Scholar] [CrossRef]
- Lu, T.L.; Tsai, Y.C. Electrocatalytic oxidation of acetylsalicylic acid at multiwalled carbon nanotube-alumina-coated silica nanocomposite modified glassy carbon electrodes. Sens. Actuators B Chem. 2010, 148, 590–594. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Azab, S.M. Determination of morphine at gold nanoparticles/Nafion® carbon paste modified sensor electrode. Analyst 2011, 136, 4682–4691. [Google Scholar] [CrossRef]
- Babaei, A.; Babazadeh, M. Multi-walled carbon nanotubes/chitosan polymer composite modified glassy carbon electrode for sensitive simultaneous determination of levodopa and morphine. Anal. Methods 2011, 3, 2400–2405. [Google Scholar] [CrossRef]
- Navaee, A.; Salimi, A.; Teymourian, H. Graphene nanosheets modified glassy carbon electrode for simultaneous detection of heroine, morphine and noscapine. Biosens. Bioelectron. 2012, 31, 205–211. [Google Scholar] [CrossRef]
- Atta, N.F.; Ahmed, R.A.; Amin, H.M.; Galal, A. Monodispersed gold nanoparticles decorated carbon nanotubes as an enhanced sensing platform for nanomolar detection of tramadol. Electroanalysis 2012, 24, 2135–2146. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Srivastava, A.K. Simultaneous voltammetric determination of acetaminophen and tramadol using Dowex50wx2 and gold nanoparticles modified glassy carbon paste electrode. Anal. Chim. Acta 2011, 706, 246–254. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Shahrokhian, S.; Ghorbani-Bidkorbeh, F. Voltammetric studies of sumatriptan on the surface of pyrolytic graphite electrode modified with multi-walled carbon nanotubes decorated with silver nanoparticles. Talanta 2009, 80, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhian, S.; Kamalzadeh, Z.; Saberi, R.S. Application of glassy carbon electrode modified with a bilayer of multiwalled carbon nanotube and polypyrrole doped with nitrazine yellow for voltammetric determination of naltrexone. Electroanalysis 2011, 23, 2925–2934. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Kalambate, P.K.; Karna, S.P.; Srivastava, A.K. Voltammetric determination of sumatriptan based on a gra-phene/gold nanoparticles/Nafion composite modified glassy carbon electrode. Talanta 2014, 120, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Babaei, A.; Dehdashti, A.; Afrasiabi, M.; Babazadeh, M.; Farshbaf, M.; Bamdad, F. A sensor for simultaneous determination of acetaminophen and codeine at glassy carbon electrode modified with multi-walled carbon nanotubes. Sens. Lett. 2012, 10, 1039–1046. [Google Scholar] [CrossRef]
- Habibi, B.; Abazari, M.; Pournaghi-Azar, M.H. Simultaneous determination of codeine and caffeine using single-walled carbon nanotubes modified carbon-ceramic electrode. Colloids Surf. B Biointerfaces 2014, 114, 89–95. [Google Scholar] [CrossRef]
- Wang, C.Y.; Hu, X.Y. Determination of benorilate in pharmaceutical formulations and its metabolite in urine at carbon paste electrode modified by silver nanoparticles. Talanta 2005, 67, 625–633. [Google Scholar] [CrossRef]
- Veiga, A.; Dordio, A.; Carvalho, A.P.; Teixeira, D.M.; Teixeira, J.G. Ultra-sensitive voltammetric sensor for trace analysis of carbamazepine. Anal. Chim. Acta 2010, 674, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Kalanur, S.S.; Jaldappagari, S.; Balakrishnan, S. Enhanced electrochemical response of carbamazepine at a nano-structured sensing film of fullerene-C60 and its analytical applications. Electrochim. Acta 2011, 56, 5295–5301. [Google Scholar] [CrossRef]
- Yari, A.; Papi, F.; Farhadi, S. Voltammetric Determination of trace antiepileptic gabapentin with a silver-nanoparticle modified multiwalled carbon nanotube paste electrode. Electroanalysis 2011, 23, 2949–2954. [Google Scholar] [CrossRef]
- Heli, H.; Faramarzi, F.; Sattarahmady, N. Oxidation and determination of Gabapentin on nanotubes of nickel oxide-modified carbon paste electrode. J. Solid State Electrochem. 2012, 16, 45–52. [Google Scholar] [CrossRef]
- Saberi, R.S.; Shahrokhian, S. Highly sensitive voltammetric determination of lamotrigine at highly oriented pyrolytic graphite electrode. Bioelectrochemistry 2012, 84, 38–43. [Google Scholar] [CrossRef]
- Daneshgar, P.; Ghaheri, N.; Moosavi-Movahedi, A.A.; Rezayat, S.M.; Norouzi, P.; Ahmad, F.; Ganjali, M.R. Nanosilver-DNA hybrid modified electrode for electrochemical sensing of buspirone in biological samples and pharmaceutical preparation. Sens. Lett. 2012, 10, 814–820. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Hsu, P.C.; Chen, S.M. A multipurpose voltammetric sensor for the determination of chlorpromazine in presence of acetaminophen, uric acid, dopamine and ascorbic acid. Int. J. Electrochem. Sci. 2012, 7, 11414–11425. [Google Scholar] [CrossRef]
- Parvin, M.H.; Golivand, M.B.; Najafi, M.; Shariaty, S.M. Carbon paste electrode modified with cobalt nanoparticles and its application to the electrocatalytic determination of chlorpromazine. J. Electroanal. Chem. 2012, 683, 31–36. [Google Scholar] [CrossRef]
- Mashhadizadeh, M.H.; Afshar, E. Electrochemical investigation of clozapine at TiO2 nanoparticles modified carbon paste electrode and simultaneous adsorptive voltammetric determination of two antipsychotic drugs. Electrochim. Acta 2013, 87, 816–823. [Google Scholar] [CrossRef]
- Afkhami, A.; Ghaedi, H. Multiwalled carbon nanotube paste electrode as an easy, inexpensive and highly selective sensor for voltammetric determination of Risperidone. Anal. Methods 2012, 4, 1415–1420. [Google Scholar] [CrossRef]
- Arvand, M.; Pourhabib, A. Adsorptive Stripping Differential Pulse Voltammetric Determination of Risperidone with a Mul-ti-Walled Carbon Nanotube-Ionic Liquid Paste Modified Glassy Carbon Electrode. J. Chin. Chem. Soc. 2013, 60, 63–72. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Ghalkhani, M.; Adeli, M.; Amini, M.K. Multi-walled carbon nanotubes with immobilised cobalt nanoparticle for modification of glassy carbon electrode: Application to sensitive voltammetric determination of thioridazine. Biosens. Bioelectron. 2009, 24, 3235–3241. [Google Scholar] [CrossRef] [PubMed]
- Mashhadizadeh, M.H.; Afshar, E. Electrochemical studies and selective detection of thioridazine using a carbon paste electrode modified with ZnS nanoparticles and simultaneous determination of thioridazine and olanzapine. Electroanalysis 2012, 24, 2193–2202. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, A.K.; Shoora, S.K. Multiwall carbon nanotube modified glassy carbon electrode as voltammetric sensor for the simultaneous determination of ascorbic acid and caffeine. Electrochim. Acta 2013, 93, 248–253. [Google Scholar] [CrossRef]
- Goyal, R.N.; Bishnoi, S. A novel multi-walled carbon nanotube modified sensor for the selective determination of epinephrine in smokers. Electrochim. Acta 2011, 56, 2717–2724. [Google Scholar] [CrossRef]
- Ivandini, T.A.; Sarada, B.V.; Terashima, C.; Rao, T.N.; Tryk, D.A.; Ishiguro, H.; Kubota, Y.; Fujishima, A. Electrochemical detection of tricyclic antidepressant drugs by HPLC using highly boron-doped diamond electrodes. J. Electroanal. Chem. 2002, 521, 117–126. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Srivastava, A.K. Adsorptive stripping voltammetric determination of imipramine, trimipramine and desipra-mine employing titanium dioxide nanoparticles and an Amberlite XAD-2 modified glassy carbon paste electrode. Analyst 2013, 138, 1395–1404. [Google Scholar] [CrossRef]
- Hegde, R.N.; Hosamani, R.R.; Nandibewoor, S.T. Voltammetric oxidation and determination of cinnarizine at glassy carbon electrode modified with multi-walled carbon nanotubes. Colloids Surf. B Biointerfaces 2009, 72, 259–265. [Google Scholar] [CrossRef]
- Babaei, A.; Taheri, A.R. Nafion/Ni(OH)2 nanoparticles-carbon nanotube composite modified glassy carbon electrode as a sensor for simultaneous determination of dopamine and serotonin in the presence of ascorbic acid. Sens. Actuators B Chem. 2013, 176, 543–551. [Google Scholar] [CrossRef]
- Lima, J.L.; Loo, D.V.; Delerue-Matos, C.; da Silva, A.S.R. Electrochemical behaviour of Venlafaxine and its determination in pharmaceutical products using square wave voltammetry. Il Farm. 1999, 54, 145–148. [Google Scholar] [CrossRef]
- Morais, S.; Ryckaert, C.P.; Delerue-Matos, C. Adsorptive stripping voltammetric determination of venlafaxine in urine with a mercury film microelectrode. Anal. Lett. 2003, 36, 2515–2526. [Google Scholar] [CrossRef]
- El-Sayed, G.O.; Yasin, S.A.; El Badawy, A.A. Voltammetric behavior and determination of cinnarizine in pharmaceutical formulations and serum. Anal. Lett. 2008, 41, 3021–3033. [Google Scholar] [CrossRef]
- Hegde, R.N.; Shetti, N.P.; Nandibewoor, S.T. Electro-oxidation and determination of trazodone at multi-walled carbon nano-tube-modified glassy carbon electrode. Talanta 2009, 79, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Heli, H.; Majdi, S.; Jabbari, A.; Sattarahmady, N.; Moosavi-Movahedi, A.A. Electrooxidation of dextromethorphan on a carbon nanotube–carbon microparticle–ionic liquid composite: Applied to determination in pharmaceutical forms. J. Solid State Elec-trochem. 2010, 14, 1515–1523. [Google Scholar] [CrossRef]
- Ghorbani-Bidkorbeh, F.; Shahrokhian, S.; Mohammadi, A.; Dinarvand, R. Electrochemical determination of naltrexone on the surface of glassy carbon electrode modified with Nafion-doped carbon nanoparticles: Application to determinations in pharmaceutical and clinical preparations. J. Electroanal. Chem. 2010, 638, 212–217. [Google Scholar] [CrossRef]
- Levent, A.; Yardim, Y.; Senturk, Z. Voltammetric behavior of nicotine at pencil graphite electrode and its enhancement determination in the presence of anionic surfactant. Electrochimica Acta 2009, 55, 190–195. [Google Scholar] [CrossRef]
- Lo, T.W.; Aldous, L.; Compton, R.G. The use of nano-carbon as an alternative to multi-walled carbon nanotubes in modified electrodes for adsorptive stripping voltammetry. Sens. Actuators B Chem. 2012, 162, 361–368. [Google Scholar] [CrossRef]
- Chauhan, N.; Tiwari, S.; Narayan, T.; Jain, U. Bienzymatic assembly formed@ Pt nano sensing framework detecting acetylcholine in aqueous phase. Appl. Surf. Sci. 2019, 474, 154–160. [Google Scholar] [CrossRef]
- Kannan, P.; Dolinska, J.; Maiyalagan, T.; Opallo, M. Facile and rapid synthesis of Pd nanodendrites for electrocatalysis and surface-enhanced Raman scattering applications. Nanoscale 2014, 6, 11169–11176. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Li, W.; Ma, B.; Chen, X. Rational material design for ultrafast rechargeable lithium-ion batteries. Chem. Soc. Rev. 2015, 44, 5926–5940. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Beitollahi, H.; Amini, M.K.; Mirkhalaf, F.; Abdollahi-Alibeik, M. New strategy for simultaneous and selective voltammetric determination of norepinephrine, acetaminophen and folic acid using ZrO2 nanoparticles-modified carbon paste electrode. Sens. Actuators B Chem. 2010, 151, 243–249. [Google Scholar] [CrossRef]
- Dresselhaus, M.S. Fifty years in studying carbon-based materials. Phys. Scr. 2012, 2012, 014002. [Google Scholar] [CrossRef]
- Adhikari, B.R.; Govindhan, M.; Chen, A. Carbon nanomaterials based electrochemical sensors/biosensors for the sensitive detection of pharmaceutical and biological compounds. Sensors 2015, 15, 22490–22508. [Google Scholar] [CrossRef]
- Cambria, E.; Das, D.; Bandyopadhyay, S.; Feraco, A. Affective computing and sentiment analysis. In A Practical Guide to Sentiment Analysis; Springer: Cham, Switzerland, 2017; pp. 1–10. [Google Scholar]
- Mubarak, N.M.; Abdullah, E.C.; Jayakumar, N.S.; Sahu, J.N. An overview on methods for the production of carbon nanotubes. J. Ind. Eng. Chem. 2014, 20, 1186–1197. [Google Scholar] [CrossRef]
- Duclaux, L. Review of the doping of carbon nanotubes (multiwalled and single-walled). Carbon 2002, 40, 1751–1764. [Google Scholar] [CrossRef]
- Mokhtari, A.; Karimi-Maleh, H.; Ensafi, A.A.; Beitollahi, H. Application of modified multiwall carbon nanotubes paste electrode for simultaneous voltammetric determination of morphine and diclofenac in biological and pharmaceutical samples. Sens. Actuators B Chem. 2012, 169, 96–105. [Google Scholar] [CrossRef]
- García, J.M.; Pablos, J.L.; García, F.C.; Serna, F. Sensory polymers for detecting explosives and chemical warfare agents. In Industrial Applications for Intelligent Polymers and Coatings; Springer: Cham, Switzerland, 2016; pp. 553–576. [Google Scholar]
- Emerich, D.F.; Thanos, C.G. Targeted nanoparticle-based drug delivery and diagnosis. J. Drug Target. 2007, 15, 163–183. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Li, L.; Guo, Z.; Xue, Z.; Lu, X. An electrochemical paracetamol sensor based on layer-by-layer covalent attachment of MWCNTs and a G4.0 PAMAM modified GCE. Anal. Methods 2016, 8, 2218–2225. [Google Scholar] [CrossRef]
- Miodek, A.; Mejri, N.; Gomgnimbou, M.; Sola, C.; Korri-Youssoufi, H. E-DNA sensor of Mycobacterium tuberculosis based on electrochemical assembly of nanomaterials (MWCNTs/PPy/PAMAM). Anal. Chem. 2015, 87, 9257–9264. [Google Scholar] [CrossRef]
- Alexander, C.; Andersson, H.S.; Andersson, L.I.; Ansell, R.J.; Kirsch, N.; Nicholls, I.A.; O’Mahony, J.; Whitcombe, M.J. Mo-lecular imprinting science and technology: A survey of the literature for the years up to and including 2003. J. Mol. Recognit. Interdiscip. J. 2006, 19, 106–180. [Google Scholar] [CrossRef] [PubMed]
- Whitcombe, M.J.; Kirsch, N.; Nicholls, I.A. Molecular imprinting science and technology: A survey of the literature for the years 2004–2011. J. Mol. Recognit. 2014, 27, 297–401. [Google Scholar] [PubMed]
- Haupt, K.; Mosbach, K. Molecularly imprinted polymers and their use in biomimetic sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef]
- Prasad, B.B.; Prasad, A.; Tiwari, M.P.; Madhuri, R. Multiwalled carbon nanotubes bearing ‘terminal monomeric unit’ for the fabrication of epinephrine imprinted polymer-based electrochemical sensor. Biosens. Bioelectron. 2013, 45, 114–122. [Google Scholar] [CrossRef]
- Hu, G.; Chen, L.; Guo, Y.; Wang, X.; Shao, S. Selective determination of L-dopa in the presence of uric acid and ascorbic acid at a gold nanoparticle self-assembled carbon nanotube-modified pyrolytic graphite electrode. Electrochim. Acta 2010, 55, 4711–4716. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, T.; Zhao, H.; Fei, T. Preparation of organic-inorganic hybrid polymers and their humidity sensing properties. Sens. Actuators B Chem. 2017, 242, 1108–1114. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Ganjipour, B.; Beitollahi, H.; Amini, M.K.; Mirkhalaf, F.; Naeimi, H.; Nejati-Barzoki, M. Simultaneous determination of levodopa, carbidopa and tryptophan using nanostructured electrochemical sensor based on novel hydro-quinone and carbon nanotubes: Application to the analysis of some real samples. Electrochim. Acta 2011, 56, 9113–9120. [Google Scholar] [CrossRef]
- Beitollah, H.; Goodarzian, M.; Khalilzadeh, M.A.; Karimi-Maleh, H.; Hassanzadeh, M.; Tajbakhsh, M. Electrochemical be-haviors and determination of carbidopa on carbon nanotubes ionic liquid paste electrode. J. Mol. Liq. 2012, 173, 137–143. [Google Scholar] [CrossRef]
- Yaghoubian, H.; Karimi-Maleh, H.; Khalilzadeh, A.M.; Karimi, F. Electrochemical detection of carbidopa using a ferrocene-modified carbon nanotube paste electrode. J. Serbian Chem. Soc. 2009, 74, 1443–1453. [Google Scholar] [CrossRef]
- Xiao, N.; Venton, B.J. Rapid, sensitive detection of neurotransmitters at microelectrodes modified with self-assembled SWCNT forests. Anal. Chem. 2012, 84, 7816–7822. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, R.; Chai, Y.; Zhang, Y.; Hu, F.; Zhang, M. Au-nanoclusters incorporated 3-amino-5-mercapto-1, 2, 4-triazole film modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosens. Bioelectron. 2011, 30, 315–319. [Google Scholar] [CrossRef]
- Sun, Y.; Fei, J.; Hou, J.; Zhang, Q.; Liu, Y.; Hu, B. Simultaneous determination of dopamine and serotonin using a carbon nanotubes-ionic liquid gel modified glassy carbon electrode. Microchim. Acta 2009, 165, 373–379. [Google Scholar] [CrossRef]
- Kumar, Y.; Pramanik, S.; Das, D.P. Lanthanum ortho-ferrite (LaFeO3) nanoparticles based electrochemical sensor for the detection of dopamine. Biointerface Res. Appl. Chem. 2020, 10, 6182–6188. [Google Scholar]
- Niu, L.M.; Lian, K.Q.; Shi, H.M.; Wu, Y.B.; Kang, W.J.; Bi, S.Y. Characterization of an ultrasensitive biosensor based on a nano-Au/DNA/nano-Au/poly (SFR) composite and its application in the simultaneous determination of dopamine, uric acid, guanine, and adenine. Sens. Actuators B Chem. 2013, 178, 10–18. [Google Scholar] [CrossRef]
- Reddy, S.; Swamy, B.E.K.; Jayadevappa, H. CuO nanoparticle sensor for the electrochemical determination of dopamine. Electrochim. Acta 2012, 61, 78–86. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.A.P.; Asiri, A.M.; Rub, M.A.; Rahman, M.M.; Ghani, S.A. In vitro studies of carbon fiber microbiosensor for dopamine neurotransmitter supported by copper-graphene oxide composite. Microchim. Acta 2014, 181, 1049–1057. [Google Scholar] [CrossRef]
- Goyal, R.N.; Bishnoi, S.; Agrawal, B. Electrochemical sensor for the simultaneous determination of caffeine and aspirin in human urine samples. J. Electroanal. Chem. 2011, 655, 97–102. [Google Scholar] [CrossRef]
- Yi, H.; Zheng, D.; Hu, C.; Hu, S. Functionalized multiwalled carbon nanotubes through in situ electropolymerization of brilliant cresyl blue for determination of epinephrine. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2008, 20, 1143–1146. [Google Scholar] [CrossRef]
- Huang, S.H.; Liao, H.H.; Chen, D.H. Simultaneous determination of norepinephrine, uric acid, and ascorbic acid at a screen printed carbon electrode modified with polyacrylic acid-coated multi-wall carbon nanotubes. Biosens. Bioelectron. 2010, 25, 2351–2355. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Z.; Wang, X.; Xu, L. Electrochemical determination of norepinephrine on the membrane of silver nanoparticles doped poly-glycine eliminating the interference of ascorbic acid. J. Solid State Electrochem. 2013, 17, 661–665. [Google Scholar] [CrossRef]
- Beitollahi, H.; Sheikhshoaie, I. Selective voltammetric determination of norepinephrine in the presence of acetaminophen and folic acid at a modified carbon nanotube paste electrode. J. Electroanal. Chem. 2011, 661, 336–342. [Google Scholar] [CrossRef]
- Han, H.S.; Lee, H.K.; You, J.M.; Jeong, H.; Jeon, S. Electrochemical biosensor for simultaneous determination of dopamine and serotonin based on electrochemically reduced GO-porphyrin. Sens. Actuators B Chem. 2014, 190, 886–895. [Google Scholar] [CrossRef]
- Babaei, A.; Babazadeh, M. A selective simultaneous determination of levodopa and serotonin using a glassy carbon electrode modified with multiwalled carbon nanotube/chitosan composite. Electroanalysis 2011, 23, 1726–1735. [Google Scholar] [CrossRef]
- Pal, R.K.; Farghaly, A.A.; Wang, C.; Collinson, M.M.; Kundu, S.C.; Yadavalli, V.K. Conducting polymer-silk biocomposites for flexible and biodegradable electrochemical sensors. Biosens. Bioelectron. 2016, 81, 294–302. [Google Scholar] [CrossRef]
- Farjami, E.; Campos, R.; Nielsen, J.S.; Gothelf, K.V.; Kjems, J.; Ferapontova, E.E. RNA aptamer-based electrochemical biosensor for selective and label-free analysis of dopamine. Anal. Chem. 2013, 85, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Jankowska-Śliwińska, J.; Dawgul, M.; Pijanowska, D.G. DNA-based electrochemical biosensor for imipramine detection. Procedia Eng. 2015, 120, 574–577. [Google Scholar] [CrossRef]
- Roushani, M.; Shahdost-fard, F. A novel ultrasensitive aptasensor based on silver nanoparticles measured via enhanced voltammetric response of electrochemical reduction of riboflavin as redox probe for cocaine detection. Sens. Actuators B Chem. 2015, 207, 764–771. [Google Scholar] [CrossRef]
- Choo, K.H.; Tan, C.L.; Taufik, A.; Kumar, S.S.D.; Lim, H.N. Highly sensitive electrochemical sensor based on 3D porous gra-phene/gold nanocomposite for dopamine detection. Sci. Rep. 2018, 8, 32477. [Google Scholar]
- Mariyappan, V.; Sundaresan, A. A high-performance aptasensing interface based on pseudo-AuNBs/TI3C2TX MXene nano-composite for non-invasive measurement of carbamazepine in human biofluids. J. Pharm. Biomed. Anal. 2024, 237, 115512. [Google Scholar]
- Li, C.; Shi, G. Carbon nanotube-based fluorescence sensors. J. Photochem. Photobiol. C Photochem. Rev. 2014, 19, 20–34. [Google Scholar] [CrossRef]
- Cha, T.G.; Baker, B.A.; Sauffer, M.D.; Salgado, J.; Jaroch, D.; Rickus, J.L.; Porterfield, D.M.; Choi, J.H. Optical nanosensor architecture for cell-signaling molecules using DNA aptamer-coated carbon nanotubes. ACS Nano 2011, 5, 4236–4244. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, X.; Li, Z.; Chen, H.; Zhang, Q. Plasmon-enhanced digital fluoroimmunoassay for subfemtomolar detection of biomarkers in complex biological samples. Nano Lett. 2023, 23, 1234–1242. [Google Scholar]
- Patel, R.; Singh, M.; Kumar, A. DNA aptamer-functionalized gold nanoparticles for colorimetric detection of cancer biomarkers. Biosensors 2023, 13, 45. [Google Scholar] [CrossRef]
- Soler, M.; Belushkin, A.; Cavallini, A.; Kebbi-Beghdadi, C.; Greub, G.; Altug, H. Multiplexed nanoplasmonic biosensor for one-step simultaneous detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine. ACS Nano 2017, 11, 4060–4067. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Cooper, D.R.; Suffern, D.; Carlini, L.; Clarke, S.J.; Parbhoo, R.; Bradforth, S.E.; Nadeau, J.L. Photoenhancement of lifetimes in CdSe/ZnS and CdTe quantum dot-dopamine conjugates. Phys. Chem. Chem. Phys. 2009, 11, 4298–4310. [Google Scholar] [CrossRef]
- Freeman, R.; Bahshi, L.; Finder, T.; Gill, R.; Willner, I. Competitive analysis of saccharides or dopamine by boronic acid-functionalized CdSe–ZnS quantum dots. Chem. Commun. 2009, 764–766. [Google Scholar] [CrossRef]
- Okumoto, S.; Looger, L.L.; Micheva, K.D.; Reimer, R.J.; Smith, S.J.; Frommer, W.B. Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors. Proc. Natl. Acad. Sci. USA 2005, 102, 8740–8745. [Google Scholar] [CrossRef] [PubMed]
- Secor, K.E.; Glass, T.E. Selective amine recognition: Development of a chemosensor for dopamine and norepinephrine. Org. Lett. 2004, 6, 3727–3730. [Google Scholar] [CrossRef]
- Kruss, S.; Landry, M.P.; Vander Ende, E.; Lima, B.M.; Reuel, N.F.; Zhang, J.; Nelson, J.; Mu, B.; Hilmer, A.; Strano, M. Neuro-transmitter detection using corona phase molecular recognition on fluorescent single-walled carbon nanotube sensors. J. Am. Chem. Soc. 2014, 136, 713–724. [Google Scholar] [CrossRef]
- Reglero Ruiz, J.A.; Sanjuán, A.M.; Vallejos, S.; García, F.C.; García, J.M. Smart polymers in micro and nano sensory devices. Chemosensors 2018, 6, 12. [Google Scholar] [CrossRef]
- Guan, G.; Liu, B.; Wang, Z.; Zhang, Z. Imprinting of molecular recognition sites on nanostructures and its applications in chemosensors. Sensors 2008, 8, 8291–8320. [Google Scholar] [CrossRef] [PubMed]
- Andersson, L.I.; Mandenius, C.F.; Mosbach, K. Studies on guest selective molecular recognition on an octadecyl silylated silicon surface using ellipsometry. Tetrahedron Lett. 1988, 29, 5437–5440. [Google Scholar] [CrossRef]
- Suriyanarayanan, S.; Cywinski, P.J.; Moro, A.J.; Mohr, G.J.; Kutner, W. Chemosensors based on molecularly imprinted polymers. In Molecular Imprinting; Springer: Berlin/Heidelberg, Germany, 2010; pp. 165–265. [Google Scholar]
- Lofgreen, J.E.; Ozin, G.A. Controlling morphology and porosity to improve performance of molecularly imprinted sol–gel silica. Chem. Soc. Rev. 2014, 43, 911–933. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, D.; Lanni, L.M.; Shimizu, K.D. Importance of functional monomer dimerization in the molecular imprinting process. Macromolecules 2010, 43, 6284–6294. [Google Scholar] [CrossRef]
- Wang, D.; Gan, N.; Zhang, H.; Li, T.; Qiao, L.; Cao, Y.; Su, X.; Jiang, S. Simultaneous electrochemical immunoassay using graphene–Au grafted recombinant apoferritin-encoded metallic labels as signal tags and dual-template magnetic molecular imprinted polymer as capture probes. Biosens. Bioelectron. 2015, 65, 78–82. [Google Scholar] [CrossRef]
- Hagrman, P.J.; Hagrman, D.; Zubieta, J. Organic–Inorganic Hybrid Materials: From “Simple” Coordination Polymers to Organodiamine-Templated Molybdenum Oxides. Angew. Chem. Int. Ed. 1999, 38, 2638–2684. [Google Scholar] [CrossRef]
- Jordan, J.; Jacob, K.I.; Tannenbaum, R.; Sharaf, M.A.; Jasiuk, I. Experimental trends in polymer nanocomposites—A review. Mater. Sci. Eng. A 2005, 393, 1–11. [Google Scholar] [CrossRef]
- Ali, M.A.; Singh, C.; Mondal, K.; Srivastava, S.; Sharma, A.; Malhotra, B.D. Mesoporous few-layer graphene platform for affinity biosensing application. ACS Appl. Mater. Interfaces 2016, 8, 7646–7656. [Google Scholar] [CrossRef]
- Ballard, N.; Asua, J.M. Radical polymerization of acrylic monomers: An overview. Prog. Polym. Sci. 2018, 79, 40–60. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Xu, H.; Holzwarth, J.M.; Yan, Y.; Xu, P.; Zheng, H.; Yin, Y.; Li, S.; Ma, P.X. Conductive PPY/PDLLA conduit for peripheral nerve regeneration. Biomaterials 2014, 35, 225–235. [Google Scholar] [CrossRef]
- Garcia-Etxarri, A.; Yuste, R. Time for nanoneuro. Nat. Methods 2021, 18, 1287–1293. [Google Scholar] [CrossRef]
- Nabovati, E.; Vakili-Arki, H.; Taherzadeh, Z.; Hasibian, M.R.; Abu-Hanna, A.; Eslami, S. Drug-drug interactions in inpatient and outpatient settings in Iran: A systematic review of the literature. DARU J. Pharm. Sci. 2014, 22, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chvilicek, M.M.; Titos, I.; Rothenfluh, A. The neurotransmitters involved in Drosophila alcohol-induced behaviors. Front. Behav. Neurosci. 2020, 14, 607700. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Lutomia, D.; Poria, R.; Kala, D.; Singh, A.K.; Gupta, M.K.; Kumar, D.; Kaushal, A.; Gupta, S. Unlocking the potential of 2D nanomaterial-based biosensors in biomarker-based detection of Helicobacter pylori. Mater. Adv. 2025, 6, 117–142. [Google Scholar] [CrossRef]
- Ghorbian, M.; Ghobaei-Arani, M.; Babaei, M.R.; Ghorbian, S. Nanotechnology and nanosensors in personalized healthcare: A comprehensive review. Sens. Bio-Sens. Res. 2025, 41, 100740. [Google Scholar] [CrossRef]
- Birtwistle, J.; Baldwin, D. Role of dopamine in schizophrenia and Parkinson’s disease. Br. J. Nurs. 1998, 7, 832–841. [Google Scholar] [CrossRef]
- Cretich, M.; Daaboul, G.G.; Sola, L.; Ünlü, M.S.; Chiari, M. Digital detection of biomarkers assisted by nanoparticles: Applica-tion to diagnostics. Trends Biotechnol. 2015, 33, 343–351. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Chiodi, E.; Celebi, I.; Saylan, Y.; Ünlü, N.L.; Ünlü, M.S.; Denizli, A. Sensitive and real-time detection of IgG using interferometric reflecting imaging sensor system. Biosens. Bioelectron. 2022, 201, 113961. [Google Scholar] [CrossRef]
- Lortlar Ünlü, N.; Bakhshpour-Yucel, M.; Chiodi, E.; Diken-Gür, S.; Emre, S.; Ünlü, M.S. Characterization of Receptor Binding Affinity for Vascular Endothelial Growth Factor with Interferometric Imaging Sensor. Biosensors 2024, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Alam, M.M.; Kim, Y.S. Gold–silver core–shell nanoparticles as multifunctional platforms for biosensing and catalysis. Sensors 2022, 22, 3355. [Google Scholar]
- Liang, G.; Liu, M.; Liu, X.; Zhang, W. Graphene–gold nanoparticle hybrid for highly sensitive electrochemical detection of cancer biomarkers. Sens. Actuators B Chem. 2023, 388, 133567. [Google Scholar]
- Guo, Y.; Chen, Y.; Zhao, Q.; Shuang, S.; Dong, C. Cyclodextrin–graphene hybrid nanosheets for ultrasensitive electrochemical determination of doxorubicin and methotrexate. Electroanalysis 2011, 23, 2400–2407. [Google Scholar] [CrossRef]
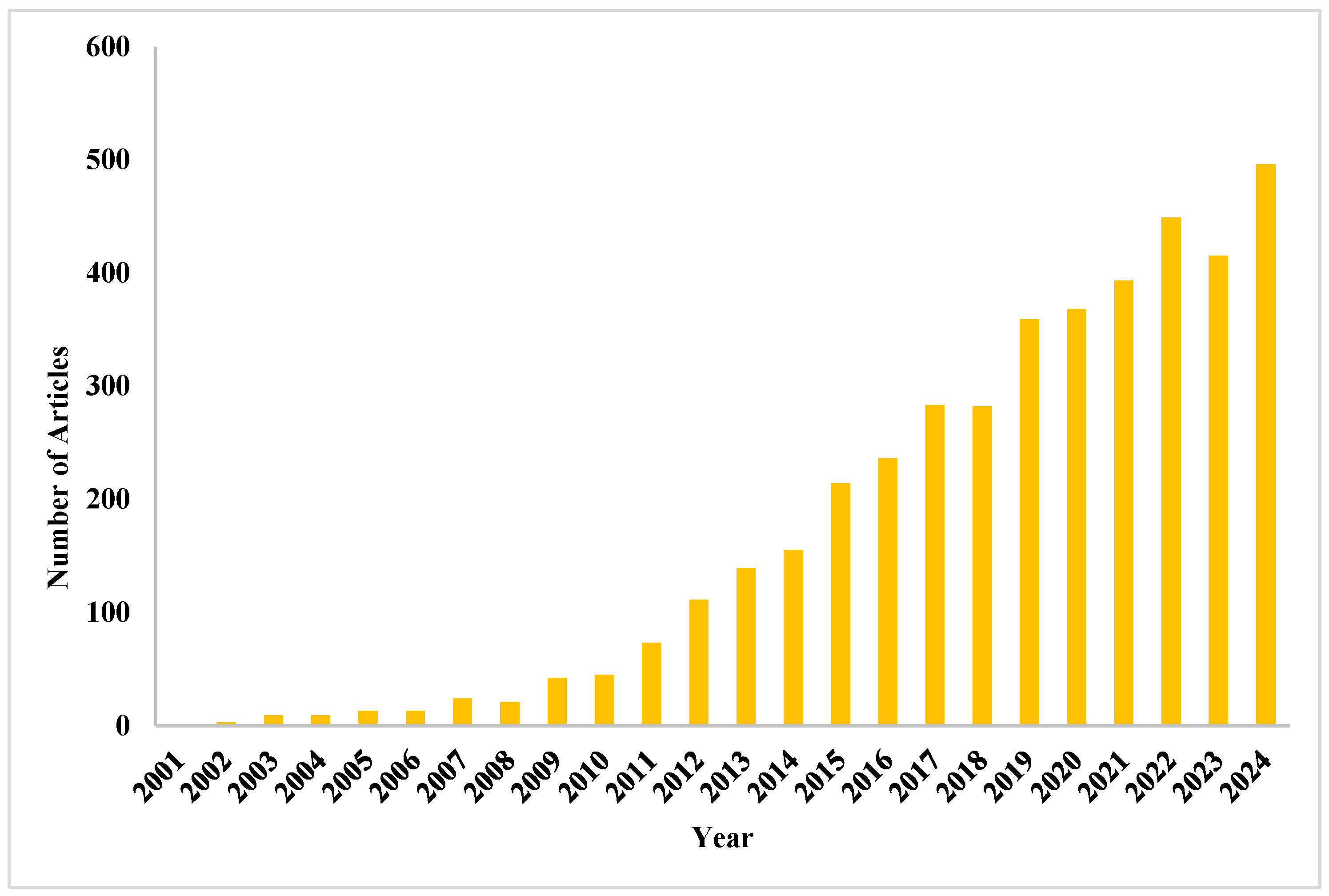





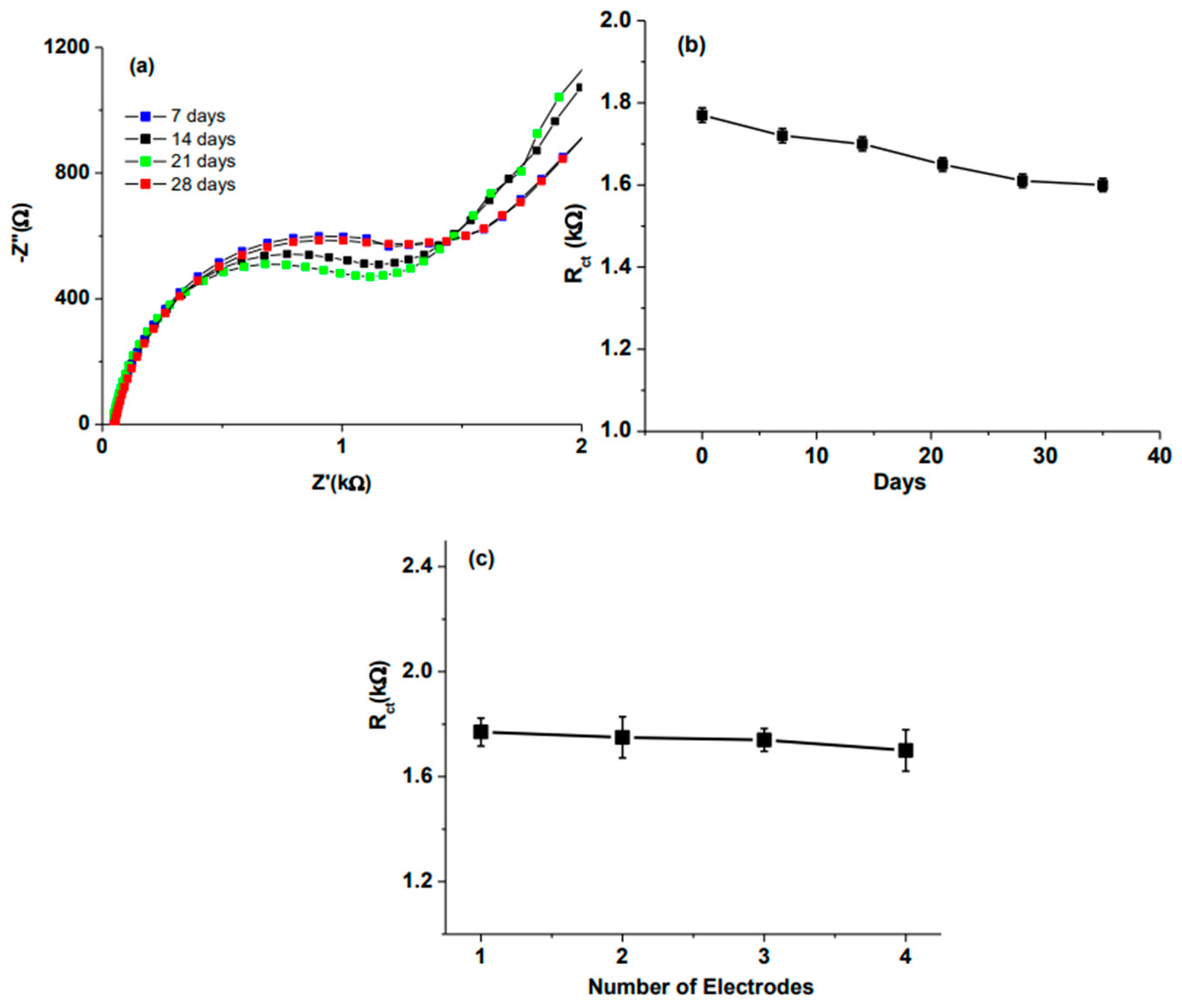
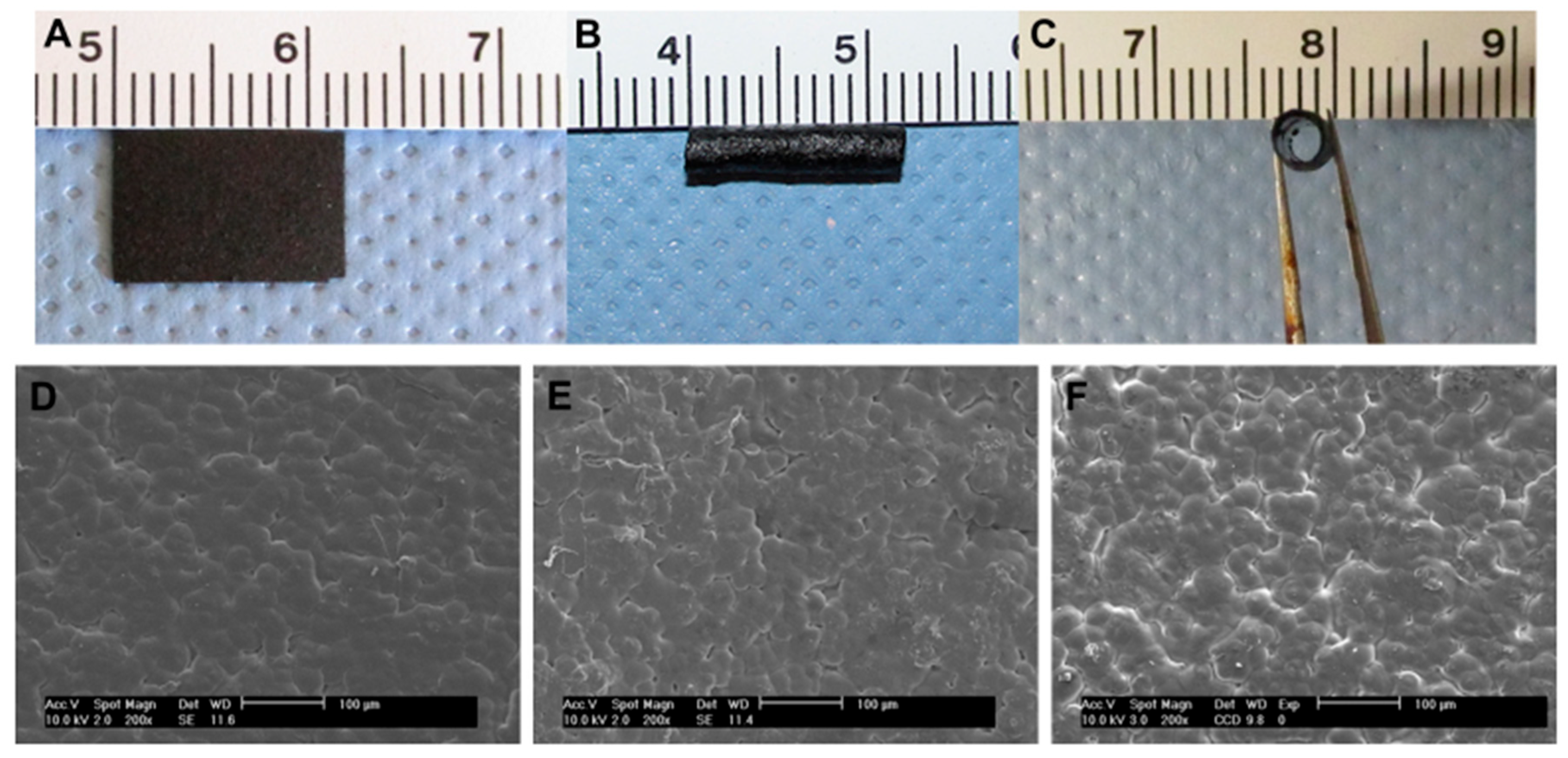

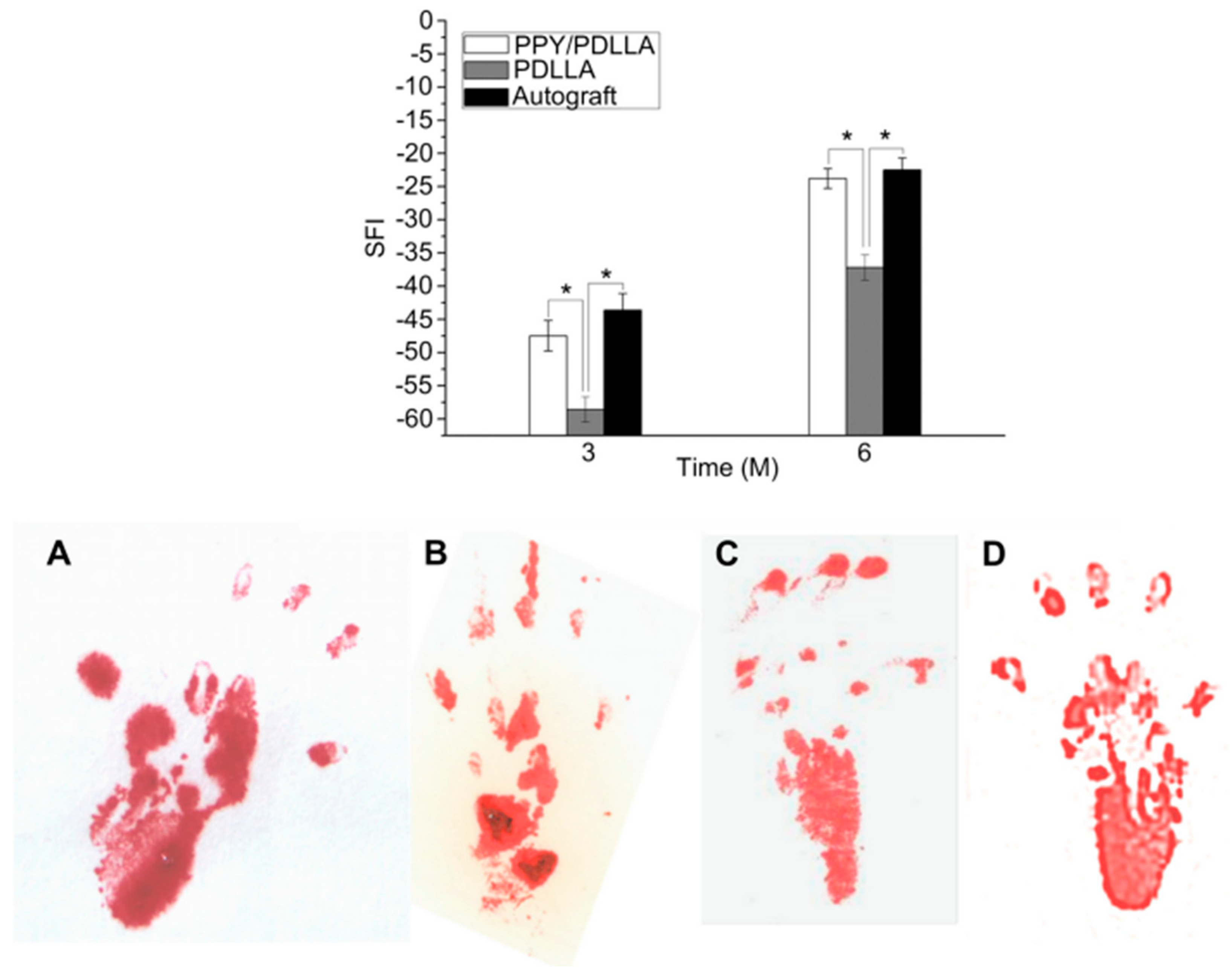
| Sensor Types | Advantages and Applications | Disadvantages | Examples | Refs. |
|---|---|---|---|---|
| Electrochemical | -Simple sample preparation and fast sampling time -High selectivity resulting from electrical signals at distinctive formal potentials -Can analyze various biological matrices -Can provide significant information about the way a drug is metabolized at specific dosage levels and its interactions with living cells | -Limited selectivity in complex biological matrices -Signal drift and electrode fouling -Stability issues in vivo applications -Challenges in calibration and reproducibility | -Carbon-based electrodes -Chemically modified electrodes | [13,14,15,16,17,18] |
| Optical | -Devices can be miniaturized -Lacks direct contact (non-invasive) -High spatial resolution -Nondestructive | -Photobleaching and signal instability -Interference from autofluorescence -Limited penetration depth | -Fluorescent carbon nanotubes -Quantum dots -Engineered proteins -Enzyme-conjugated nanoparticles -Small organic fluorophores | [6,19,20,21,22,23] |
| Nanomaterial | Example | Functional and Structural Features | Refs. |
|---|---|---|---|
| Noble metal nanomaterials | -Au nanoparticles | -High chemical stability -Easy preparation and fabrication methods -Wide electrochemical potential range -Biocompatibility -High catalytic activity | [28,29,30,31,32,33] |
| -Ag nanoparticles | -High conductivity -Biocompatibility -Amplified electrochemical signal | ||
| -Platinum nanoparticles | -Distinctive electrocatalytic and electronic properties | ||
| -Palladium nanoparticles | -High catalytic and sensor activity | ||
| Metal oxide nanomaterials | -Cerium oxide nanoparticles | -Enzymes and proteins can be easily immobilized on its surface -High catalytic activity | [34,35] |
| -Copper oxide nanoparticles | -Various valence states -Tunable electron-transport performance -High surface area | ||
| -Magnetic nanoparticles | -Highly active and accessible surface are -Unique electron-transfer behavior | ||
| Carbon-based nanomaterials | -Carbon nanotubes | -Their chemical properties can be easily modified and conjugated due to their tubular structure | [1,36,37,38] |
| -Graphene | -High sensitivity -High selectivity -Low over potential -High electrocatalytic activity -Wide potential range | ||
| Polymeric nanomaterials | -Dendrimers | -Structurally consistent and organized -Accurate -Biocompatible | [1] |
| -Conducting polymers | -Unique electronic properties | ||
| -Molecularly imprinted polymers | -High selectivity | ||
| Bionanomaterials | -Aptamers | -High affinity -High selectivity | [1] |
| -DNA nanostructures | -Reusability -Simple synthesis process -High affinity and selectivity -Low cost |
| Drug Family | Drug/Compound Name | Some Nanomaterial-Based Sensor System Used for Detection of the Drug | Refs. |
|---|---|---|---|
| Anesthetics | Procaine | -MWCNT coated glassy carbon electrode | [39] |
| Capsaicin | -Carbon nanotube based electrode | [40] | |
| Analgesics | Paracetamol | -Carbon nanotubes and graphene | [41,42,43,44,45,46] |
| -Metallic nanoparticles Metal oxide and metal hydroxide nanoparticle composite electrodes | [47,48,49,50] | ||
| Aspirin | -Boron-doped diamond electrode | [51] | |
| -Alumina coated MWCNT nanocomposite modified glassy carbon electrode | [52] | ||
| Morphine | -Gold nanoparticle and Nafion modified carbon paste electrode | [53] | |
| -MWCNT and a chitosan modified glassy carbon electrode. | [53] | ||
| -GCE modified with graphene nanosheet | [54] | ||
| -Carbon paste electrode modified with vinylferrocene and MWCNT | [55] | ||
| Tramadol | -Carbon nanotube and Au nanoparticles glassy carbon electrode | [56] | |
| -Glassy carbon paste modified with Dowex and Au nanoparticles | [57] | ||
| Sumatriptan | -Pyrolytic graphite electrode modified with MWCNT and Ag nano particles | [58] | |
| -Glassy carbon electrode modified with MWCNT and polypyrrole doped with new coccine | [59] | ||
| -Au nanoparticle and graphene modified glassy carbon electrode | [60] | ||
| Cadeine | -MWCNT modified glassy carbon electrode | [61] | |
| -SWCNT modified carbon-ceramic electrode | [62] | ||
| Benorilate | -Carbon paste electrode with Ag nanoparticles | [63] | |
| Anti-epileptics | Carbamazepine | -MWCNT on a glassy carbon electrode | [64,65] |
| Fullerene-C60-modified glassy carbon electrode | |||
| Gabapentin | -Ag nanoparticles modified MWCNT | [66,67] | |
| -Carbon paste electrode modified with nanotubes of nickel oxide was carried out | [68] | ||
| Lamotrigine | -Ag nano modified carbon screen-printed electrode | [69] | |
| Psycholeptics | Buspirone | -DNA-templated Ag nanoparticle placed on a glassy carbon electrode | [70] |
| Chlorpromazine | -Glassy carbon electrode modified with a MWCNTs-polyethyleneimine | [71] | |
| -Carbon paste electrode was modified with cobalt nanoparticles | [72] | ||
| Clozapine | -Carbon paste electrode modified with TiO2 | [73] | |
| -Carbon paste electrode modified with polypyrrole that was doped with MWCNTs | [74] | ||
| Risperidone | -Carbon paste electrode modified MWCNTs | [75] | |
| -Carbon paste electrode coated with MWCNTs and the ionic liquid n-octylpyridinum hexafluorophosphate | [76] | ||
| Thioridazine | -Electrode modified with MWCNT and Co nanoparticles | [77] | |
| -Carbon paste electrode modified with ZnS nanoparticles | |||
| Psychoanaleptics | Caffeine | -Glassy carbon electrode modified MWCNTs | [78] |
| -Pyrolytic graphite electrode | [79] | ||
| Clomipramine | -Glassy carbon electrode that was modified with poly (aminobenzene sulfonic acid) and Pt nano-clusters | [80] | |
| Desipramine, imipramine and trimipramine | -Glassy carbon paste electrode modified with an Amberlite (XAD2) and TiO2 nanoparticles | [81] | |
| -Boron-doped diamond electrode | [82] | ||
| Trazodone | -Glassy carbon electrode modified MWCNTs | [83] | |
| Venlafaxine and desvenlafaxine | -GCE modified with a nafion–carbon nanotube composite | [84] | |
| -Hanging mercury dropping electrode | [85] | ||
| -Mercury film electrode | [86] | ||
| Other nervous system drugs | Cinnarizine | -Glassy carbon electrode | [87] |
| -Glassy carbon electrode with MWCNTs | [88] | ||
| Dextromethorphan | -Glassy carbon electrode modified with carbon nanotubes and an ionic liquid | [59] | |
| Naltrexone | -Glassy carbon electrode with Nafion-doped carbon nanoparticles | [89] | |
| -Glassy carbon electrode with a bilayer of MWCNT and polypyrrole doped with nitrazine yellow | [90] | ||
| Nicotine | -Pencil graphite electrode in the presence of the anionic surfactant | [91] | |
| -Basal plane pyrolytic graphite electrode modified with layers of MWCNTs | [92] |
| Neurotransmitter | Neurotransmitter Function | Detecting Technique | Ref. |
|---|---|---|---|
| Levodopa and carbidopa | -Used for the medication of Parkinson’s disease | -MWCNTs and polypyrrole modified glassy carbon electrode doped with tiron | [109] |
| -MWCNTs covered graphite electrode that were modified with Au nanoparticles | [110] | ||
| -Carbon paste electrode modified with a bis (nitriloethylidyne)-bis-hydroquinone and carbon nanotube | [111] | ||
| -Carbon nanotube paste electrode modified with an ionic liquid. | [112] | ||
| -Ferrocene-modified carbon nanotube paste electrode | [113] | ||
| Dopamine | -Plays a huge role in functions of central nervous, hormonal, renal, and cardiovascular systems | -Self assembled carbon nanotubes | [114] |
| -A gold nanocluster was incorporated into a glassy carbon electrode modified with 3-amino-5-mercapto-1,2,4 triazole film. | [115] | ||
| -A Pt/reduced graphene oxide modified glassy carbon electrode | [116] | ||
| -LaFeO3 nanoparticles | [117] | ||
| -Nano Au/DNA/nano Au/poly safranine T composite deposited on glassy carbon electrode | [118] | ||
| -CuO nanoparticles | [119] | ||
| -Carbon fiber microbiosensor modified with copper-graphene oxide | [120] | ||
| Epinephrine | -Regulates heart rate, blood vessel and air passage diameters along with metabolic shifts | -MWCNT-modified edge-plane pyrolytic graphite electrode | [121] |
| -Its release plays a big role in the fight-or-flight response of the sympathetic nervous system. | -Functionalized MWCNTs | [122] | |
| Norepinephrine | -It is used for treating myocardial infarction hypertension, bronchial asthma and organic heart disease | -Screen printed carbon electrode modified with poly (acrylic acid)-coated MWCNTs | [123] |
| -Poly-glycine membrane containing silver nanoparticles | [124] | ||
| -Carbon paste electrode modified with carbon nanotubes and a molybdenum (VI) complex | [125] | ||
| Serotonin | -Plays a crucial role in the emotional system such as regulation of mood, sleep, vomiting, appetite and sexuality. | -MWCNTs /ionic liquid | [118] |
| -Reduced graphene oxide in a porphyrine-modified glassy carbon electrode | [126] | ||
| -Glassy carbon electrode modified with MWCNT/chitosan | [127] | ||
| -Nafion/Ni(OH)2 nanoparticles and MWCNTs modified glassy carbon electrode | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhshpour-Yücel, M.; Aljayyousi, N.; Osman, B.; Lortlar Ünlü, N.; Denizli, A.; Ünlü, M.S. Nanomaterial-Based Sensing Systems to Detect Neuropharmaceutical Compounds and Neurotransmitters. Sensors 2025, 25, 3256. https://doi.org/10.3390/s25113256
Bakhshpour-Yücel M, Aljayyousi N, Osman B, Lortlar Ünlü N, Denizli A, Ünlü MS. Nanomaterial-Based Sensing Systems to Detect Neuropharmaceutical Compounds and Neurotransmitters. Sensors. 2025; 25(11):3256. https://doi.org/10.3390/s25113256
Chicago/Turabian StyleBakhshpour-Yücel, Monireh, Nawal Aljayyousi, Bilgen Osman, Nese Lortlar Ünlü, Adil Denizli, and M. Selim Ünlü. 2025. "Nanomaterial-Based Sensing Systems to Detect Neuropharmaceutical Compounds and Neurotransmitters" Sensors 25, no. 11: 3256. https://doi.org/10.3390/s25113256
APA StyleBakhshpour-Yücel, M., Aljayyousi, N., Osman, B., Lortlar Ünlü, N., Denizli, A., & Ünlü, M. S. (2025). Nanomaterial-Based Sensing Systems to Detect Neuropharmaceutical Compounds and Neurotransmitters. Sensors, 25(11), 3256. https://doi.org/10.3390/s25113256









