Advancements in Electrochemical Biosensors for Comprehensive Glycosylation Assessment of Biotherapeutics
Abstract
1. Introduction
2. Electrochemical Techniques for Glycosylation Analysis
2.1. Mechanism of Glycosylation Assessment Using Electrochemical Sensors
2.2. Electrochemical Sensor Components and Setup
2.2.1. Differential Pulse Voltammetry (DPV) in Glycosylation Analysis
2.2.2. Amperometry in Glycosylation Analysis
2.2.3. Voltammetry in Glycosylation Analysis
2.2.4. Impedance Spectroscopy in Glycosylation Analysis
2.3. Fabrication Methods for Electrochemical Glycosylation Sensors
3. Real-Time Monitoring of Glycosylation in Bioprocessing Using Electrochemical Sensors
3.1. Electrochemical Monitoring of Environmental Factors Affecting Glycosylation
3.2. Mediated Electrochemistry for Glycosylation Profiling and Targeted Modulation
3.3. Electrochemical Biosensors for Glycosylation Profiling of Biotherapeutics: Challenges and Emerging Strategies
Electrochemical Glycobiosensors Utilizing Biological Recognition Elements
4. Challenges of Transformative Electrochemical Glycobiosensors
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Christianson, J.C.; Jarosch, E.; Sommer, T. Mechanisms of substrate processing during ER-associated protein degradation. Nat. Rev. Mol. Cell Biol. 2023, 24, 777–796. [Google Scholar] [PubMed]
- Brás-Costa, C.; Chaves, A.F.A.; Cajado-Carvalho, D.; da Silva Pires, D.; Andrade-Silva, D.; Serrano, S.M. Profilings of subproteomes of lectin-binding proteins of nine Bothrops venoms reveal variability driven by different glycan types. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2022, 1870, 140795. [Google Scholar]
- Walther, R.; Zelikin, A.N. Chemical (neo) glycosylation of biological drugs. Adv. Drug Deliv. Rev. 2021, 171, 62–76. [Google Scholar] [PubMed]
- Al-Rubeai, M. (Ed.) Glycosylation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006; Volume 3. [Google Scholar]
- Dammen-Brower, K.; Tan, E.; Almaraz, R.T.; Du, J.; Yarema, K.J. Protocol considerations for in vitro metabolic glycoengineering of non-natural glycans. Curr. Protoc. 2023, 3, e822. [Google Scholar]
- Wang, W.; Zhang, L.; Liu, Y.; Liu, X.; Liu, X. Oriental covalent immobilization of N-glycan binding protein via N-terminal selective modification. Anal. Chim. Acta 2024, 1330, 343311. [Google Scholar]
- Gonzalez-Rodriguez, E.; Zol-Hanlon, M.; Bineva-Todd, G.; Marchesi, A.; Skehel, M.; Mahoney, K.E.; Roustan, C.; Borg, A.; Di Vagno, L.; Kjaer, S.; et al. O-linked sialoglycans modulate the proteolysis of SARS-CoV-2 spike and likely contribute to the mutational trajectory in variants of concern. ACS Cent. Sci. 2023, 9, 393–404. [Google Scholar]
- Cogez, V.; Vicogne, D.; Schulz, C.; Portier, L.; Venturi, G.; De Ruyck, J.; Decloquement, M.; Lensink, M.F.; Brysbaert, G.; Dall’Olio, F.; et al. N-Glycan on the non-consensus NXC glycosylation site impacts activity, stability, and localization of the Sda synthase B4GALNT2. Int. J. Mol. Sci. 2023, 24, 4139. [Google Scholar]
- Rodrigues, J.G.; Duarte, H.O.; Reis, C.A.; Gomes, J. Aberrant protein glycosylation in cancer: Implications in targeted therapy. Biochem. Soc. Trans. 2021, 49, 843–854. [Google Scholar]
- Mattox, D.E.; Bailey-Kellogg, C. Comprehensive analysis of lectin-glycan interactions reveals determinants of lectin specificity. PLoS Comput. Biol. 2021, 17, e1009470. [Google Scholar]
- Hu, M.; Zhang, R.; Yang, J.; Zhao, C.; Liu, W.; Huang, Y.; Lyu, H.; Xiao, S.; Guo, D.; Zhou, C.; et al. The role of N-glycosylation modification in the pathogenesis of liver cancer. Cell Death Dis. 2023, 14, 222. [Google Scholar]
- Vicente, M.M.; Leite-Gomes, E.; Pinho, S.S. Glycome dynamics in T and B cell development: Basic immunological mechanisms and clinical applications. Trends Immunol. 2023, 44, 585. [Google Scholar] [PubMed]
- Pinho, S.S.; Alves, I.; Gaifem, J.; Rabinovich, G.A. Immune regulatory networks coordinated by glycans and glycan-binding proteins in autoimmunity and infection. Cell. Mol. Immunol. 2023, 20, 1101–1113. [Google Scholar] [PubMed]
- Magalhães, A.; Duarte, H.O.; Reis, C.A. The role of O-glycosylation in human disease. Mol. Asp. Med. 2021, 79, 100964. [Google Scholar]
- Lin, B.; Qing, X.; Liao, J.; Zhuo, K. Role of protein glycosylation in host-pathogen interaction. Cells 2020, 9, 1022. [Google Scholar] [CrossRef]
- Esmail, S.; Manolson, M.F. Advances in understanding N-glycosylation structure, function, and regulation in health and disease. Eur. J. Cell Biol. 2021, 100, 151186. [Google Scholar]
- Gao, G.; Li, C.; Fan, W.; Zhang, M.; Li, X.; Chen, W.; Li, W.; Liang, R.; Li, Z.; Zhu, X. Brilliant glycans and glycosylation: Seq and ye shall find. Int. J. Biol. Macromol. 2021, 189, 279–291. [Google Scholar]
- Fernández-Ponce, C.; Geribaldi-Doldán, N.; Sánchez-Gomar, I.; Navarro Quiroz, R.; Atencio Ibarra, L.; Gomez Escorcia, L.; Fernández-Cisnal, R.; Aroca Martinez, G.; García-Cózar, F.; Navarro Quiroz, E. The role of glycosyltransferases in colorectal cancer. Int. J. Mol. Sci. 2021, 22, 5822. [Google Scholar] [CrossRef]
- Li, P.; Liu, Z. Glycan-specific molecularly imprinted polymers towards cancer diagnostics: Merits, applications, and future perspectives. Chem. Soc. Rev. 2024, 53, 1870–1891. [Google Scholar]
- Wanyama, F.M.; Blanchard, V. Glycomic-based biomarkers for ovarian cancer: Advances and challenges. Diagnostics 2021, 11, 643. [Google Scholar] [CrossRef]
- DelaCourt, A.; Mehta, A. Beyond glyco-proteomics—Understanding the role of genetics in cancer biomarkers. In Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2023; Volume 157, pp. 57–81. [Google Scholar]
- Wolters-Eisfeld, G.; Oliveira-Ferrer, L. Glycan diversity in ovarian cancer: Unraveling the immune interplay and therapeutic prospects. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2024; Volume 46, pp. 1–20. [Google Scholar]
- Lee, S.B.; Bose, S.; Ahn, S.H.; Son, B.H.; Ko, B.S.; Kim, H.J.; Chung, I.Y.; Kim, J.; Lee, W.; Ko, M.S.; et al. Breast cancer diagnosis by analysis of serum N-glycans using MALDI-TOF mass spectroscopy. PLoS ONE 2020, 15, e0231004. [Google Scholar]
- Rocamora, F.; Peralta, A.G.; Shin, S.; Sorrentino, J.; Wu, M.Y.M.; Toth, E.A.; Fuerst, T.R.; Lewis, N.E. Glycosylation shapes the efficacy and safety of diverse protein, gene and cell therapies. Biotechnol. Adv. 2023, 67, 108206. [Google Scholar] [CrossRef] [PubMed]
- Thompson, N.; Wakarchuk, W. O-glycosylation and its role in therapeutic proteins. Biosci. Rep. 2022, 42, BSR20220094. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Joshi, S.; Guttman, A.; Rathore, A.S. N-Glycosylation of monoclonal antibody therapeutics: A comprehensive review on significance and characterization. Anal. Chim. Acta 2022, 1209, 339828. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tu, H.; Chillara, A.K.; Chang, E.; Atouf, F. More comprehensive standards for monitoring glycosylation. Anal. Biochem. 2021, 612, 113896. [Google Scholar] [CrossRef]
- Kaur, H. Characterization of glycosylation in monoclonal antibodies and its importance in therapeutic antibody development. Crit. Rev. Biotechnol. 2021, 41, 300–315. [Google Scholar] [CrossRef]
- Rathore, A.S.; Guttman, A.; Shrivastava, A.; Joshi, S. Recent progress in high-throughput and automated characterization of N-glycans in monoclonal antibodies. TrAC Trends Anal. Chem. 2023, 169, 117397. [Google Scholar] [CrossRef]
- Wang, T.; Liu, L.; Voglmeir, J. mAbs N-glycosylation: Implications for biotechnology and analytics. Carbohydr. Res. 2022, 514, 108541. [Google Scholar] [CrossRef]
- Mastrangeli, R.; Satwekar, A.; Bierau, H. Innovative metrics for reporting and comparing the glycan structural profile in biotherapeutics. Molecules 2023, 28, 3304. [Google Scholar] [CrossRef]
- Sánchez-Pomales, G.; Zangmeister, R.A. Recent advances in electrochemical glycobiosensing. Int. J. Electrochem. 2011, 2011, 825790. [Google Scholar] [CrossRef]
- Smith, B.A.; Bertozzi, C.R. The clinical impact of glycobiology: Targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 2021, 20, 217–243. [Google Scholar] [CrossRef]
- Casalino, L.; Gaieb, Z.; Goldsmith, J.A.; Hjorth, C.K.; Dommer, A.C.; Harbison, A.M.; Fogarty, C.A.; Barros, E.P.; Taylor, B.C.; McLellan, J.S.; et al. Beyond shielding: The roles of glycans in the SARS-CoV-2 spike protein. ACS Cent. Sci. 2020, 6, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Unione, L.; Ardá, A.; Jiménez-Barbero, J.; Millet, O. NMR of glycoproteins: Profiling, structure, conformation and interactions. Curr. Opin. Struct. Biol. 2021, 68, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Khalikova, M.; Jireš, J.; Horáček, O.; Douša, M.; Kučera, R.; Nováková, L. What is the role of current mass spectrometry in pharmaceutical analysis? Mass Spectrom. Rev. 2024, 43, 560–609. [Google Scholar] [CrossRef] [PubMed]
- Pralow, A.; Cajic, S.; Alagesan, K.; Kolarich, D.; Rapp, E. State-of-the-art glycomics technologies in glycobiotechnology. In Advances in Glycobiotechnology; Springer International Publishing: Cham, Switzerland, 2020; pp. 379–411. [Google Scholar]
- Habazin, S.; Štambuk, J.; Šimunović, J.; Keser, T.; Razdorov, G.; Novokmet, M. Mass spectrometry-based methods for immunoglobulin GN-glycosylation analysis. In Antibody Glycosylation; Springer International Publishing: Cham, Switzerland, 2021; pp. 73–135. [Google Scholar]
- Kiely, L.J.; Hickey, R.M. Characterization and analysis of food-sourced carbohydrates. In Glycosylation: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2022; pp. 67–95. [Google Scholar]
- Perez, S.; Makshakova, O.; Angulo, J.; Bedini, E.; Bisio, A.; de Paz, J.L.; Fadda, E.; Guerrini, M.; Hricovini, M.; Hricovini, M.; et al. Glycosaminoglycans: What remains to be deciphered? Jacs Au 2023, 3, 628–656. [Google Scholar]
- Mechref, Y.; Novotny, M.V. Structural investigations of glycoconjugates at high sensitivity. Chem. Rev. 2002, 102, 321–369. [Google Scholar]
- Echeverri, D.; Orozco, J. Glycan-based electrochemical biosensors: Promising tools for the detection of infectious diseases and cancer biomarkers. Molecules 2022, 27, 8533. [Google Scholar] [CrossRef]
- Paleček, E.; Tkáč, J.; Bartosik, M.; Bertók, T.; Ostatná, V.; Paleček, J. Electrochemistry of nonconjugated proteins and glycoproteins. Toward sensors for biomedicine and glycomics. Chem. Rev. 2015, 115, 2045–2108. [Google Scholar] [CrossRef]
- Bertok, T.; Gemeiner, P.; Mikula, M.; Gemeiner, P.; Tkac, J. Ultrasensitive impedimetric lectin based biosensor for glycoproteins containing sialic acid. Microchim. Acta 2013, 180, 151–159. [Google Scholar]
- da Silva, M.L.S. Lectins as biorecognition elements in biosensors for clinical applications in cancer. Front. Nat. Prod. Chem. 2018, 4, 156–203. [Google Scholar]
- Tkac, J.; Nahalka, J.; Gemeiner, P.; Bertok, T. Chapter perspectives in glycomics and lectin engineering. In Lectins. Methods and Protocols; Springer Nature: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Janghorban, M.; Kazemi, S.; Tormon, R.; Ngaju, P.; Pandey, R. Methods and analysis of biological contaminants in the biomanufacturing industry. Chemosensors 2023, 11, 298. [Google Scholar] [CrossRef]
- Quinchia, J.; Echeverri, D.; Cruz-Pacheco, A.F.; Maldonado, M.E.; Orozco, J. Electrochemical biosensors for determination of colorectal tumor biomarkers. Micromachines 2020, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.L.; Nguyen, S.H.; Tran, M.T. A comprehensive review of transduction methods of lectin-based biosensors in biomedical applications. Heliyon 2024, 10, e38371. [Google Scholar] [PubMed]
- Klukova, L.; Bertok, T.; Kasák, P.; Tkac, J. Nanoscale-controlled architecture for the development of ultrasensitive lectin biosensors applicable in glycomics. Anal. Methods 2014, 6, 4922–4931. [Google Scholar] [PubMed]
- Li, J.; Liu, Y.; Kim, E.; March, J.C.; Bentley, W.E.; Payne, G.F. Electrochemical reverse engineering: A systems-level tool to probe the redox-based molecular communication of biology. Free Radic. Biol. Med. 2017, 105, 110–131. [Google Scholar]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical biosensors-sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Ratheesh, A.; Elias, L.; Aboobakar Shibli, S.M. Tuning of electrode surface for enhanced bacterial adhesion and reactions: A review on recent approaches. ACS Appl. Bio Mater. 2021, 4, 5809–5838. [Google Scholar]
- Drogui, P.; Blais, J.F.; Mercier, G. Review of electrochemical technologies for environmental applications. Recent Pat. Eng. 2007, 1, 257–272. [Google Scholar]
- Sandhyarani, N. Surface modification methods for electrochemical biosensors. In Electrochemical Biosensors; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–75. [Google Scholar]
- D’Orazio, P.; Meyerhoff, M.E. Electrochemistry and Chemical Sensors; Elsevier: St. Louis, MO, USA, 2014; p. 151. [Google Scholar]
- Ujjain, S.K.; Das, A.; Srivastava, G.; Ahuja, P.; Roy, M.; Arya, A.; Bhargava, K.; Sethy, N.; Singh, S.K.; Sharma, R.K.; et al. Nanoceria based electrochemical sensor for hydrogen peroxide detection. Biointerphases 2014, 9, 031011. [Google Scholar]
- Ujjain, S.K.; Ahuja, P.; Sharma, R.K. Facile preparation of graphene nanoribbon/cobalt coordination polymer nanohybrid for non-enzymatic H2O2 sensing by dual transduction: Electrochemical and fluorescence. J. Mater. Chem. B 2015, 3, 7614–7622. [Google Scholar]
- Ahuja, P.; Ujjain, S.K.; Kanojia, R. MnOx/C nanocomposite: An insight on high-performance supercapacitor and non-enzymatic hydrogen peroxide detection. Appl. Surf. Sci. 2017, 404, 197–205. [Google Scholar]
- Zhang, J.J.; Cheng, F.F.; Zheng, T.T.; Zhu, J.J. Design and Implementation of Electrochemical Cytosensor for Evaluation of Cell Surface Carbohydrate and Glycoprotein. Anal. Chem. 2010, 82, 3547–3555. [Google Scholar]
- Xue, Y.; Ding, L.; Lei, J.; Ju, H. A simple electrochemical lectin-probe for in situ homogeneous cytosensing and facile evaluation of cell surface glycan. Biosens. Bioelectron. 2010, 26, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Molazemhosseini, A.; Magagnin, L.; Vena, P.; Liu, C.C. Single-use disposable electrochemical label-free immunosensor for detection of glycated hemoglobin (HbA1c) using differential pulse voltammetry (DPV). Sensors 2016, 16, 1024. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Carrascosa, L.G.; Sina, A.A.I.; Shiddiky, M.J.A.; Hill, M.M.; Trau, M. Electrochemical detection of protein glycosylation using lecting and protein-gold affinity interactions. Analyst 2016, 141, 2356. [Google Scholar] [CrossRef]
- Zhang, Y.; Figueroa-Miranda, G.; Zafiu, C.; Willbold, D.; Offenhäusser, A.; Mayer, D. Amperometric aptasensor for amyloid-β oligomer detection by optimized stem-loop structures with an adjustable detection range. ACS Sens. 2019, 4, 3042–3050. [Google Scholar] [CrossRef]
- He, P.; Oncescu, V.; Lee, S.; Choi, I.; Erickson, D. Label-free electrochemical monitoring of vasopressin in aptamer-based microfluidic biosensors. Anal. Chim. Acta 2013, 759, 74–80. [Google Scholar] [CrossRef]
- Sierra, T.; Henry, C.S.; Crevillén, A.G.; Escarpa, A. Disposable Passive Electrochemical Microfluidic Device for Diagnosis of Congenital Disorders of Glycosylation. Anal. Sens. 2022, 2, e202100038. [Google Scholar] [CrossRef]
- Ahn, K.S.; Kim, B.K.; Lee, W.Y. Cyclic Voltammetric studies of carbohydrate-protein interactions on gold surface. Electrochem. Commun. 2015, 58, 69–72. [Google Scholar] [CrossRef]
- Trefulka, M.; Paleček, E. Direct chemical modification and voltammetric detection of glycans in glycoproteins. Electrochem. Commun. 2014, 48, 52–55. [Google Scholar] [CrossRef]
- Trefulka, M.; Paleček, E. Distinguishing glycan isomers by voltammetry. Modification of 2, 3-sialyllactose and 2, 6-sialyllactose by osmium (VI) complexes. Electrochem. Commun. 2017, 85, 19–22. [Google Scholar] [CrossRef]
- Paleček, E.; Dorčák, V. Label-free electrochemical analysis of biomacromolecules. Appl. Mater. Today 2017, 9, 434–450. [Google Scholar] [CrossRef]
- Alshanski, I.; Sukhran, Y.; Mervinetsky, E.; Unverzagt, C.; Yitzchaik, S.; Hurevich, M. Electrochemical biosensing platform based on complex biantennary N-glycan for detecting enzymatic sialylation processes. Biosens. Bioelectron. 2021, 172, 112762. [Google Scholar]
- Khorshed, A.A.; Savchenko, O.; Liu, J.; Shoute, L.; Zeng, J.; Ren, S.; Gu, J.; Jha, N.; Yang, Z.; Wang, J.; et al. Development of an impedance-based biosensor for determination of IgG galactosylation levels. Biosens. Bioelectron. 2024, 245, 115793. [Google Scholar] [CrossRef] [PubMed]
- Motabar, D.; Li, J.; Wang, S.; Tsao, C.Y.; Tong, X.; Wang, L.X.; Payne, G.F.; Bentley, W.E. Simple, rapidly electroassembled thiolated PEG-based sensor interfaces enable rapid interrogation of antibody titer and glycosylation. Biotechnol. Bioeng. 2021, 118, 2744–2758. [Google Scholar]
- Neupane, D.; Stine, K.J. Electrochemical Sandwich Assays for Biomarkers Incorporating Aptamers, Antibodies and Nanomaterials for Detection of Specific Protein Biomarkers. Appl. Sci. 2021, 11, 7087. [Google Scholar] [CrossRef]
- Tonelli, D.; Scavetta, E.; Gualandi, I. Electrochemical Deposition of Nanomaterials for Electrochemical Sensing. Sensors 2019, 19, 1186. [Google Scholar] [CrossRef]
- Rabbani, G.; Ahmad, A.; Zamzami, M.A.; Baothman, O.A.; Hosawi, S.A.; Hisham Altayeb, H.; Muhammad Shahid Nadeem, M.S.; Ahmad, V. Fabrication of an affordable and sensitive corticosteroid-binding globulin immunosensor based on electrodeposited gold nanoparticles modified glassy carbon electrode. Bioelectrochemistry 2024, 157, 108671. [Google Scholar] [CrossRef]
- Seferos, D.S.; Lai, R.Y.; Plaxco, K.W.; Bazan, G.C. α,ω-Dithiol Oligo(phenylene vinylene)s for the Preparation of High-Quality π-Conjugated Self-Assembled Monolayers and Nanoparticle- Functionalized Electrodes. Adv. Funct. Mater. 2006, 16, 2387–2392. [Google Scholar]
- Ferrari, A.G.M.; Rowley-Neale, S.J.; Banks, C.E. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open 2021, 3, 100032. [Google Scholar]
- Acquino, A.K.; Manzer, Z.A.; Daniel, S.; DeLisa, M.P. Glycosylation-on-a-Chip: A Flow-Based Microfluidic System for Cell-Free Glycoprotein Biosynthesis. Front. Mol. Biosci. 2021, 8, 782905. [Google Scholar]
- Alshanski, I.; Shitrit, A.; Sukhran, Y.; Unverzagt, C.; Hurevich, M.; Yitzchaik, S. Effect of interfacial properties on impedimetric biosensing of the sialylation process with a biantennary N-glycan-based monolayer. Langmuir 2022, 38, 849–855. [Google Scholar] [PubMed]
- Azimzadeh, M.; Khashayar, P.; Amereh, M.; Tasnim, N.; Hoorfar, M.; Akbari, M. Microfluidic-based oxygen (O2) sensors for on-chip monitoring of cell, tissue and organ metabolism. Biosensors 2021, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Gargalo, C.L.; Lopez, P.C.; Hasanzadeh, A.; Udugama, I.A.; Gernaey, K.V. On-line monitoring of process parameters during fermentation. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 117–164. [Google Scholar]
- Ivarsson, M.; Villiger, T.K.; Morbidelli, M.; Soos, M. Evaluating the impact of cell culture process parameters on monoclonal antibody N-glycosylation. J. Biotechnol. 2014, 188, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Dekker, L.; Polizzi, K.M. Sense and sensitivity in bioprocessing—Detecting cellular metabolites with biosensors. Curr. Opin. Chem. Biol. 2017, 40, 31–36. [Google Scholar]
- Ashrafi, A.M.; Mukherjee, A.; Saadati, A.; Matysik, F.M.; Richtera, L.; Adam, V. Enhancing the substrate selectivity of enzyme mimetics in biosensing and bioassay: Novel approaches. In Advances in Colloid and Interface Science; Elsevier: Amsterdam, The Netherlands, 2024; p. 103233. [Google Scholar]
- Motabar, D.; Li, J.; Payne, G.F.; Bentley, W.E. Mediated electrochemistry for redox-based biological targeting: Entangling sensing and actuation for maximizing information transfer. Curr. Opin. Biotechnol. 2021, 71, 137–144. [Google Scholar]
- Wang, R.; Jiang, Q.H.; Wang, H.X.; Zhang, X.W.; Yan, N. Electrochemically Mediated S-Glycosylation of 1-Thiosugars with Xanthene Derivatives. Org. Lett. 2023, 25, 4252–4257. [Google Scholar]
- Lv, J.; Wu, D.; Ma, Y.; Zhang, X.; Xu, W.; Wang, M.; Chen, S.; Hu, Q.; Han, D.; Niu, L. Glycan-Evocated Metallization for Amplification-Free Electrochemical Detection of Glycoproteins at Low Concentration Levels. Anal. Chem. 2024, 96, 17739–17745. [Google Scholar]
- Sirén, H. Research of saccharides and related biocomplexes: A review with recent techniques and applications. J. Sep. Sci. 2024, 47, 2300668. [Google Scholar]
- Ma, X.; Li, M.; Tong, P.; Zhao, C.; Li, J.; Xu, G. A strategy for construction of highly sensitive glycosyl imprinted electrochemical sensor based on sandwich-like multiple signal enhancement and determination of neural cell adhesion molecule. Biosens. Bioelectron. 2020, 156, 112150. [Google Scholar]
- Magar, H.S.; Hassan, R.Y.; Mulchandani, A. Electrochemical impedance spectroscopy (EIS): Principles, construction, and biosensing applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Babazad, M.A.; Foroozandeh, A.; Abdouss, M.; SalarAmoli, H.; Babazad, R.A.; Hasanzadeh, M. Recent progress and challenges in biosensing of carcinoembryonic antigen. In TrAC Trends in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2024; p. 117964. [Google Scholar]
- Ganguly, A.; Lin, K.C.; Muthukumar, S.; Nagaraj, V.J.; Prasad, S. Label-free protein glycosylation analysis using NanoMonitor—An ultrasensitive electrochemical biosensor. Curr. Protoc. 2021, 1, e150. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Liang, Y.; Hu, Q.; Liang, Z.; Feng, W.; Tian, Y.; Li, S.; Ye, Z.; Hong, M.; Han, D.; et al. Amplification-free ratiometric electrochemical aptasensor for point-of-care detection of therapeutic monoclonal antibodies. Anal. Chem. 2023, 95, 14094–14100. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, I.; Sharma, N.; Vasilescu, A.; Iancu, M.; Badea, G.; Boukherroub, R.; Ogale, S.; Szunerits, S. Electrochemical aptamer-based biosensors for the detection of cardiac biomarkers. ACS Omega 2018, 3, 12010–12018. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.; Wan, S.; Cansiz, S.; Cui, C.; Liu, Y.; Cai, R.; Hong, C.; Teng, I.T.; Shi, M.; et al. Aptasensor with expanded nucleotide using DNA nanotetrahedra for electrochemical detection of cancerous exosomes. ACS Nano 2017, 11, 3943–3949. [Google Scholar] [CrossRef]
- Feng, K.; Liao, F.; Yang, M. Analysis of glycan expression on cell surfaces by using a glassy carbon electrode modified with MnO2 nanosheets and DNA-generated electrochemical current. Microchim. Acta 2020, 187, 148. [Google Scholar] [CrossRef]
- Losada-Garcia, N.; Garcia-Sanz, C.; Andreu, A.; Velasco-Torrijos, T.; Palomo, J.M. Glyconanomaterials for human virus detection and inhibition. Nanomaterials 2021, 11, 1684. [Google Scholar] [CrossRef]
- Anilkumar, T.R. Carbon nanotubes and their biotechnological and biomedical applications. In Carbon Composites; Apple Academic Press: Palm Bay, FL, USA, 2023; pp. 135–161. [Google Scholar]
- Chia, C.H.; Talele, S.G.; Abraham, A.R.; Haghi, A.K. (Eds.) Carbon Nanotubes for Biomedical Applications and Healthcare; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Rodríguez, M.C.; Dalmasso, P.; Rubianes, M.D.; Dragnic, S.B.; Gallay, P.; Mujica, M.L.; Montemerlo, A.; Perrachione, F.; Tamborelli, L.A.; Vaschetti, V.; et al. Carbon nanomaterials-based electrochemical cancer biomarkers biosensors. In The Detection of Biomarkers; Academic Press: Cambridge, MA, USA, 2022; pp. 225–253. [Google Scholar]
- Wu, R.; Feng, Z.; Zhang, J.; Jiang, L.; Zhu, J.J. Quantum dots for electrochemical cytosensing. TrAC Trends Anal. Chem. 2022, 148, 116531. [Google Scholar] [CrossRef]
- Kour, R.; Arya, S.; Young, S.J.; Gupta, V.; Bandhoria, P.; Khosla, A. Recent advances in carbon nanomaterials as electrochemical biosensors. J. Electrochem. Soc. 2020, 167, 037555. [Google Scholar] [CrossRef]
- Wani, A.K.; Akhtar, N.; Katoch, V.; Shukla, S.; Kadam, U.S.; Hong, J.C. Advancing biological investigations using portable sensors for detection of sensitive samples. Heliyon 2023, 9, e22679. [Google Scholar]

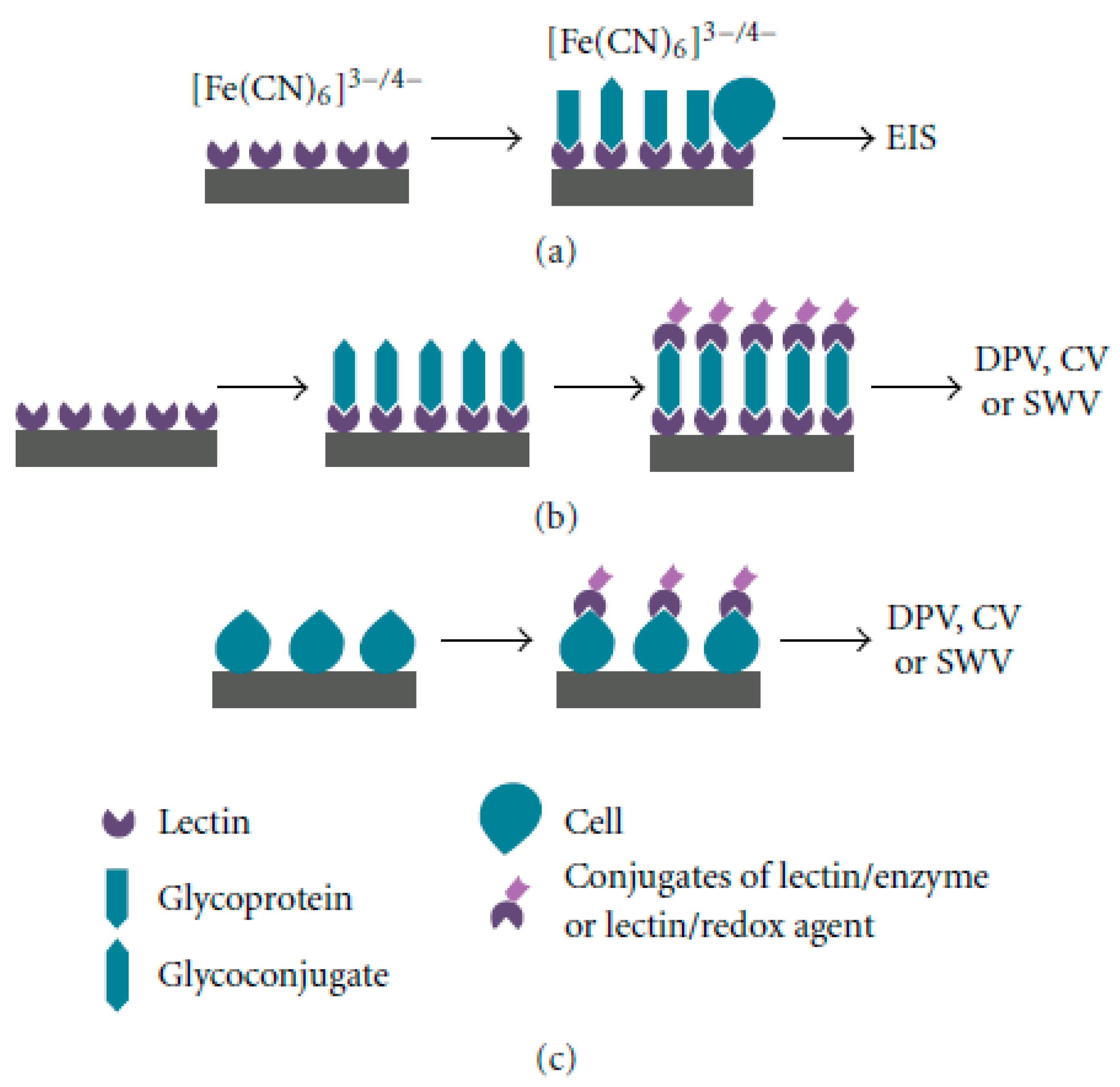

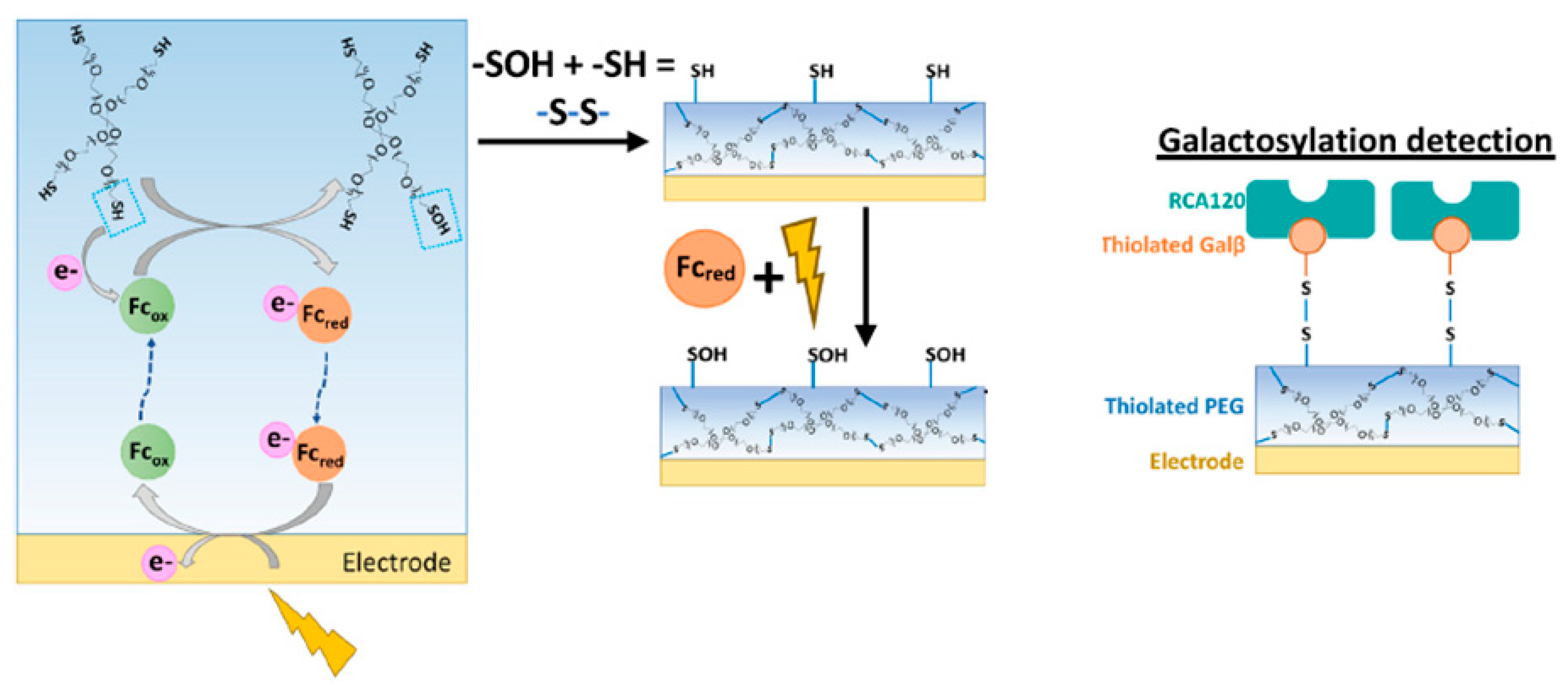
| Ref No | Fabrication Method | Sensitivity | Sensor Type | Application Area | Additional Parameters |
|---|---|---|---|---|---|
| [81] | Microfluidic-based design | 1.4 µM (O2) | Oxygen sensor | Cell, tissue, and organ metabolism | Real-time monitoring |
| [85] | Enzyme-mimetic biosensors | - | Biosensor | Substrate selectivity | Enhanced bioassay detection |
| [87] | Electrochemical dehydrogenative cross-coupling of benzylic C–H bonds with 1-thiosugars | Yield: up to 91% | Electrochemical sensor | Glycosylation | No oxidant used, mild reaction conditions, environmentally benign, room temperature |
| [90] | Glycosyl imprinted electrochemical sensor | 0.1 nM | Electrochemical sensor | Neural cell adhesion | Signal enhancement for glycosylation detection |
| [93] | Label-free glycosylation analysis | 0.1 pM | Electrochemical biosensor | Glycosylation analysis | NanoMonitor technology |
| [94] | Ratiometric electrochemical aptasensor | 0.5 nM | Aptasensor | Therapeutic monoclonal antibodies | Point-of-care detection |
| [95] | Electrochemical aptamer-based sensor | 0.05 nM | Cardiac biomarkers | High sensitivity for biomarker detection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahuja, P.; Singh, M.; Ujjain, S.K. Advancements in Electrochemical Biosensors for Comprehensive Glycosylation Assessment of Biotherapeutics. Sensors 2025, 25, 2064. https://doi.org/10.3390/s25072064
Ahuja P, Singh M, Ujjain SK. Advancements in Electrochemical Biosensors for Comprehensive Glycosylation Assessment of Biotherapeutics. Sensors. 2025; 25(7):2064. https://doi.org/10.3390/s25072064
Chicago/Turabian StyleAhuja, Preety, Manpreet Singh, and Sanjeev Kumar Ujjain. 2025. "Advancements in Electrochemical Biosensors for Comprehensive Glycosylation Assessment of Biotherapeutics" Sensors 25, no. 7: 2064. https://doi.org/10.3390/s25072064
APA StyleAhuja, P., Singh, M., & Ujjain, S. K. (2025). Advancements in Electrochemical Biosensors for Comprehensive Glycosylation Assessment of Biotherapeutics. Sensors, 25(7), 2064. https://doi.org/10.3390/s25072064







