Preparation of NiO NWs by Thermal Oxidation for Highly Selective Gas-Sensing Applications
Abstract
1. Introduction
2. Materials and Methods
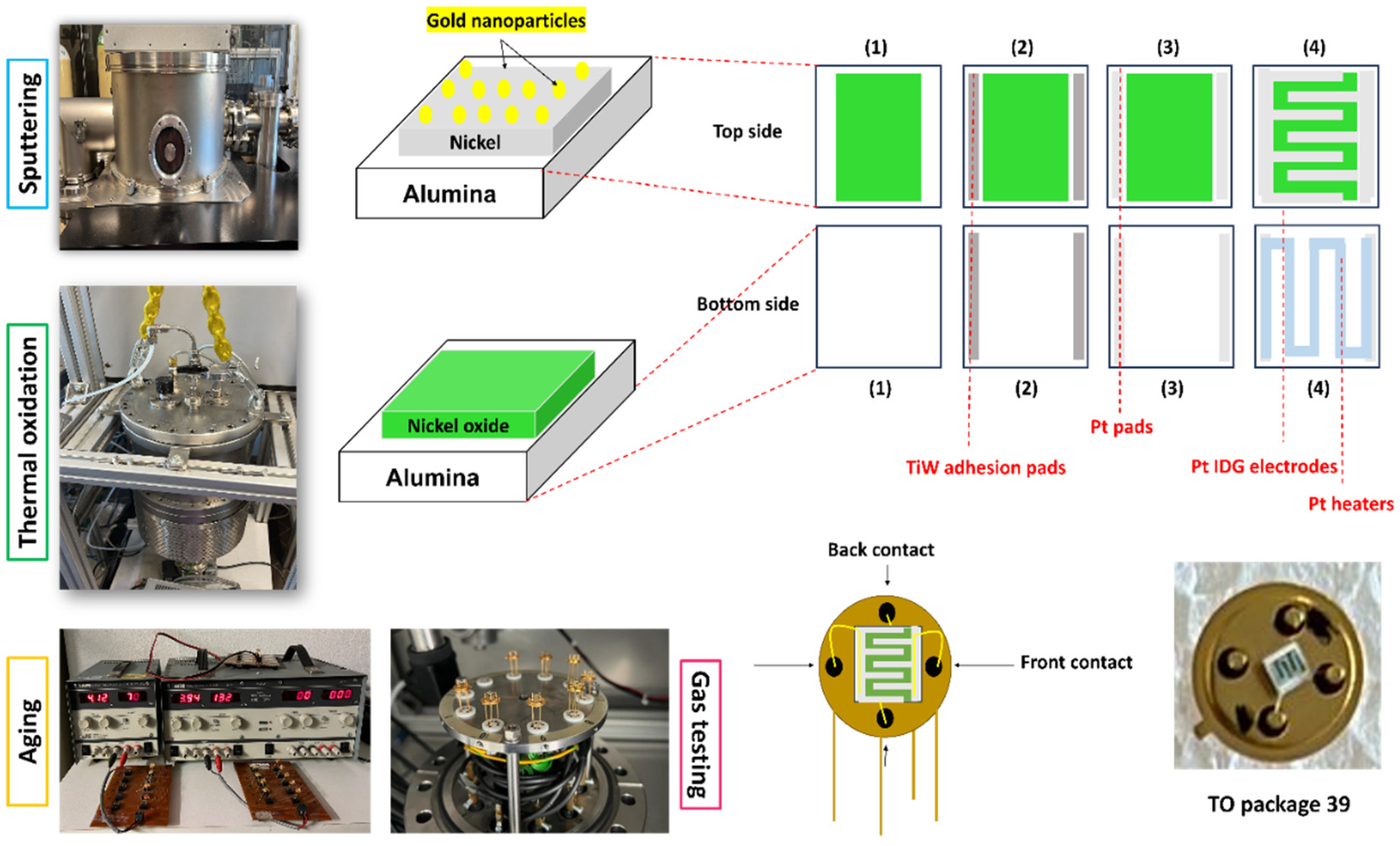
2.1. Samples
2.2. Sensor Preparation
2.3. Gas-Testing Conditions
3. Results and Discussion
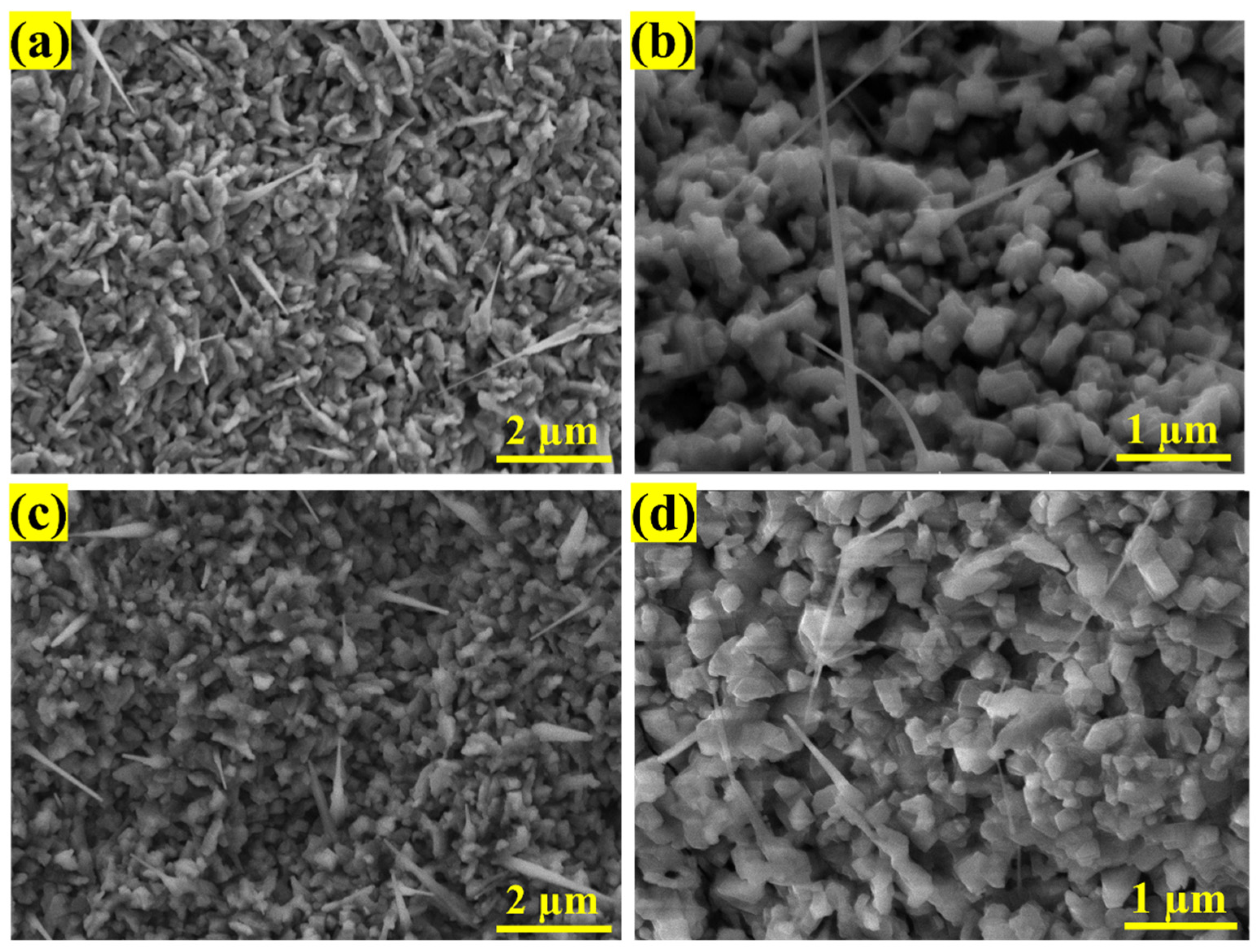
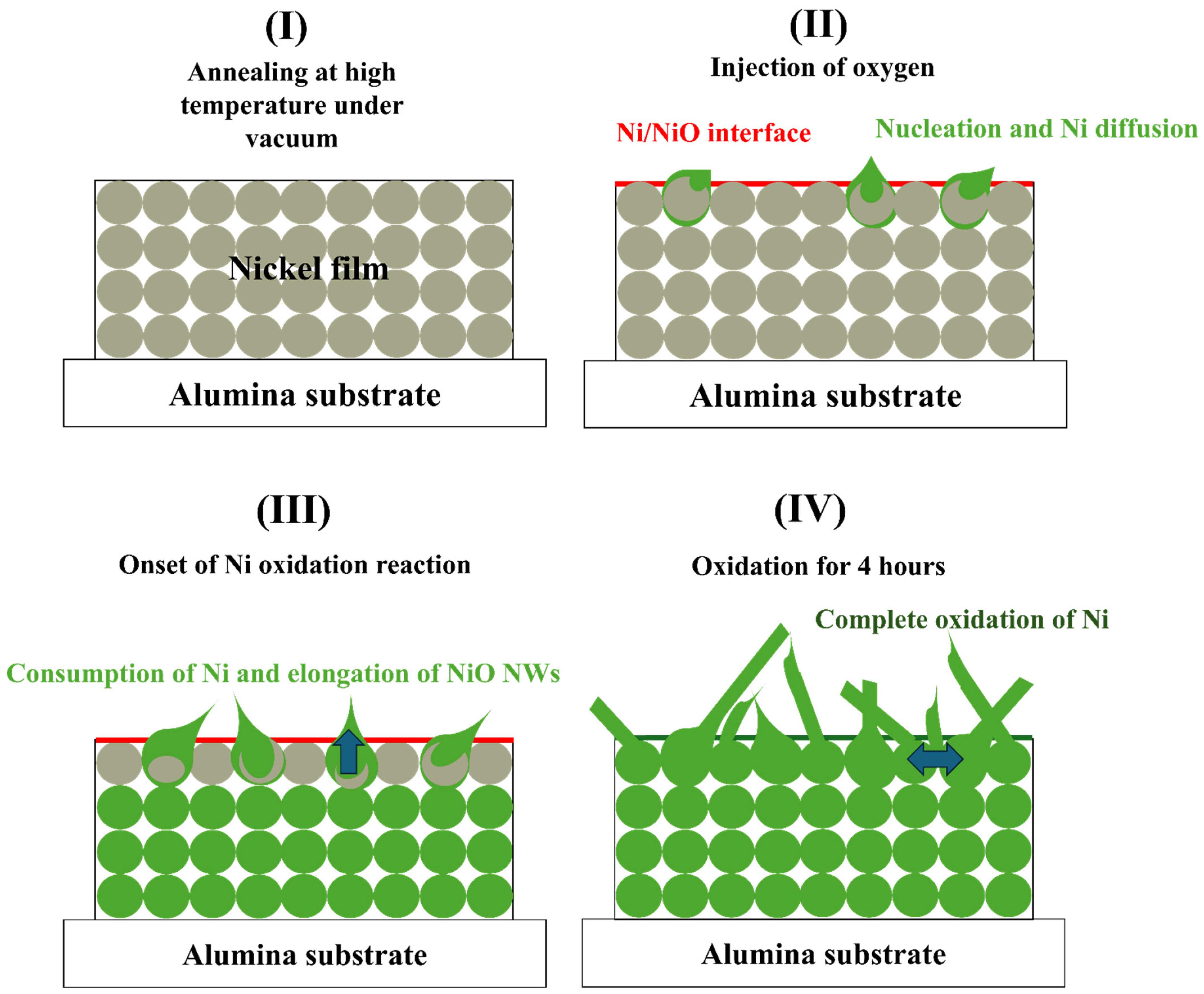
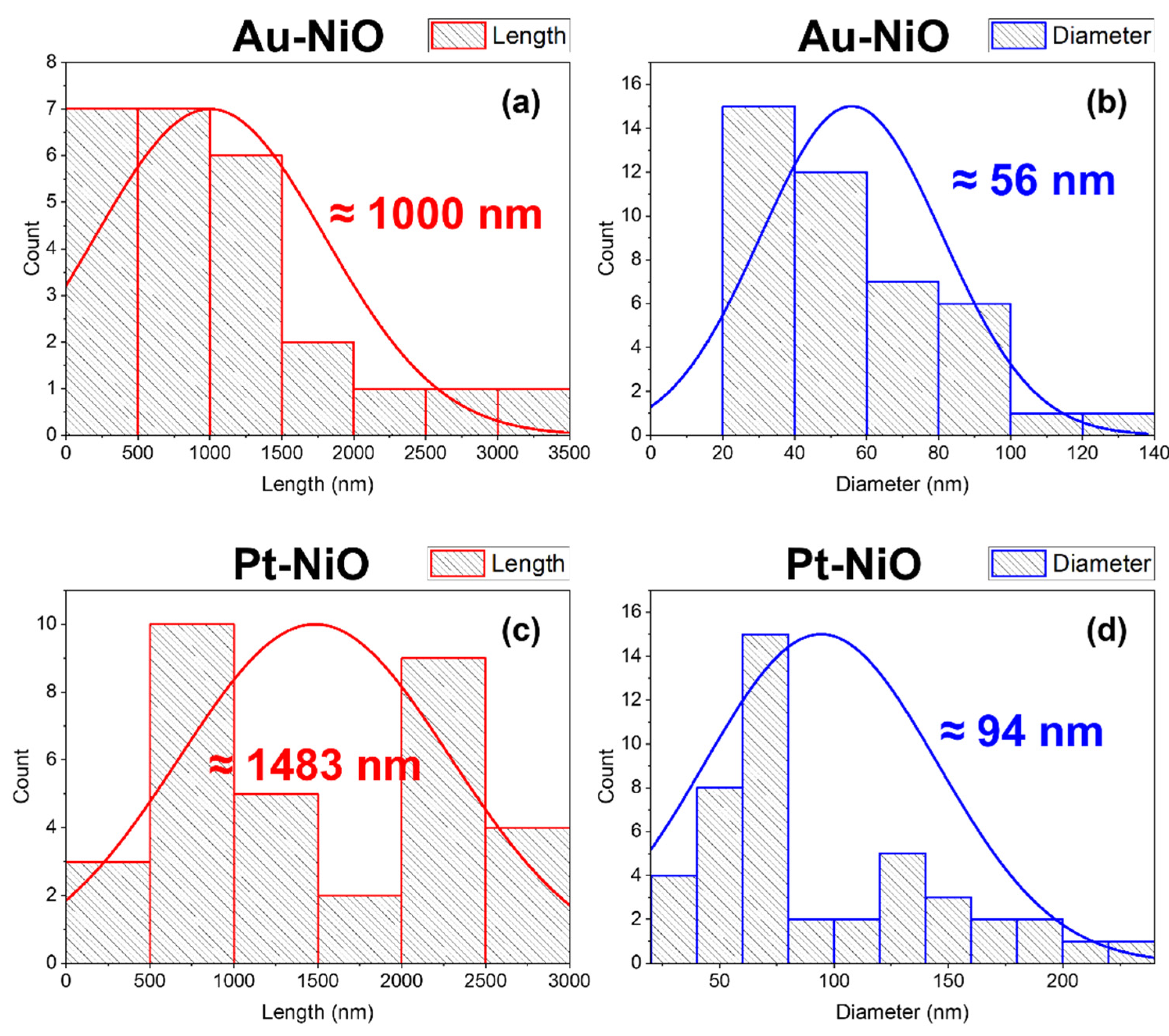
3.1. Morphology and Structural Properties
3.2. Gas-Sensing Performance
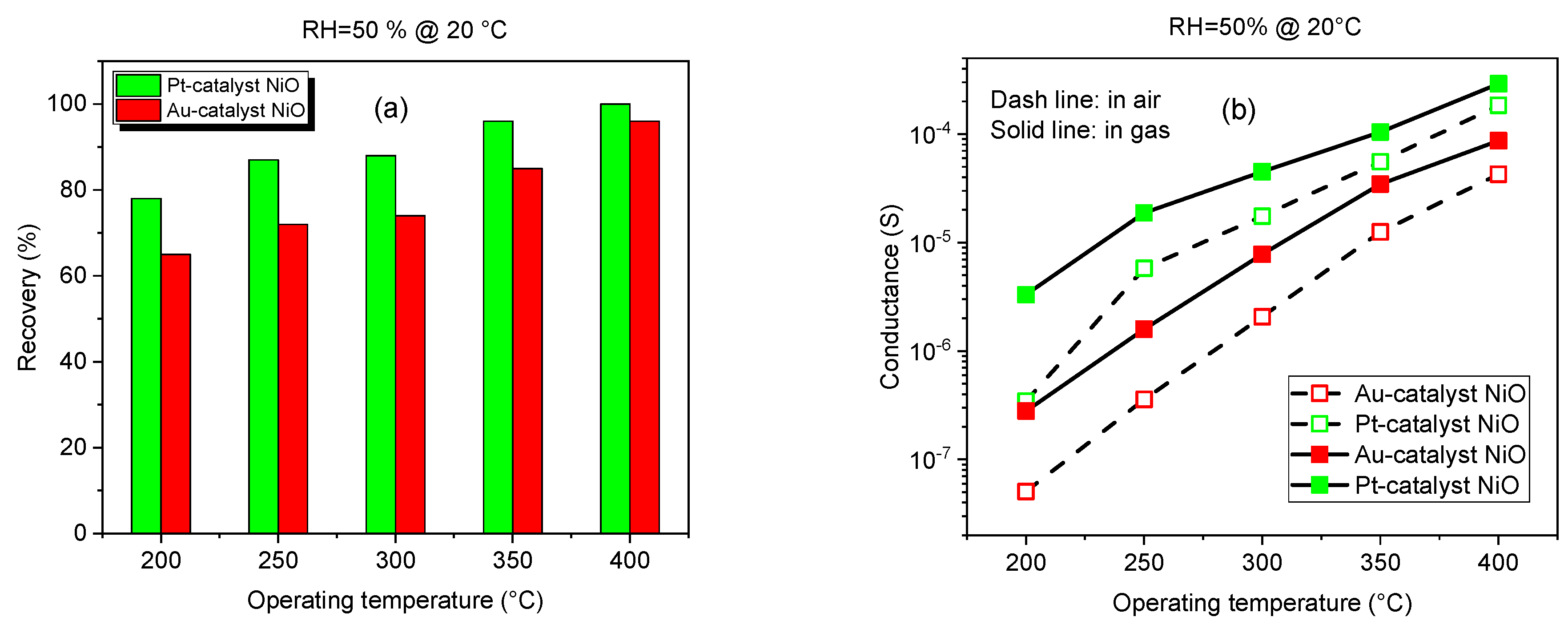
3.2.1. Gas Response at Different Operating Temperatures
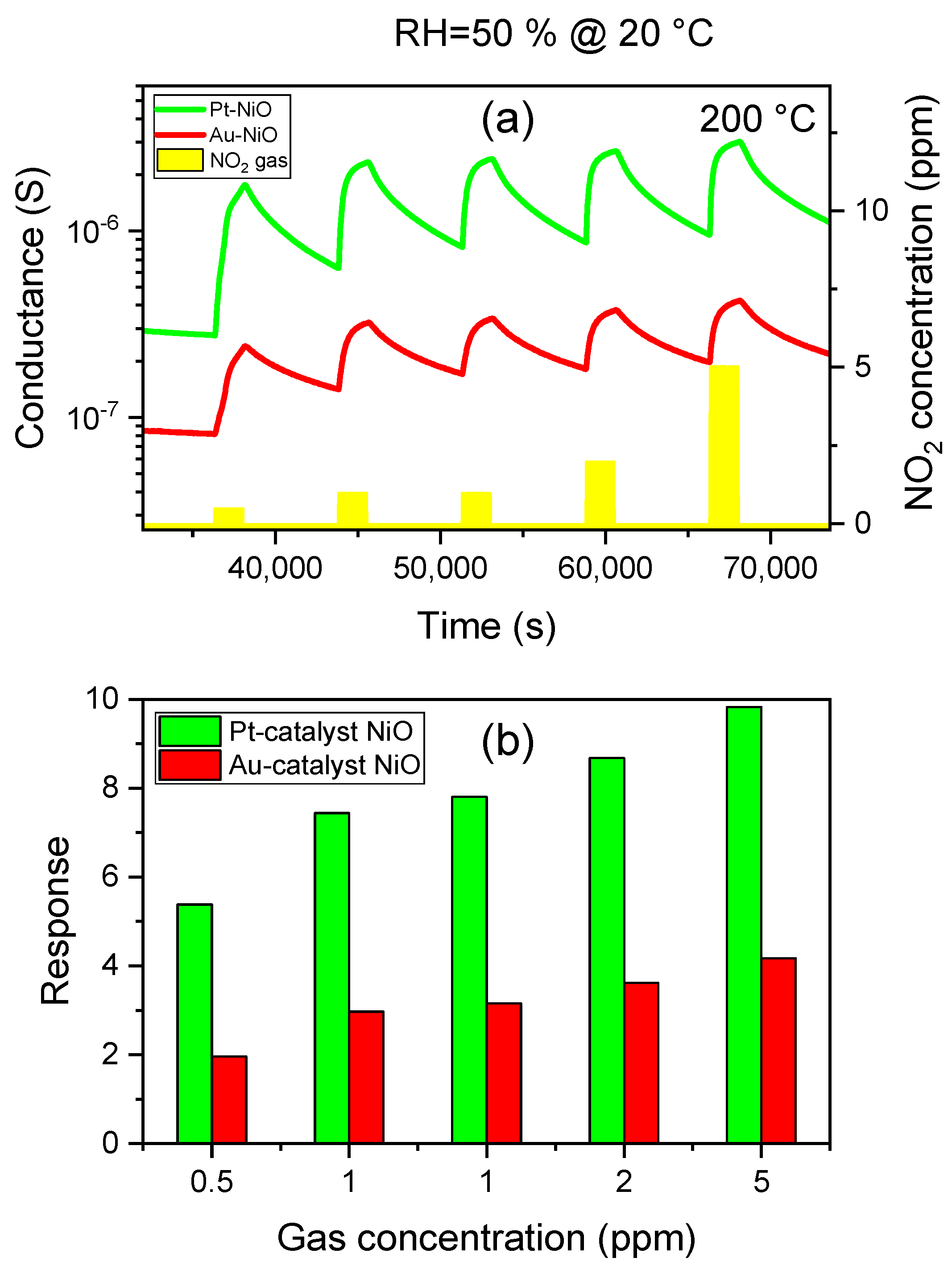
3.2.2. Dynamic Response to NO2
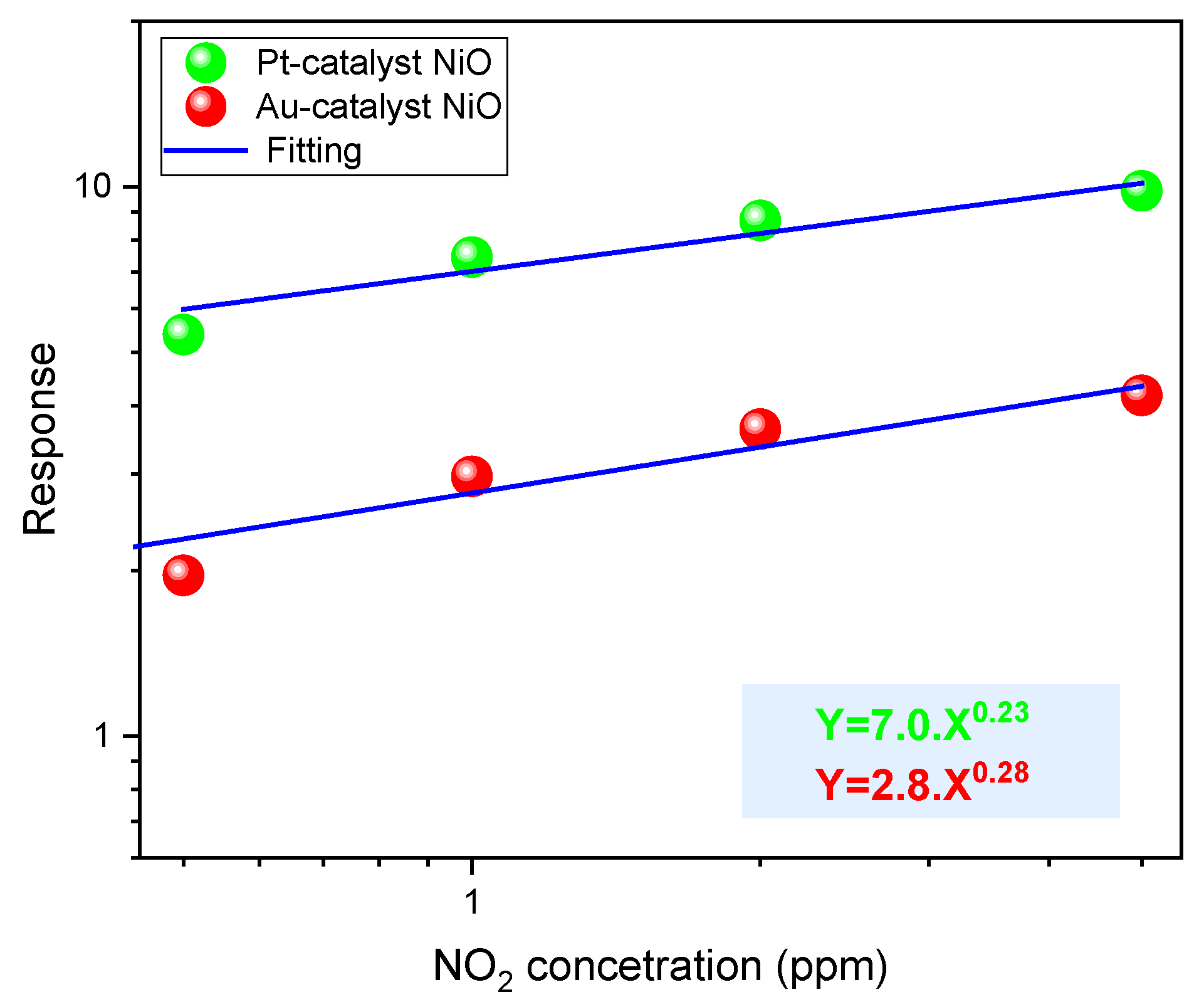
3.2.3. Mechanism of NO2 Sensing
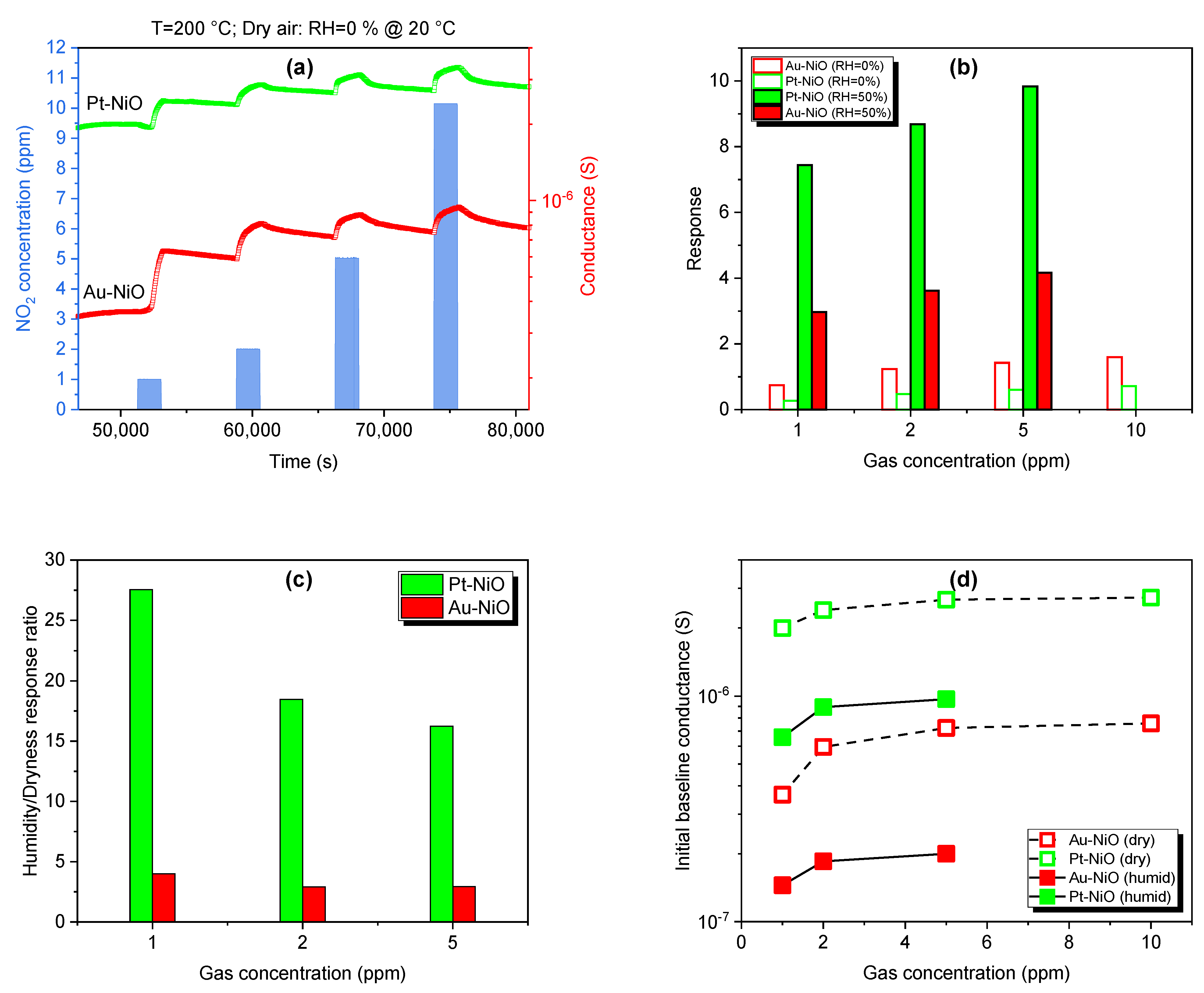
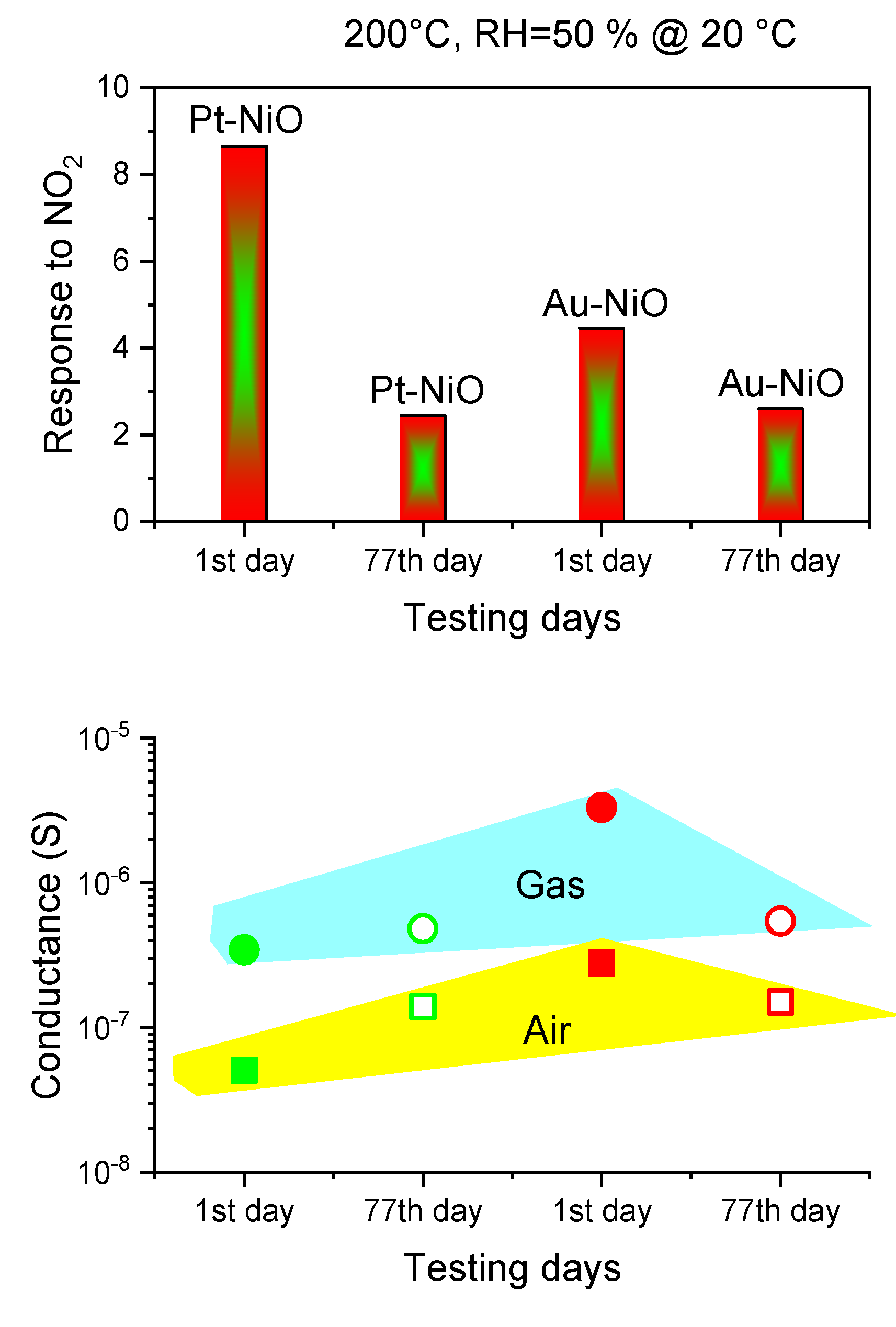
3.2.4. Moisture Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, L.; Yu, L.; Li, S.; Nan, N.; Zhang, C.; Fan, X. In-situ growth of well-ordered ZnO nanowire-networks with interconnected junctions for enhanced SO2 gas sensing properties. Appl. Surf. Sci. 2024, 646, 158899. [Google Scholar] [CrossRef]
- Tonezzer, M. Selective gas sensor based on one single SnO2 nanowire. Sens. Actuators B Chem. 2019, 288, 53–59. [Google Scholar] [CrossRef]
- Tonezzer, M.; Hieu, N.V. Size-dependent response of single-nanowire gas sensors. Sens. Actuators B Chem. 2012, 163, 146–152. [Google Scholar] [CrossRef]
- Vallejos, S.; Di Maggio, F.; Shujah, T.; Blackman, C. Chemical vapour deposition of gas sensitive metal oxides. Chemosensors 2016, 4, 4. [Google Scholar] [CrossRef]
- Chen, L.; Yu, Q.; Pan, C.; Song, Y.; Dong, H.; Xie, X.; Li, Y.; Liu, J.; Wang, D.; Chen, X. Chemiresistive gas sensors based on electrospun semiconductor metal oxides: A review. Talanta 2022, 246, 123527. [Google Scholar] [CrossRef]
- Li, R.; Chen, S.; Lou, Z.; Li, L.; Huang, T.; Song, Y.; Chen, D.; Shen, G. Fabrication of porous SnO2 nanowires gas sensors with enhanced sensitivity. Sens. Actuators B Chem. 2017, 252, 79–85. [Google Scholar] [CrossRef]
- Dang, T.T.L.; Tonezzer, M.; Nguyen, V.H. Hydrothermal growth and hydrogen selective sensing of nickel oxide nanowires. J. Nanomater. 2015, 2015, 785856. [Google Scholar] [CrossRef]
- Ramgir, N.; Datta, N.; Kaur, M.; Kailasaganapathi, S.; Debnath, A.K.; Aswal, D.; Gupta, S. Metal oxide nanowires for chemiresistive gas sensors: Issues, challenges and prospects. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 101–116. [Google Scholar] [CrossRef]
- Kaur, N.; Comini, E.; Zappa, D.; Poli, N.; Sberveglieri, G. Nickel oxide nanowires: Vapor liquid solid synthesis and integration into a gas sensing device. Nanotechnology 2016, 27, 205701. [Google Scholar] [CrossRef]
- Supab, C.; Niyom, H.; Ekasiddh, W. Metal-Oxide Nanowires by Thermal Oxidation Reaction Technique. In Nanowires; Paola, P., Ed.; IntechOpen: Rijeka, Croatia, 2010; Chapter 5. [Google Scholar]
- Zappa, D.; Briand, D.; Comini, E.; Courbat, J.; De Rooij, N.; Sberveglieri, G. Zinc oxide nanowires deposited on polymeric hotplates for low-power gas sensors. Procedia Eng. 2012, 47, 1137–1140. [Google Scholar] [CrossRef]
- Spanu, D.; Recchia, S.; Schmuki, P.; Altomare, M. Thermal-Oxidative Growth of Substoichiometric WO3−x Nanowires at Mild Conditions. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2020, 14, 2000235. [Google Scholar]
- Zappa, D.; Comini, E.; Zamani, R.; Arbiol, J.; Morante, J.; Sberveglieri, G. Copper oxide nanowires prepared by thermal oxidation for chemical sensing. Procedia Eng. 2011, 25, 753–756. [Google Scholar]
- Zappa, D.; Bertuna, A.; Comini, E.; Molinari, M.; Poli, N.; Sberveglieri, G. Tungsten oxide nanowires for chemical detection. Anal. Methods 2015, 7, 2203–2209. [Google Scholar]
- Zappa, D.; Comini, E.; Zamani, R.; Arbiol, J.; Morante, J.; Sberveglieri, G. Preparation of copper oxide nanowire-based conductometric chemical sensors. Sens. Actuators B Chem. 2013, 182, 7–15. [Google Scholar]
- Munasinghe Arachchige, H.M.; Zappa, D.; Poli, N.; Gunawardhana, N.; Attanayake, N.H.; Comini, E. Seed-assisted growth of TiO2 nanowires by thermal oxidation for chemical gas sensing. Nanomaterials 2020, 10, 935. [Google Scholar] [CrossRef]
- Moumen, A.; Kumarage, G.C.W.; Comini, E. P-Type Metal Oxide Semiconductor Thin Films: Synthesis and Chemical Sensor Applications. Sensors 2022, 22, 1359. [Google Scholar] [CrossRef]
- Ren, Y.; Chiam, S.; Chim, W. Diameter dependence of the void formation in the oxidation of nickel nanowires. Nanotechnology 2011, 22, 235606. [Google Scholar]
- Xiang, W.; Dong, Z.; Luo, Y.; Zhao, J.; Wang, J.-o.; Ibrahim, K.; Zhan, H.; Yue, W.; Guo, H. Synthesis of NiO Nanotubes via a Dynamic Thermal Oxidation Process. Materials 2019, 12, 805. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, W.; Liu, Y.; Hu, M.; Zhao, K. Synthesis of High-Aspect-Ratio Nickel Nanowires by Dropping Method. Nanoscale Res. Lett. 2016, 11, 118. [Google Scholar] [CrossRef]
- Hajakbari, F. Characterization of nanocrystalline nickel oxide thin films prepared at different thermal oxidation temperatures. J. Nanostruct. Chem. 2020, 10, 97–103. [Google Scholar] [CrossRef]
- Ravikumar, P.; Taparia, D.; Alagarsamy, P. Thickness-Dependent Thermal Oxidation of Ni into NiO Thin Films. J. Supercond. Nov. Magn. 2018, 31, 3761–3775. [Google Scholar] [CrossRef]
- Abubakar, D.; Ahmed, N.M.; Mahmud, S.; Algadri, N.A. Properties of NiO nanostructured growth using thermal dry oxidation of nickel metal thin film for hydrogen gas sensing at room temperature. Mater. Res. Express 2017, 4, 075009. [Google Scholar] [CrossRef]
- Raghavan, L.; Ojha, S.; Sulania, I.; Mishra, N.C.; Ranjith, K.M.; Baenitz, M.; Kanjilal, D. Thermal annealing induced competition of oxidation and grain growth in nickel thin films. Thin Solid Film. 2019, 680, 40–47. [Google Scholar] [CrossRef]
- Unutulmazsoy, Y.; Merkle, R.; Fischer, D.; Mannhart, J.; Maier, J. The oxidation kinetics of thin nickel films between 250 and 500 °C. Phys. Chem. Chem. Phys. 2017, 19, 9045–9052. [Google Scholar] [CrossRef]
- Zhu, W.; Winterstein, J.P.; Sharma, R.; Zhou, G. The Growth of Catalyst-free NiO Nanowires. Microsc. Microanal. 2016, 22, 1620–1621. [Google Scholar]
- Qu, B.; van Benthem, K. In situ anisotropic NiO nanostructure growth at high temperature and under water vapor. J. Am. Ceram. Soc. 2022, 105, 2454–2464. [Google Scholar] [CrossRef]
- Qu, B.; van Benthem, K. In-situ anisotropic growth of nickel oxide nanostructures through layer-by-layer metal oxidation. Scr. Mater. 2022, 214, 114660. [Google Scholar] [CrossRef]
- Koga, K.; Hirasawa, M. Anisotropic growth of NiO nanorods from Ni nanoparticles by rapid thermal oxidation. Nanotechnology 2013, 24, 375602. [Google Scholar] [CrossRef]
- Xiang, L.; Guo, J.; Wu, C.; Cai, M.; Zhou, X.; Zhang, N. A brief review on the growth mechanism of CuO nanowires via thermal oxidation. J. Mater. Res. 2018, 33, 2264–2280. [Google Scholar] [CrossRef]
- Ponzoni, A. Metal Oxide Chemiresistors: A Structural and Functional Comparison between Nanowires and Nanoparticles. Sensors 2022, 22, 3351. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A. An electronic nose based on carbon nanotube -titanium dioxide hybrid nanostructures for detection and discrimination of volatile organic compounds. Sens. Actuators B Chem. 2022, 357, 131418. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of temperature and humidity on the sensing performance of TiO2 nanowire-based ethanol vapor sensors. Nanotechnology 2021, 32, 325501. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Yang, Y.-S. Effect of crystallographic orientations on electrical properties of sputter-deposited nickel oxide thin films. Thin Solid Film. 2008, 516, 5590–5596. [Google Scholar] [CrossRef]
- Penza, M.; Cassano, G.; Rossi, R.; Alvisi, M.; Rizzo, A.; Signore, M.A.; Dikonimos, T.; Serra, E.; Giorgi, R. Enhancement of sensitivity in gas chemiresistors based on carbon nanotube surface functionalized with noble metal (Au, Pt) nanoclusters. Appl. Phys. Lett. 2007, 90, 173123. [Google Scholar] [CrossRef]
- Kim, J.; Park, W.H.; Doh, W.H.; Lee, S.W.; Noh, M.C.; Gallet, J.-J.; Bournel, F.; Kondoh, H.; Mase, K.; Jung, Y. Adsorbate-driven reactive interfacial Pt-NiO1−x nanostructure formation on the Pt3Ni (111) alloy surface. Sci. Adv. 2018, 4, eaat3151. [Google Scholar] [CrossRef]
- Nikolaev, S.; Pichugina, D.; Mukhamedzyanova, D. Sites for the selective hydrogenation of ethyne to ethene on supported NiO/Au catalysts. Gold Bull. 2012, 45, 221–231. [Google Scholar] [CrossRef]
- Burns, A.R.; Jennison, D.R.; Stechel, E.B. Electronically stimulated dissociation of NO2 on Pt(111). Phys. Rev. B Condens. Matter. 1989, 40, 9485–9497. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, S.; Meng, F.; Zhang, H.; Zuo, K. Investigation of grain radius dependence of sensitivity for porous thin film semiconducting metal oxide gas sensor. IEEE Sens. J. 2019, 20, 4275–4282. [Google Scholar] [CrossRef]
- Gomaa, M.M.; Sayed, M.H.; Patil, V.L.; Boshta, M.; Patil, P.S. Gas sensing performance of sprayed NiO thin films toward NO2 gas. J. Alloys Compd. 2021, 885, 160908. [Google Scholar] [CrossRef]
- Mokoena, T.P.; Swart, H.C.; Motaung, D.E. A review on recent progress of p-type nickel oxide based gas sensors: Future perspectives. J. Alloys Compd. 2019, 805, 267–294. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Kheel, H.; Lee, C. Oxidizing gas sensing properties of the n-ZnO/p-Co3O4 composite nanoparticle network sensor. Sens. Actuators B Chem. 2016, 222, 1193–1200. [Google Scholar] [CrossRef]
- Wilson, R.L.; Simion, C.E.; Stanoiu, A.; Taylor, A.; Guldin, S.; Covington, J.A.; Carmalt, C.J.; Blackman, C.S. Humidity-Tolerant Ultrathin NiO Gas-Sensing Films. ACS Sens. 2020, 5, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, D.; Tian, S.; Qin, Z.; Zeng, D.; Xie, C. CuInS2 QDs decorated ring-like NiO for significantly enhanced room-temperature NO2 sensing performances via effective interfacial charge transfer. Sens. Actuators B Chem. 2018, 256, 1001–1010. [Google Scholar] [CrossRef]
- Gawali, S.R.; Patil, V.L.; Deonikar, V.G.; Patil, S.S.; Patil, D.R.; Patil, P.S.; Pant, J. Ce doped NiO nanoparticles as selective NO2 gas sensor. J. Phys. Chem. Solids 2018, 114, 28–35. [Google Scholar] [CrossRef]
- Walker, J.M.; Akbar, S.A.; Morris, P.A. Synergistic effects in gas sensing semiconducting oxide nano-heterostructures: A review. Sens. Actuators B Chem. 2019, 286, 624–640. [Google Scholar] [CrossRef]
- Pakdel, H.; Borsi, M.; Ponzoni, M.; Comini, E. Enhanced Gas Sensing Performance of CuO-ZnO Composite Nanostructures for Low-Concentration NO2 Detection. Chemosensors 2024, 12, 54. [Google Scholar] [CrossRef]
- Behera, B.; Chandra, S. Synthesis of WO3 nanorods by thermal oxidation technique for NO2 gas sensing application. Mater. Sci. Semicond. Process. 2018, 86, 79–84. [Google Scholar] [CrossRef]
- Han, T.-H.; Bak, S.-Y.; Kim, S.; Lee, S.H.; Han, Y.-J.; Yi, M. Decoration of CuO NWs Gas Sensor with ZnO NPs for Improving NO2 Sensing Characteristics. Sensors 2021, 21, 2103. [Google Scholar] [CrossRef]
- Hou, J.-L.; Lin, Y.-T.; Hsueh, T.-J. Employing Thermal Oxidation and Through-Silicon Via Technologies in CuO-Based Sensors for Room-Temperature NO2 Detection. J. Electrochem. Soc. 2024, 171, 057512. [Google Scholar] [CrossRef]
- Navale, Y.H.; Navale, S.T.; Ramgir, N.S.; Stadler, F.J.; Gupta, S.K.; Aswal, D.K.; Patil, V.B. Zinc oxide hierarchical nanostructures as potential NO2 sensors. Sens. Actuators B Chem. 2017, 251, 551–563. [Google Scholar] [CrossRef]
- Liu, W.; Gu, D.; Li, X. Ultrasensitive NO2 Detection Utilizing Mesoporous ZnSe/ZnO Heterojunction-Based Chemiresistive-Type Sensors. ACS Appl. Mater. Interfaces 2019, 11, 29029–29040. [Google Scholar] [CrossRef]
- Hou, J.-L.; Lin, Y.-T.; Hsueh, T.-J. Room-Temperature TiO2 Gas Sensor with Conical-TSV Using a Thermal Oxidation Process. ACS Appl. Electron. Mater. 2023, 5, 6542–6548. [Google Scholar] [CrossRef]
- Dastan, D.; Shan, K.; Jafari, A.; Marszalek, T.; Mohammed, M.K.A.; Tao, L.; Shi, Z.; Chen, Y.; Yin, X.-T.; Alharbi, N.D.; et al. Influence of heat treatment on H2S gas sensing features of NiO thin films deposited via thermal evaporation technique. Mater. Sci. Semicond. Process. 2023, 154, 107232. [Google Scholar] [CrossRef]
- Nakate, U.T.; Ahmad, R.; Patil, P.; Yu, Y.T.; Hahn, Y.-B. Ultra thin NiO nanosheets for high performance hydrogen gas sensor device. Appl. Surf. Sci. 2020, 506, 144971. [Google Scholar] [CrossRef]
- Baek, J.; Kim, Y.; Baik, K.H.; Jang, S. H2S Sensing Characteristics of NiO Nanopetal Film. ECS J. Solid. State Sci. Technol. 2023, 12, 085002. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, D.; Zhao, S.; Wu, J.; Xu, K.; Zhu, Q.; Zhang, G.; Xie, C. Room temperature NO2 sensing: What advantage does the rGO-NiO nanocomposite have over pristine NiO? Phys. Chem. Chem. Phys. 2015, 17, 14903–14911. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, S.; Vishwanadh, B.; Girija, K.G. Cost-effective synthesis of NiO nanoparticles and realization of highly selective NO2 sensors. Appl. Phys. A 2022, 128, 630. [Google Scholar] [CrossRef]
- Chou, P.-C.; Chen, H.-I.; Liu, I.P.; Chen, C.-C.; Liou, J.-K.; Hsu, K.-S.; Liu, W.-C. Hydrogen sensing performance of a nickel oxide (NiO) thin film-based device. Int. J. Hydrogen Energy 2015, 40, 729–734. [Google Scholar] [CrossRef]
- Chou, P.-C.; Chen, H.-I.; Liu, I.-P.; Chen, C.-C.; Liou, J.-K.; Hsu, K.-S.; Liu, W.-C. On the ammonia gas sensing performance of a RF sputtered NiO thin-film sensor. IEEE Sens. J. 2015, 15, 3711–3715. [Google Scholar]
- Prajesh, R.; Goyal, V.; Nahid, M.; Saini, V.; Singh, A.K.; Sharma, A.K.; Bhargava, J.; Agarwal, A. Nickel oxide (NiO) thin film optimization by reactive sputtering for highly sensitive formaldehyde sensing. Sens. Actuators B Chem. 2020, 318, 128166. [Google Scholar] [CrossRef]
- Zappa, D.; Bertuna, A.; Comini, E.; Kaur, N.; Poli, N.; Sberveglieri, V.; Sberveglieri, G. Metal oxide nanostructures: Preparation, characterization and functional applications as chemical sensors. Beilstein J. Nanotechnol. 2017, 8, 1205–1217. [Google Scholar] [CrossRef]
- Osburn, C.M.; Vest, R.W. Defect structure and electrical properties of NiO—I. High temperature. J. Phys. Chem. Solids 1971, 32, 1331–1342. [Google Scholar] [CrossRef]
- Agrawal, A.V.; Kumar, N.; Kumar, M. Strategy and Future Prospects to Develop Room-Temperature-Recoverable NO2 Gas Sensor Based on Two-Dimensional Molybdenum Disulfide. Nano-Micro Lett. 2021, 13, 38. [Google Scholar] [CrossRef]
- Brophy, R.E.; Junker, B.; Fakhri, E.A.; Árnason, H.Ö.; Svavarsson, H.G.; Weimar, U.; Bârsan, N.; Manolescu, A. Ultra Responsive NO2 silicon nanowires gas sensor. Sens. Actuators B Chem. 2024, 410, 135648. [Google Scholar] [CrossRef]
- Tit, N.; Said, K.; Mahmoud, N.M.; Kouser, S.; Yamani, Z.H. Ab-initio investigation of adsorption of CO and CO2 molecules on graphene: Role of intrinsic defects on gas sensing. Appl. Surf. Sci. 2017, 394, 219–230. [Google Scholar] [CrossRef]
- Bang, J.H.; Choi, M.S.; Mirzaei, A.; Kwon, Y.J.; Kim, S.S.; Kim, T.W.; Kim, H.W. Selective NO2 sensor based on Bi2O3 branched SnO2 nanowires. Sens. Actuators B Chem. 2018, 274, 356–369. [Google Scholar] [CrossRef]
- Wang, B.; Nisar, J.; Ahuja, R. Molecular Simulation for Gas Adsorption at NiO (100) Surface. ACS Appl. Mater. Interfaces 2012, 4, 5691–5697. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, D.; Zhu, Q.; Wu, J.; Huang, Q.; Xie, C. Effect of Nickel Vacancies on the Room-Temperature NO2 Sensing Properties of Mesoporous NiO Nanosheets. J. Phys. Chem. C 2016, 120, 3936–3945. [Google Scholar] [CrossRef]
- Nie, S.; Li, J.; He, Y.; Yin, X. Selective adsorption behavior of reducing gases on the NiO–In2O3 heterojunction under the interfacial effect. Ceram. Int. 2024, 50, 41397–41406. [Google Scholar] [CrossRef]

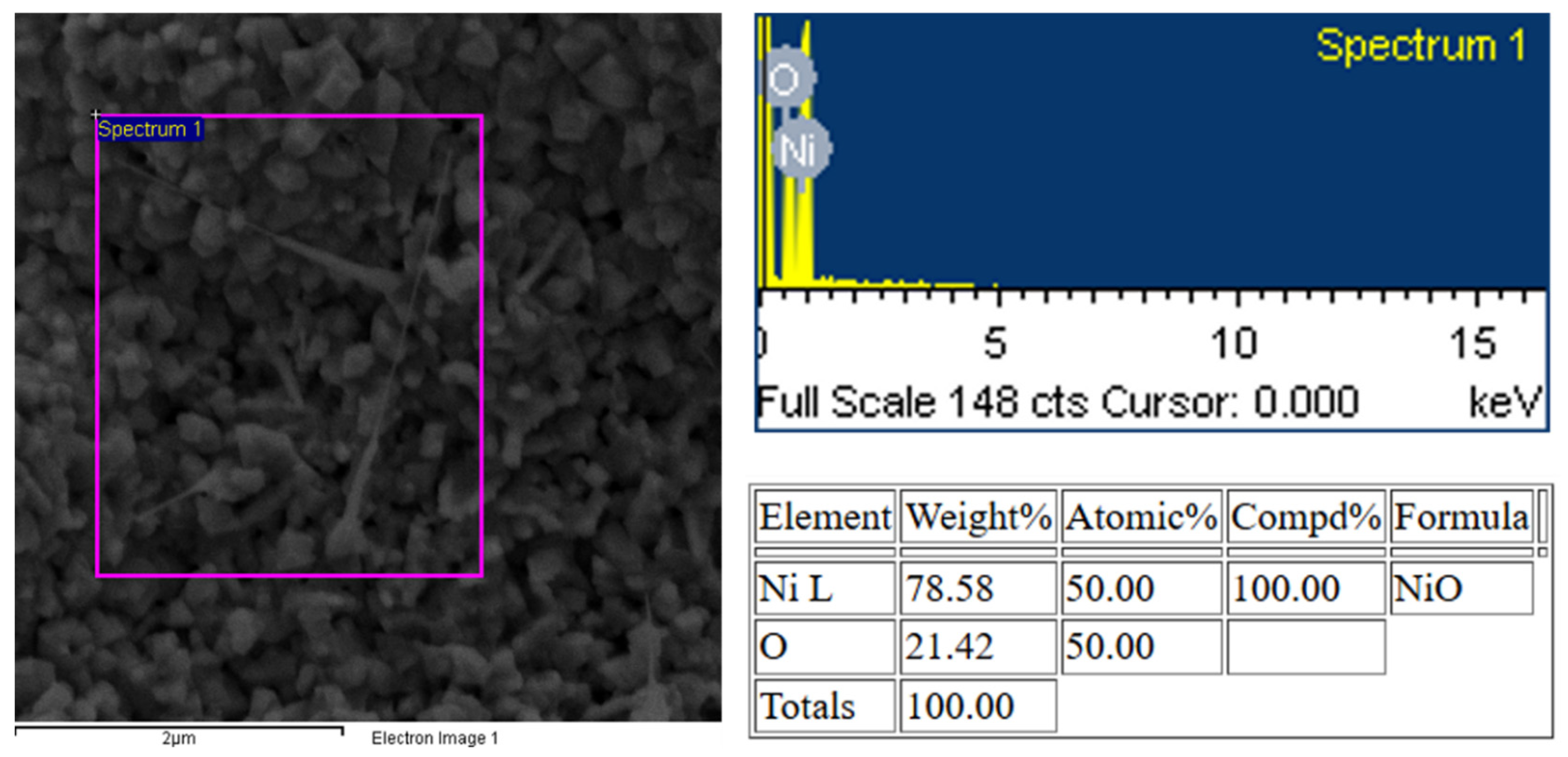

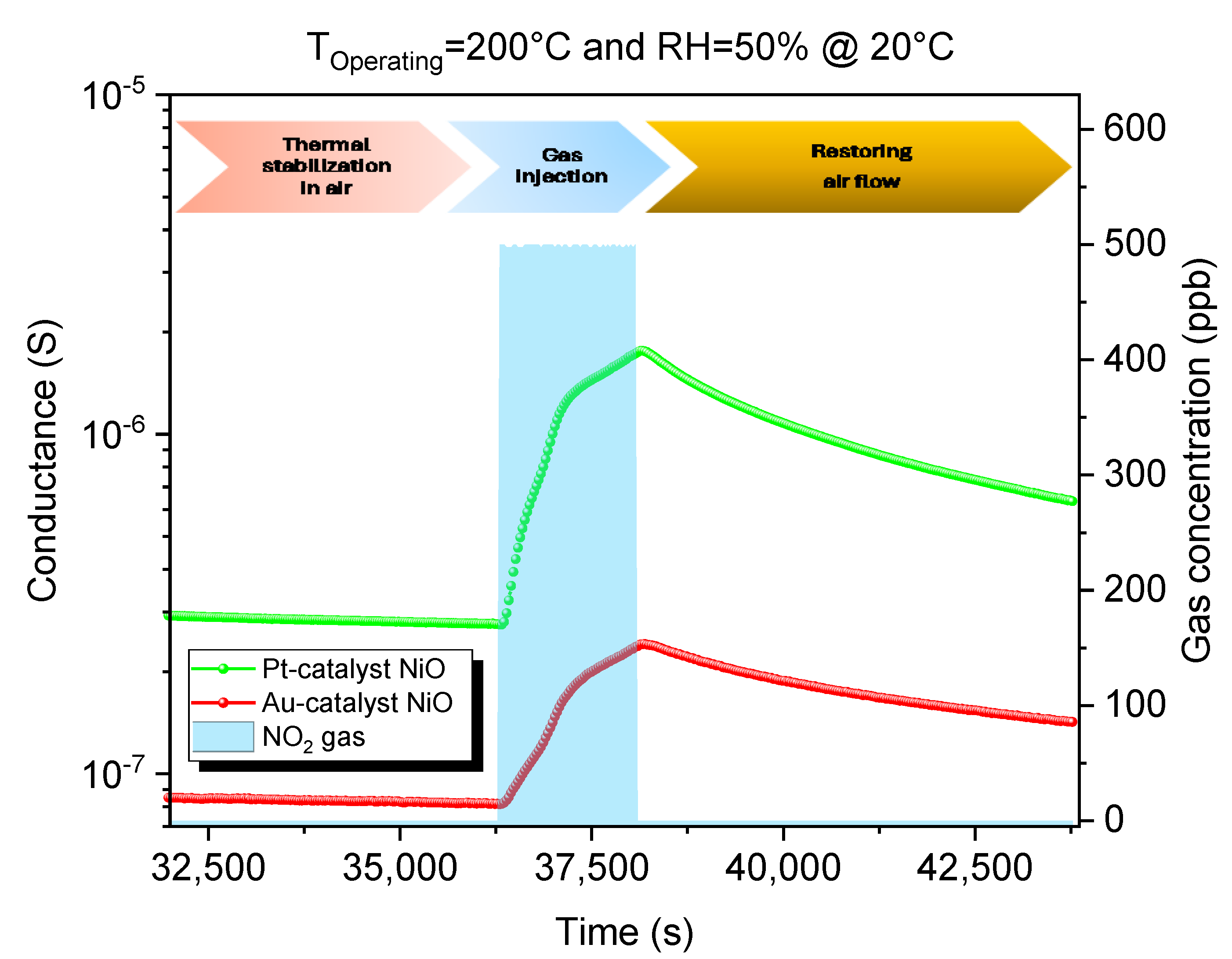
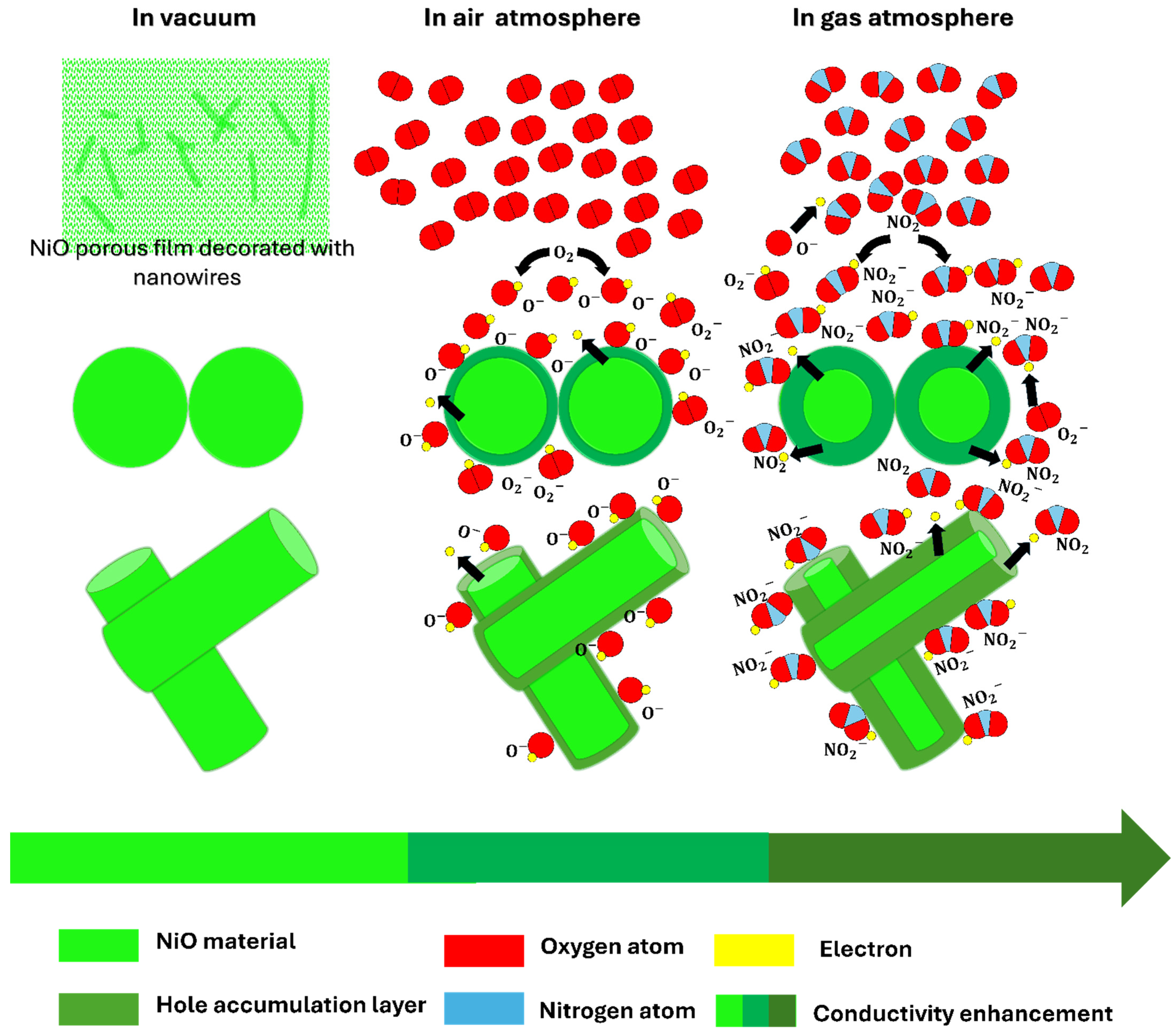
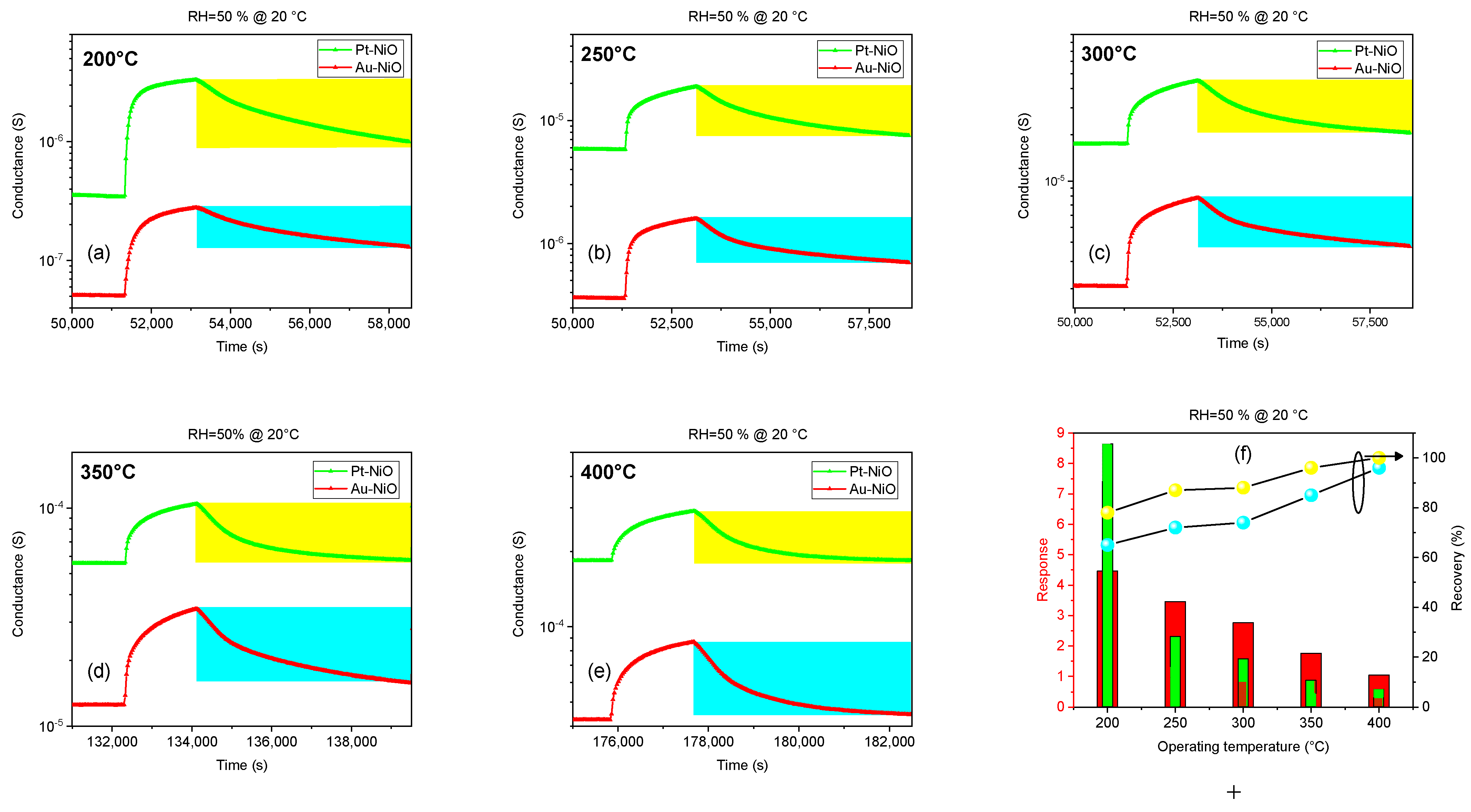

| Parameter | Value |
|---|---|
| (degree) | 43.38 |
| (degree) | 43.29 |
| (degree) | 0.373 |
| D (nm) | 229 |
| ) | 19 |
| ) | 1.5 |
| d (Å) | 2.083 |
| (Å) | 2.088 |
| a (Å) | 4.166 |
| (Å) | 4.176 |
| Sensing Material | Morphology | Gas Concentration/Gas Response | Working Temperature |
|---|---|---|---|
| NiO (Present work) | NWs + NPs | 5 ppm/G = 8.65 | 200 °C |
| WO3 [48] | Nanorods | 10 ppm/R = 1.16 | 225 °C |
| CuO [49] | NWs | 100 ppm/R* = 1.58 | 250 °C |
| CuO [50] | Thin film | 5 ppm/R = 0.463 | 25 °C |
| ZnO [51] | Bunch of NWs | 100 ppm/R = 6.22 | 200 °C |
| ZnO [51] | Nanorods | 100 ppm/R = 1.01 | 200 °C |
| ZnSe/ZnO [52] | Mesoporous microstructure (nanoflakes+ crystals) | 5 ppm/R** < 7 | 200 °C |
| TiO2 [53] | Thin film | 5 ppm/R** = 0.31 | 25 °C |
| Synthesis Method | Morphology | Gas Concentration/Gas Response | Working Temperature |
|---|---|---|---|
| Thermal oxidation (Present work) | NWs + NPs | NO2: 5 ppm/G = 8.65 | 200 °C |
| Thermal oxidation [54] | Granular film | H2S: 5 ppm/R′ = 2 | RT |
| Hydrothermal [55] | Nanosheets | H2: 150 ppm/R = 1.91 | 250 °C |
| Hydrothermal [56] | Nano-petal film | H2S: 500 ppm/I = 4 | 300 °C |
| Hydrothermal [57] | hexagonal nanosheets | NO2: 15 ppm/G = 0.39 | RT |
| Co-precipitation [58] | NPs | NO2: 5 ppm/1.75 < R″ < 2.25 | 180 °C |
| Sputtering [59] | Granular film | H2: 50 ppm/R = 0.23 | 250 °C |
| Sputtering [60] | Thin film | NH3: 1000 ppm/R = 2.89 | 250 °C |
| Sputtering [61] | Thin film | HCHO: 0.5 ppm/R = 0.168 | 200 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arbia, M.B.; Kim, S.-H.; Yoon, J.-B.; Comini, E. Preparation of NiO NWs by Thermal Oxidation for Highly Selective Gas-Sensing Applications. Sensors 2025, 25, 2075. https://doi.org/10.3390/s25072075
Arbia MB, Kim S-H, Yoon J-B, Comini E. Preparation of NiO NWs by Thermal Oxidation for Highly Selective Gas-Sensing Applications. Sensors. 2025; 25(7):2075. https://doi.org/10.3390/s25072075
Chicago/Turabian StyleArbia, Marwa Ben, Sung-Ho Kim, Jun-Bo Yoon, and Elisabetta Comini. 2025. "Preparation of NiO NWs by Thermal Oxidation for Highly Selective Gas-Sensing Applications" Sensors 25, no. 7: 2075. https://doi.org/10.3390/s25072075
APA StyleArbia, M. B., Kim, S.-H., Yoon, J.-B., & Comini, E. (2025). Preparation of NiO NWs by Thermal Oxidation for Highly Selective Gas-Sensing Applications. Sensors, 25(7), 2075. https://doi.org/10.3390/s25072075







