Dynamic Interference Testing—Unexpected Results Obtained with the Abbott Libre 2 and Dexcom G6 Continuous Glucose Monitoring Devices †
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Camerlingo, N.; Vettoretti, M.; Sparacino, G.; Facchinetti, A.; Mader, J.K.; Choudhary, P.; Del Favero, S.; Hypo-RESOLVE Consortium. Choosing the duration of continuous glucose monitoring for reliable assessment of time in range: A new analytical approach to overcome the limitations of correlation-based methods. Diabet Med. 2022, 39, e14758. [Google Scholar]
- Advani, A. Positioning time in range in diabetes management. Diabetologia 2020, 63, 242–252. [Google Scholar] [PubMed]
- Maiorino, M.I.; Signoriello, S.; Maio, A.; Chiodini, P.; Bellastella, G.; Scappaticcio, L.; Longo, M.; Giugliano, D.; Esposito, K. Effects of Continuous Glucose Monitoring on Metrics of Glycemic Control in Diabetes: A Systematic Review With Meta-analysis of Randomized Controlled Trials. Diabetes Care 2020, 43, 1146–1156. [Google Scholar]
- Beck, R.W.; Riddlesworth, T.; Ruedy, K.; Ahmann, A.; Bergenstal, R.; Haller, S.; Kollman, C.; Kruger, D.; McGill, J.B.; Polonsky, W.; et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: The DIAMOND randomized clinical trial. JAMA 2017, 317, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, L.; Guido Freckmann, G.; Gabriele Faber-Heinemann, G.; Faber-Heinemann, G.; Guerra, S.; Waldenmaier, D.; Hermanns, N. Benefits of continuous glucose monitoring use in adults with type 1 diabetes and impaired hypoglycaemia awareness and/or severe hypoglycaemia treated with multiple daily insulin injections: Results of the multicentre, randomised controlled HypoDE study. Lancet 2018, 391, 1367–1377. [Google Scholar]
- Bolinder, J.; Antuna, R.; Geelhoed-Duijvestijn, P.; Kröger, J.; Weitgasser, R. Novel glucose-sensing technology and hypoglycemia in type 1 diabetes: A multicentre, non-masked, randomised controlled trial. Lancet 2016, 388, 2254–2263. [Google Scholar]
- Beck, R.W.; Riddlesworth, T.D.; Ruedy, K.; Ahmann, A.; Haller, S.; Kruger, D.; McGill, J.B.; Polonsky, W.; Price, D.; Aronoff, S.; et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: A randomized trial. Ann. Intern. Med. 2017, 167, 365–374. [Google Scholar] [PubMed]
- Lind, M.; Polonsky, W.; Hirsch, I.B. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: The GOLD randomized clinical trial. JAMA 2017, 317, 379–387. [Google Scholar]
- Aleppo, G.; Ruedy, K.J.; Riddlesworth, T.D.; Kruger, D.F.; Peters, A.L.; Hirsch, I.; Bergenstal, R.M.; Toschi, E.; Ahmann, A.J.; Shah, V.N.; et al. REPLACE-BG: A randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care 2017, 40, 538–545. [Google Scholar] [CrossRef]
- Charleer, S.; De Block, C.; Van Huffel, L.; Broos, B.; Fieuws, S.; Nobels, F.; Mathieu, C.; Gillard, P. Quality of Life and Glucose Control After 1 Year of Nationwide Reimbursement of Intermittently Scanned Continuous Glucose Monitoring in Adults Living With Type 1 Diabetes (FUTURE): A Prospective Observational Real-World Cohort Study. Diabetes Care 2020, 43, 389–397. [Google Scholar]
- Charleer, S.; Mathieu, C.; Nobels, F.; De Block, C.; Radermecker, R.P.; Hermans, M.P.; Taes, Y.; Vercammen, C.; T’Sjoen, G.; Crenier, L.; et al. Effect of Continuous Glucose Monitoring on Glycemic Control, Acute Admissions, and Quality of Life: A Real-World Study. Clin Endocrinol Metab. 2018, 103, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Fokkert, M.; van Dijk, P.; Edens, M.; Barents, E.; Mollema, J.; Slingerland, R.; Gans, R.; Bilo, H. Improved well-being and decreased disease burden after 1-year use of flash glucose monitoring (FLARE-NL4). BMJ Open Diabetes Res. Care 2019, 7, e000809. [Google Scholar] [PubMed]
- Almurashi, A.M.; Rodriguez, E.; Garg, S.K. Emerging Diabetes Technologies: Continuous Glucose Monitors/Artificial Pancreases. J. Indian Inst. Sci. 2023, 28, 1–26. [Google Scholar]
- Klonoff, D.C.; Gabbay, M.; Moon, S.J.; Wilmot, E.G. Importance of FDA-Integrated Continuous Glucose Monitors to Ensure Accuracy of Continuous Glucose Monitoring. J. Diabetes Sci. Technol. online first. 2024. [Google Scholar]
- Shapiro, A.R. Nonadjunctive Use of Continuous Glucose Monitors for Insulin Dosing: Is It Safe? J. Diabetes Sci. Technol. 2017, 11, 833–838. [Google Scholar]
- Shapiro, A.R. The Safety of Nonadjunctive Use of Continuous Glucose Monitors for Insulin Dosing: Still Not Resolved. J. Diabetes Sci. Technol. 2017, 11, 856–857. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.P.; Szuts, E.Z.; Malomo, K.J.; Anagnostopoulos, A.; Rao, S. Accuracy of alternate site testing--comparing arm and finger blood glucose results in glucose dynamic states. Diabetes Technol Ther. 2002, 4, 87–89. [Google Scholar] [CrossRef]
- Koschinsky, T.; Jungheim, K.; Heinemann, L. Glucose sensors and the alternate site testing-like phenomenon: Relationship between rapid blood glucose changes and glucose sensor signals. Diabetes Technol Ther. 2003, 5, 829–842. [Google Scholar] [CrossRef]
- Forst, T.; Pfützner, A.; Kunt, T.; Pohlmann, T.; Schenk, U.; Bauersachs, R.; Küstner, E.; Beyer, J. Skin microcirculation in patients with type I diabetes with and without neuropathy after neurovascular stimulation. Clin. Sci. 1998, 94, 255–261. [Google Scholar] [CrossRef]
- Ginsberg, B.H. Factors affecting blood glucose monitoring: Sources of errors in measurement. J. Diabetes Sci. Technol. 2009, 3, 903–913. [Google Scholar]
- Pfützner, A.; Schipper, C.; Ramljak, S.; Flacke, F.; Sieber, J.; Forst, T.; Musholt, P.B. Evaluation of the effects of insufficient blood volume samples on the performance of blood glucose self-test meters. J. Diabetes Sci. Technol. 2013, 7, 1522–1529. [Google Scholar] [CrossRef]
- Demircik, F.; Ramljak, S.; Hermanns, I.; Pfützner, A.; Pfützner, A. Evaluation of hematocrit interference with MyStar extra and seven competitive devices. J. Diabetes Sci. Technol. 2015, 9, 262–267. [Google Scholar] [PubMed]
- Hoss, U.; Budiman, E.S. Factory-Calibrated Continuous Glucose Sensors: The Science Behind the Technology. Diabetes Technol Ther. 2017, 19 (Suppl. S2), S44–S50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joubert, M.; Reznik, Y. Personal continuous glucose monitoring (CGM) in diabetes management: Review of the literature and implementation for practical use. Diabetes Res Clin Pract. 2012, 96, 294–305. [Google Scholar] [PubMed]
- Breton, M.D.; Kovatchev, B.P. Impact of blood glucose self-monitoring errors on glucose variability, risk for hypoglycemia, and average glucose control in type 1 diabetes: An in silico study. J. Diabetes Sci. Technol. 2010, 4, 562–570. [Google Scholar]
- Heinemann, L. Interferences With CGM Systems: Practical Relevance? J. Diabetes Sci Technol 2022, 16, 271–274. [Google Scholar]
- Boock, R.; Rixman Swinney, M.; Zhang, H.; Estes, M.; Lawrence, K. Polymer Membranes for Continuous Analyte Sensors. U.S. Patent 12/413,166, 2016. [Google Scholar]
- Pfützner, A.; Jensch, H.; Cardinal, C.; Srikanthamoorthy, G.; Riehn, E.; Thomé, N. Laboratory Protocol and Pilot Results for Dynamic Interference Testing of Continuous Glucose Monitoring Sensors. J. Diabetes Sci. Technol. 2024, 18, 59–65. [Google Scholar]
- Thomé, N.; Jensch, H.; Srikanthamoorthy, G.; Kuhl, C.; Weingärtner, L.; Grady, M.; Setford, S.; Holt, E.; Pfützner, A. Dynamic Interference Testing Results with the Dexcom G6 Continuous Glucose Monitoring Device. Diabetes 2023, 72 (Suppl. S1), 938p. [Google Scholar]
- Jensch, H.; Thomé, N.; Srikanthamoorthy, G.; Weingärtner, L.; Setford, S.; Grady, M.; Holt, E.; Pfützner, A. Dynamic Interference Testing Results with the Libre 2 Continuous Glucose Monitoring Device. Diabetes 2023, 72 (Suppl. S1), 939p. [Google Scholar]
- Collier, B.B.; McShane, M.J. Enzymatic Glucose Sensor Compensation for Variations in Ambient Oxygen Concentration. Proc. SPIE Int. Soc. Opt. Eng. 2015, 8591, 859104. [Google Scholar]
- Kausaite-Minkstimiene, A.; Kaminskas, A.; Gayda, G.; Ramanaviciene, A. Towards a Self-Powered Amperometric Glucose Biosensor Based on a Single-Enzyme Biofuel Cell. Biosensors 2024, 14, 138. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Interference Testing in Clinical Chemistry; Approved Guideline—Third Edition, CLSI document EP7-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Supplemental Tables for Interference Testing in Clinical Chemistry (CLSI Supplement EP37), 1st ed.; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Krouwer, J.S. Interference testing: Why following standards is not always the right thing to do. J. Diabetes Sci. Technol. 2012, 6, 1182–1184. [Google Scholar]
- Food and Drug Administration. Self-Monitoring Blood Glucose Test Systems for Over-the-Counter Use: Guidance for Industry and Food and Drug Administration Staff. Available online: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM380327.pdf (accessed on 21 November 2018).
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar]
- Graumlich, J.F.; Ludden, T.M.; Conry-Cantilena, C.; Cantilena, L.R.; Jr Wang, Y.; Levine, M. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm Res. 1997, 14, 1133–1139. [Google Scholar] [PubMed]
- Maahs, D.M.; DeSalvo, D.; Pyle, L.; Ly, T.; Messer, L.; Clinton, P.; Westfall, E.; Wadwa, R.P.; Buckingham, B. Effect of acetaminophen on CGM glucose in an outpatient setting. Diabetes Care 2015, 38, e158–e159. [Google Scholar] [PubMed]
- Basu, A.; Veettil, S.; Dyer, R.; Peyser, T.; Basu, R. Direct Evidence of Acetaminophen Interference with Subcutaneous Glucose Sensing in Humans: A Pilot Study. Diabetes Technol. Ther. 2018, 18 (Suppl. S2), S243–S247. [Google Scholar]
- Basu, A.; Slama, M.Q.; Nicholson, W.T.; Langman, L.; Peyser, T.; Carter, R.; Basu, R. Continuous Glucose Monitor Interference with Commonly Prescribed Medications: APilot Study. J. Diabetes Technol. Ther. 2017, 11, 936–941. [Google Scholar]
- Calhoun, P.; Johnson, T.K.; Hughes, J.; Price, D.; Balo, A.K. Resistance to acetaminophen interference in a novel continuous glucose monitoring system. J. Diabetes Sci. Technol. 2018, 12, 393–396. [Google Scholar]
- Denham, D. Effect of Repeated Doses of Acetaminophen on a Continuous Glucose Monitoring System with Permselective Membrane. J. Diabetes Sci. Technol. 2021, 15, 517–518. [Google Scholar]
- Dexcom Continuous Glucose Monitoring. Interfering Substances and Risks. Available online: https://www.dexcom.com/interference (accessed on 10 April 2021).
- Tellez, S.E.; Hornung, L.N.; Courter, J.D.; Abu-El-Haija, M.; Nathan, J.D.; Lawson, S.A.; Elder, D.A. Inaccurate Glucose Sensor Values After Hydroxyurea Administration. Diabetes Technol. Ther. 2021, 23, 443–451. [Google Scholar]
- Shi, T.; Li, D.; Li, G.; Zhang, Y.; Xu, K.; Lu, L. Modeling and Measurement of Correlation between Blood and Interstitial Glucose Changes. J. Diabetes Res. 2016, 2016, 4596316, Erratum in J. Diabetes Res. 2017, 2017, 3164027. [Google Scholar]
- Galindo, R.J.; Umpierrez, G.E.; Rushakoff, R.J.; Basu, A.; Lohnes, S.; Nichols, J.H.; Spanakis, E.K.; Espinoza, J.; Palermo, N.E.; Awadjie, D.G.; et al. Continuous Glucose Monitors and Automated Insulin Dosing Systems in the Hospital Consensus Guideline. J. Diabetes Sci. Technol. 2020, 14, 1035–1064. [Google Scholar] [CrossRef] [PubMed]

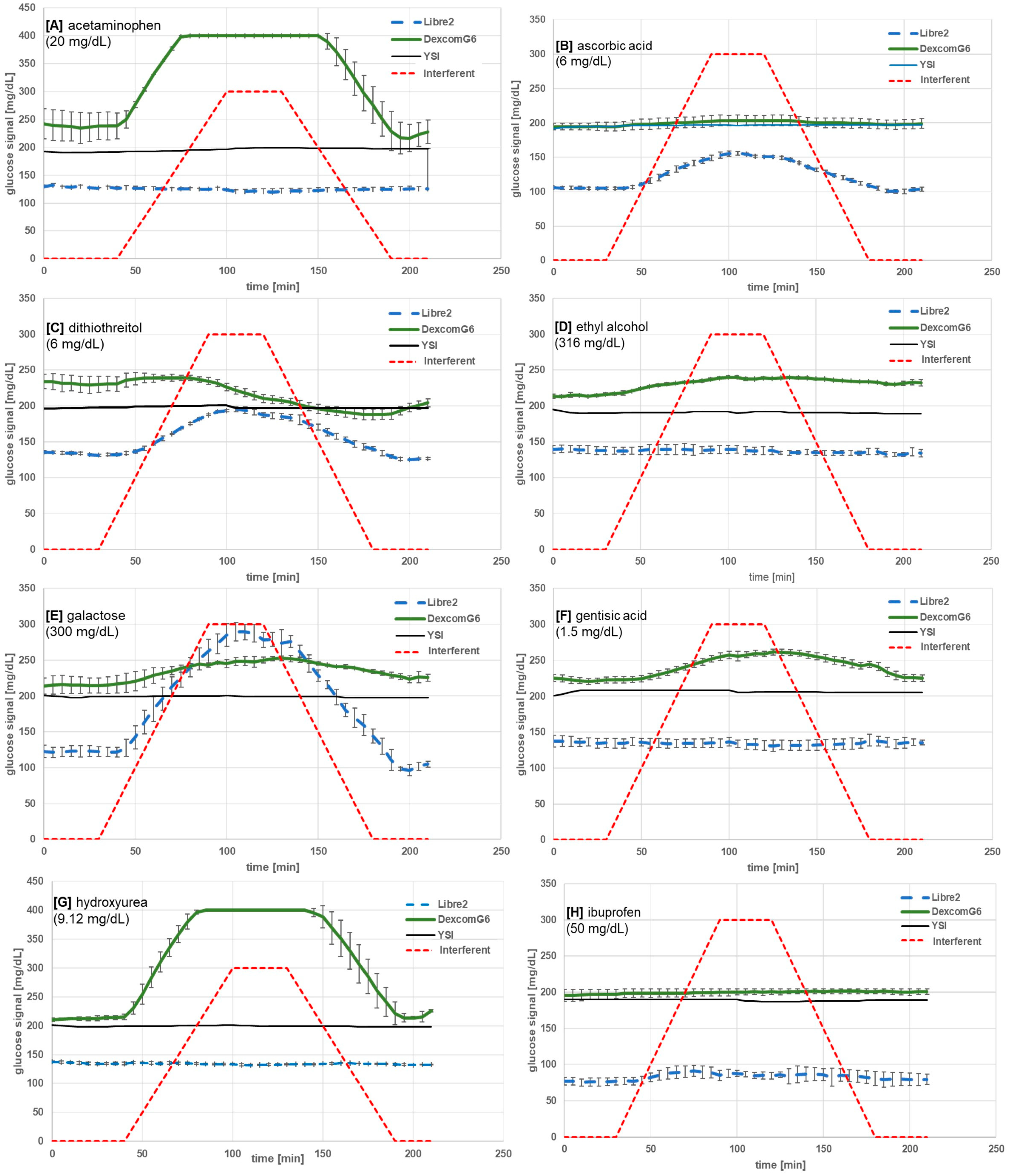

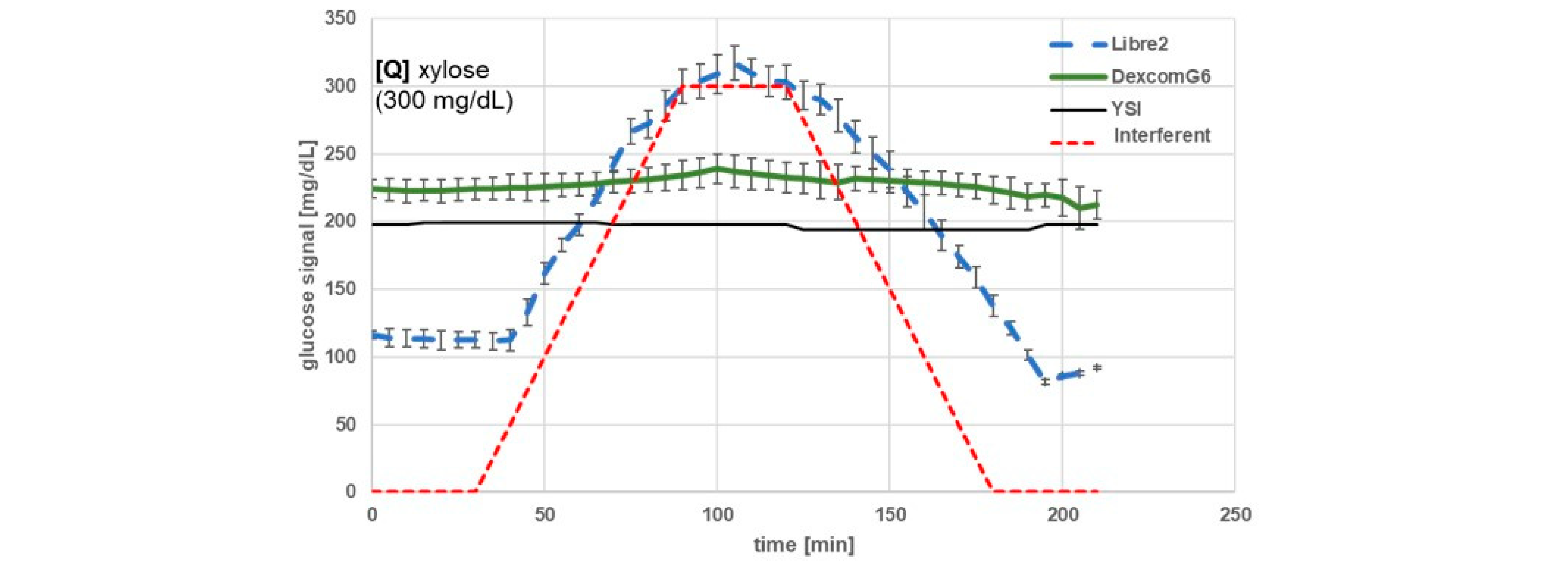
| Substance | Maximum Concentration Tested (mg/dL) | BOB (%) 1 | ICC [mg/dL] | Mean Glucose Change (SD) at 120 min vs. Baseline (mg/dL) 2 | Type of Substance | |||
|---|---|---|---|---|---|---|---|---|
| L2 | G6 | L2 | G6 | L2 | G6 | |||
| Acetaminophen | 20 | −7 | >+100 | - | 5.9 | −9 (2) | Max 3 | drug |
| Ascorbic acid | 6 | +48 | +6 | 2.5 | - | 45 (1) | 9 (3) | nutrient |
| Dithiothreitol | 6 | +46 | −18 4 fouling | 3 | 6 | 52 (3) | −24 (9) | drug |
| Ethyl alcohol | 316 | +1 | +12 | - | 300 | −2 (2) | 25 (2) | drug, nutrient |
| Galactose | 300 | >+100 | +17 | 90 | 212 | 156 (2) | 36 (16) | nutrient |
| Gentisic acid | 1.5 | 0 | +18 4 fouling | - | 1.0 | −6 (1) | 34 (4) | drug |
| Hydroxyurea | 9.12 | −3 | >+100 | - | 0.8 | −5 (0) | Max 3 | drug |
| Ibuprofen | 50 | +14 | +2 | 20 | - | 8 (2) | 5 (5) | drug |
| Icodextrin | 224 | +10 | −2 | 224 | - | 10 (2) | −4 (1) | drug |
| l-Cysteine | 5 | +4 | −25 4 fouling | - | 2.5 | 3 (2) | −54 (18) | nutrient |
| l-Dopa | 0.75 | +7 | +11 | - | 0.5 | 3 (1) | 17 (21) | drug |
| Mannose | 300 | >+100 | +20 | 77 | 155 | 163 (5) | 49 (17) | nutrient |
| Mesalazine | 0.136 | 0 3 fouling | 0 4 fouling | - | - | drug | ||
| Methyldopa | 2 | +16 | +7 | 2 | - | 15 (1) | 11 (2) | drug |
| N-acetyl-cysteine | 55.4 | +11 | +18 | 55 | 9.2 | 17 (7) | 38 (21) | drug |
| Red wine | 3.8 mL/dL | +12 4 | −5 5 | 3.5 | - | 177 (16) | 238 (6) | nutrient |
| Uric acid | 23.5 | +2 | +33 | - | 5.9 | 3 (8) | 66 (10) | endogenous |
| Xylose | 300 | >+100 | +7 | 65 | - | 188 (9) | 8 (5) | nutrient |
| Substance | Maximum Concentration Tested (mg/dL) | BOB (%) 1 | Mean Glucose Change (SD) at 120 min vs. Baseline (mg/dL) 2 | Type of Substance | ||
|---|---|---|---|---|---|---|
| L2 | G6 | L2 | G6 | |||
| Alpha-tocopherol | 2 | +4% | +4% | +5 (2) | +7 (1) | nutrient |
| Amoxicillin | 5 | +4% | +2% | +7 (1) | +4 (0) | drug |
| Atenolol | 0.09 | +2% | +3% | +4 (1) | +6 (1) | drug |
| Atorvastatin | 0.012 | +1% | −1% | +3 (5) | −2 (2) | drug |
| Bilirubin conjugated | 50 | +1% | +7% | −9 (2) | +7 (1) | endogenous |
| Bilirubin unconjugated | 40 | +1% | +2% | −1 (5) | −3 (2) | endogenous |
| Budesonide | 230 ng/dL | +9% | +2% | +5 (-) | +2 (1) | drug |
| Caffeine | 5 | +7% | +3% | +11 (1) | +8 (2) | nutrient |
| Cholesterol | 500 | +1% | +2% | +1 (1) | +1 (1) | endogenous |
| Codeine | 0.015 | +6% | +1% | +8 (1) | +3 (1) | drug |
| Creatinine | 15 | +2% | +1% | +2 (1) | −17 (2) | endogenous |
| D-fructose | 18 | +1% | +2% | +5 (2) | +5 (0) | nutrient |
| Dextromethorphan | 0.0051 | −4% | +2% | −3 (5) | +4 (1) | drug |
| Diclofenac | 11.5 | +2% | +2% | +1 (2) | +2 (4) | drug |
| Dihydrolipoate | 1 | +4% | +3% | +2 (2) | +4 (2) | drug |
| Diphenhydramine | 0.011 | −8% | +0% | +7 (1) | −4 (2) | drug |
| Dopamine | 0.09 | +1% | +1% | −1 (3) | +3 (1) | endogenous |
| Doxylamine succinate | 0.01 | +7% | +3% | +2 (2) | +2 (0) | drug |
| Dulaglutide | 0.0115 | −2% | +3% | +4 (2) | +7 (2) | drug |
| Empagliflozin | 10 | +1% | +1% | −8 (4) | −7 (1) | drug |
| Enalapril | 0.01 | 0% | −2% | +1 (1) | −3 (0) | drug |
| Ephedrine | 12 | −3% | 0% | +2 (2) | −1 (2) | drug |
| Gemfibrozil | 6.2 | 0% | +1% | +4 (3) | +2 (0) | drug |
| Glimepiride | 0.055 | +5% | +3% | +3 (1) | +5 (0) | drug |
| Guaifenesin | 0.15 | +1% | −2% | 0 (0) | −3 (0) | drug |
| Heparin | 2.78 | +3% | −1% | −6 (2) | −3 (2) | drug |
| Hydrochlorothiazide | 0.05 | +1% | −1% | +5 (2) | −3 (1) | drug |
| Insulin | 500 | −1% | +2% | −8 (0) | −15 (10) | endogenous, drug |
| Isomalt | 0.09 | −1% | +2% | −10 (4) | −8 (1) | nutrient |
| Lacitol | 0.09 | 2% | +4% | +1 (1) | +10 (5) | sugar alcohol |
| Lactate | 30 | 0% | +2% | −3 (1) | +3 (3) | endogenous |
| Lactose | 20 | −1% | +1% | −11 (2) | +1 (7) | nutrient |
| L-Glutathione reduced | 4.6 | +1% | +1% | +2 (4) | +1 (1) | endogenous |
| Losartan | 0.075 | +2% | +1% | +1 (3) | +2 (1) | drug |
| Mannitol | 1800 | +3% | −1% | +11 (1) | +2 (0) | nutrient |
| Malitol | 0.09 | +1% | +2% | −2 (0) | +3 (1) | sugar alcohol |
| Maltose | 480 | +4% | +9% | +5 (3) | +17 (22) | nutrient |
| Metformin | 0.5 | +4% | +1% | +3 (1) | +2 (0) | drug |
| Methylprednisolone | 0.05 | +6% | 0% | +5 (2) | 0 (4) | drug |
| Naproxen | 7.8 | −6% | −2% | −3 (2) | −8 (2) | drug |
| Quinidine | 6.2 | −5% | +2% | +1 (1) | +3 (1) | drug, nutrient |
| Phenylephedrine | 0.065 | +6% | +2% | +11 (6) | +4 (0) | drug |
| Reservatrol | 0.08 | +8% | −4% | +4 (1) | −6 (4) | drug |
| Rivaroxaban | 0.03 | +4% | +3% | +5 (2) | +7 (1) | drug |
| Salicylic Acid | 60 | +5% | +5% | +4 (1) | +9 (2) | drug |
| Sitagliptin | 0.0233 | −5% | +2% | +8 (1) | +3 (2) | drug |
| Sorbitol | 0.09 | +1% | +4% | +5 (1) | +5 (6) | sugar alcohol |
| Tetracycline | 0.5 | +4% | +2% | +4 (1) | +4 (2) | drug |
| Urea | 24 | +2% | +4% | −5 (-) | +7 (0) | endogenous |
| Xylitol | 0.09 | 0% | +2% | −2 (2) | −2 (5) | sugar alcohol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jensch, H.; Setford, S.; Thomé, N.; Srikanthamoorthy, G.; Weingärtner, L.; Grady, M.; Holt, E.; Pfützner, A. Dynamic Interference Testing—Unexpected Results Obtained with the Abbott Libre 2 and Dexcom G6 Continuous Glucose Monitoring Devices. Sensors 2025, 25, 1985. https://doi.org/10.3390/s25071985
Jensch H, Setford S, Thomé N, Srikanthamoorthy G, Weingärtner L, Grady M, Holt E, Pfützner A. Dynamic Interference Testing—Unexpected Results Obtained with the Abbott Libre 2 and Dexcom G6 Continuous Glucose Monitoring Devices. Sensors. 2025; 25(7):1985. https://doi.org/10.3390/s25071985
Chicago/Turabian StyleJensch, Hendrick, Steven Setford, Nicole Thomé, Geethan Srikanthamoorthy, Lea Weingärtner, Mike Grady, Elizabeth Holt, and Andreas Pfützner. 2025. "Dynamic Interference Testing—Unexpected Results Obtained with the Abbott Libre 2 and Dexcom G6 Continuous Glucose Monitoring Devices" Sensors 25, no. 7: 1985. https://doi.org/10.3390/s25071985
APA StyleJensch, H., Setford, S., Thomé, N., Srikanthamoorthy, G., Weingärtner, L., Grady, M., Holt, E., & Pfützner, A. (2025). Dynamic Interference Testing—Unexpected Results Obtained with the Abbott Libre 2 and Dexcom G6 Continuous Glucose Monitoring Devices. Sensors, 25(7), 1985. https://doi.org/10.3390/s25071985






