Investigating the Design of a Photoplethysmography Device for Vital Sign Monitoring
Abstract
1. Introduction
2. Hardware Design
2.1. Circuitry Design
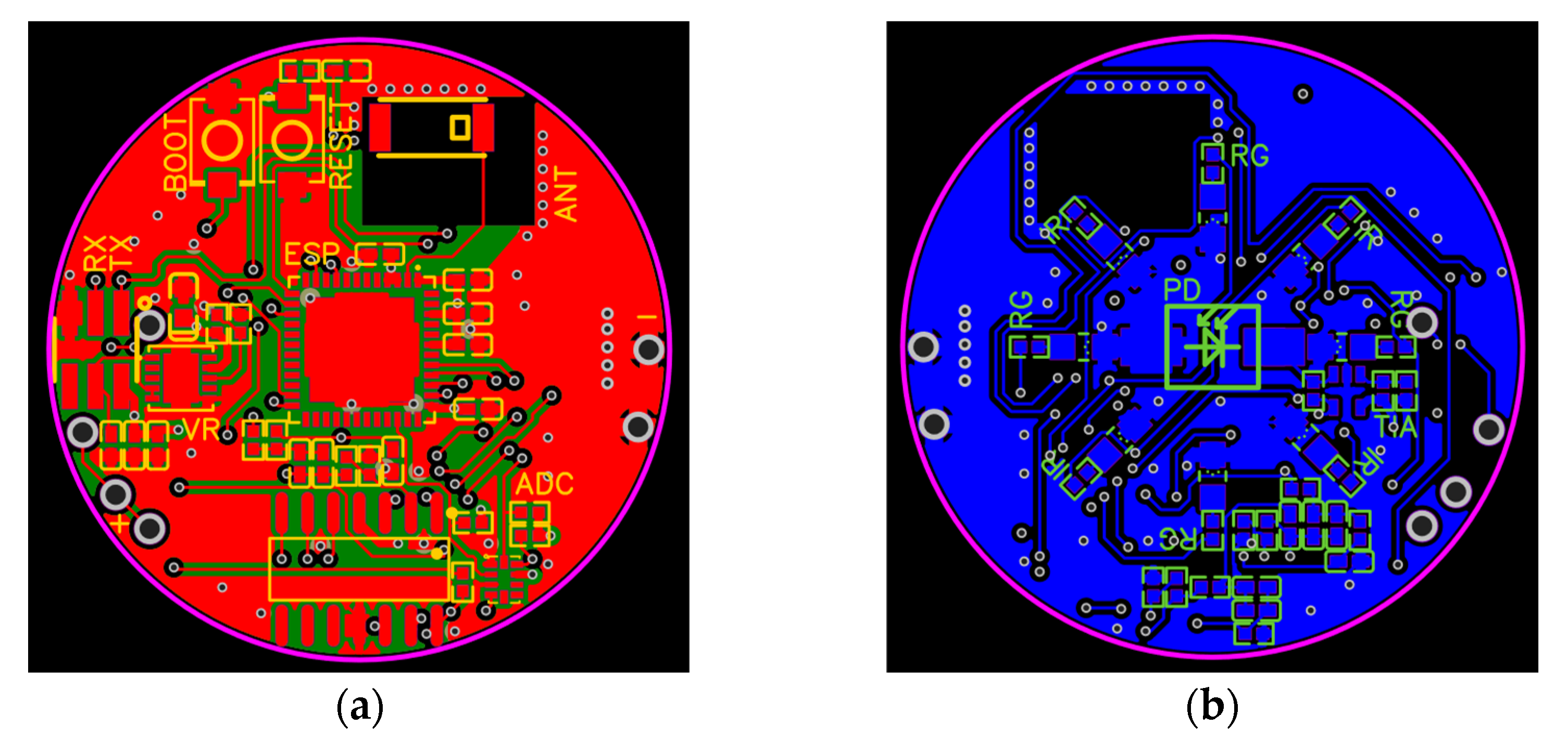
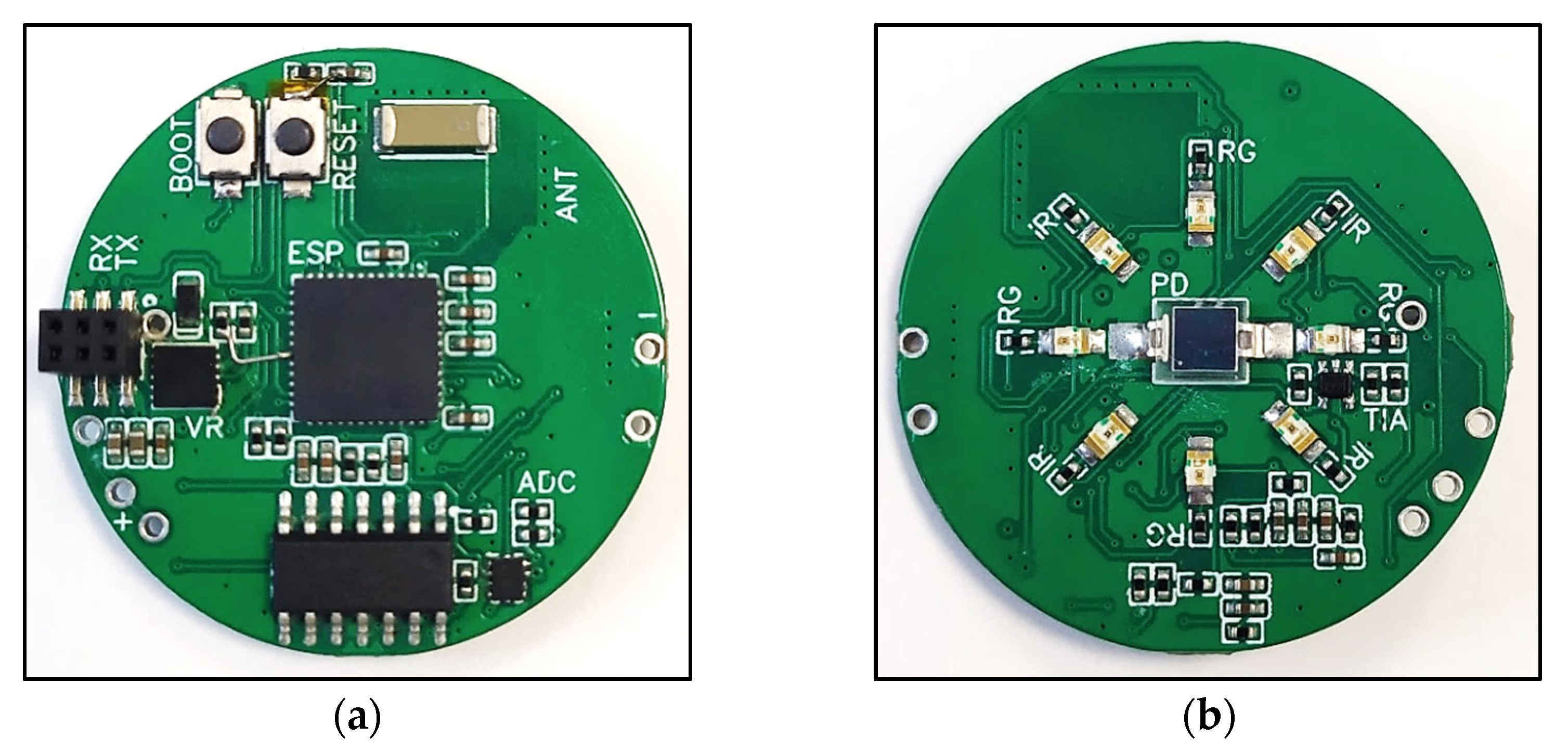
2.2. PCB Design
- The voltage-regulator circuit and accompanying connections;
- The microcontroller and its flash-circuit, where a flash-circuit allows the developer to program the microcontroller;
- The input circuit;
- The LED circuit;
- The ADC circuit;
- The antenna circuit.
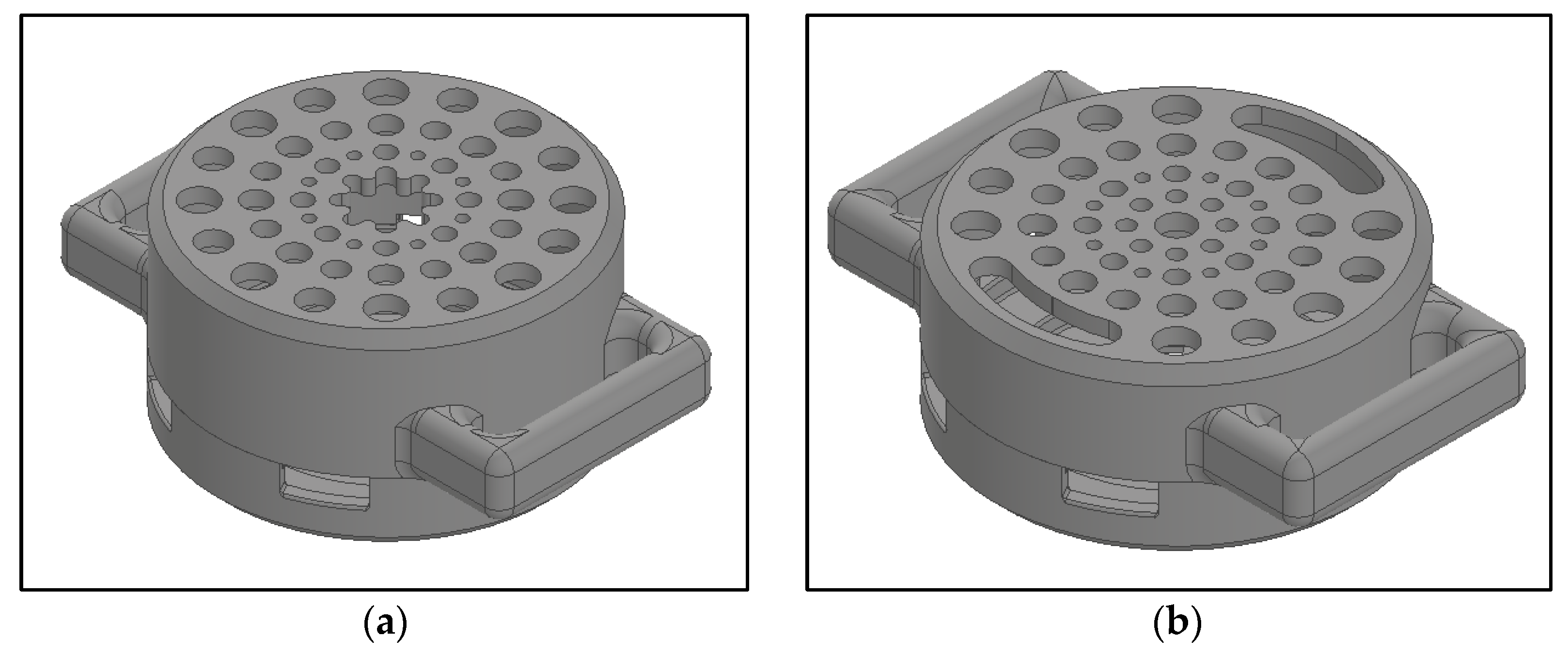
2.3. Enclosure Design
3. Software Design
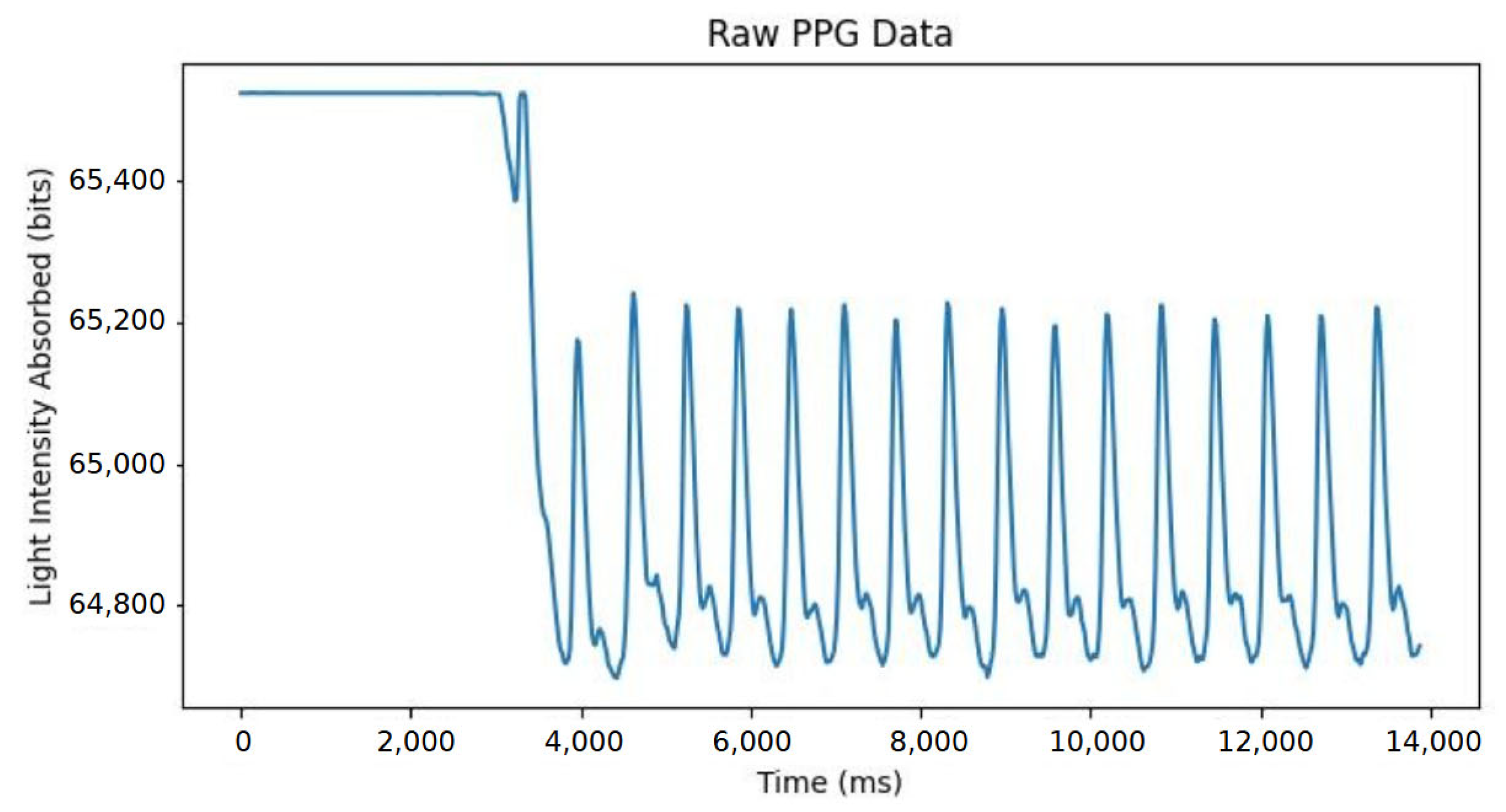
3.1. Data Acquisition Software
3.2. Data Processing Software
3.3. Cardiovascular Metric Estimation Software
4. Preliminary Performance Evaluation
4.1. Device Configuration Categories
- Light source wavelength: Red, green, or infrared;
- Light source brightness (8-bit duty cycle values): 100, 175, or 250;
- Number of light sources: One pair or two pairs;
- Photodetector lens shape: Flat or dome;
- Sensor-to-skin contact pressure: Low, medium, or high;
- Body location: Finger or wrist.
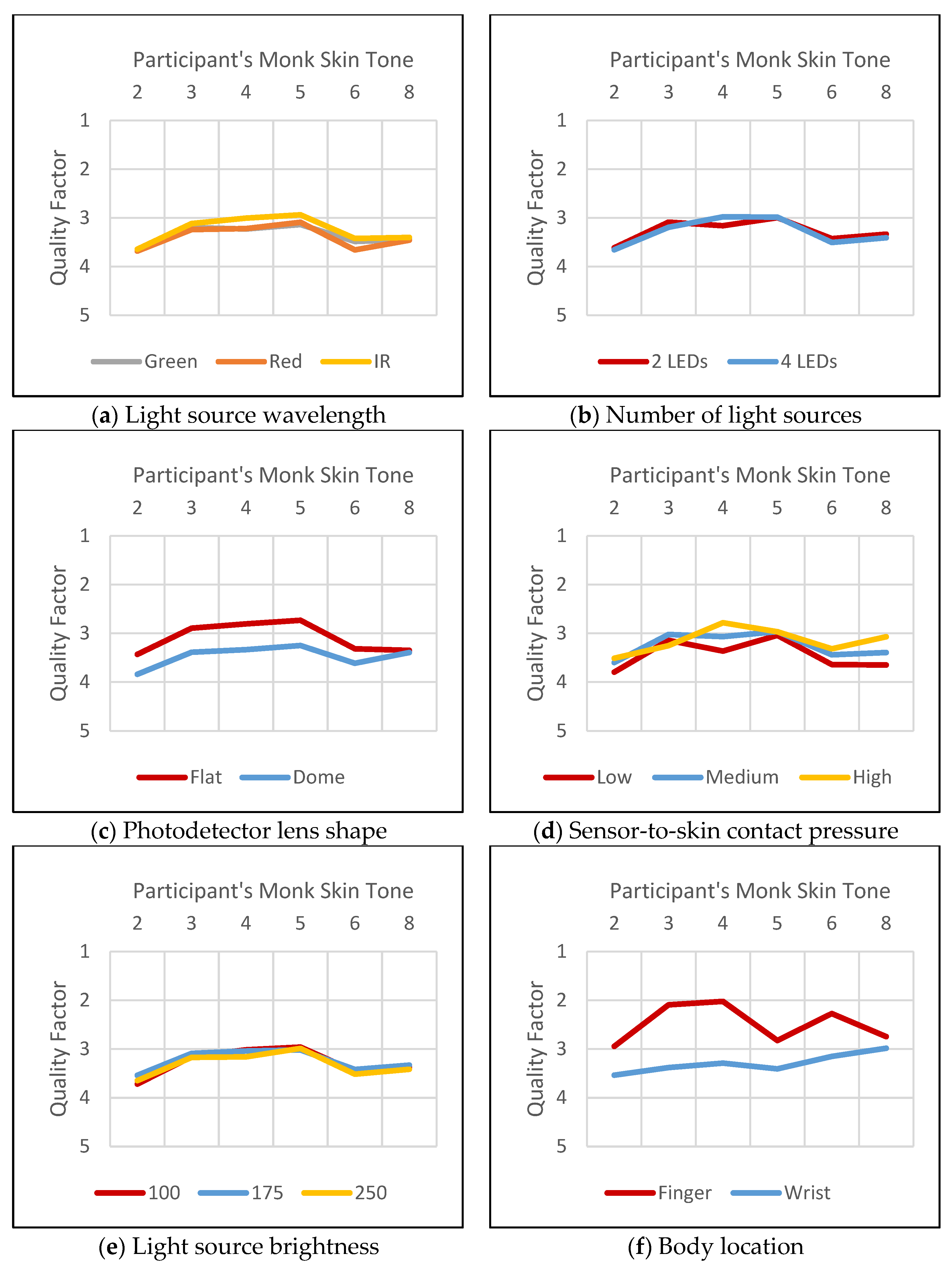
4.1.1. Light Source Wavelength
4.1.2. Light Source Brightness
4.1.3. Number of Light Sources
4.1.4. Photodetector Lens Shape
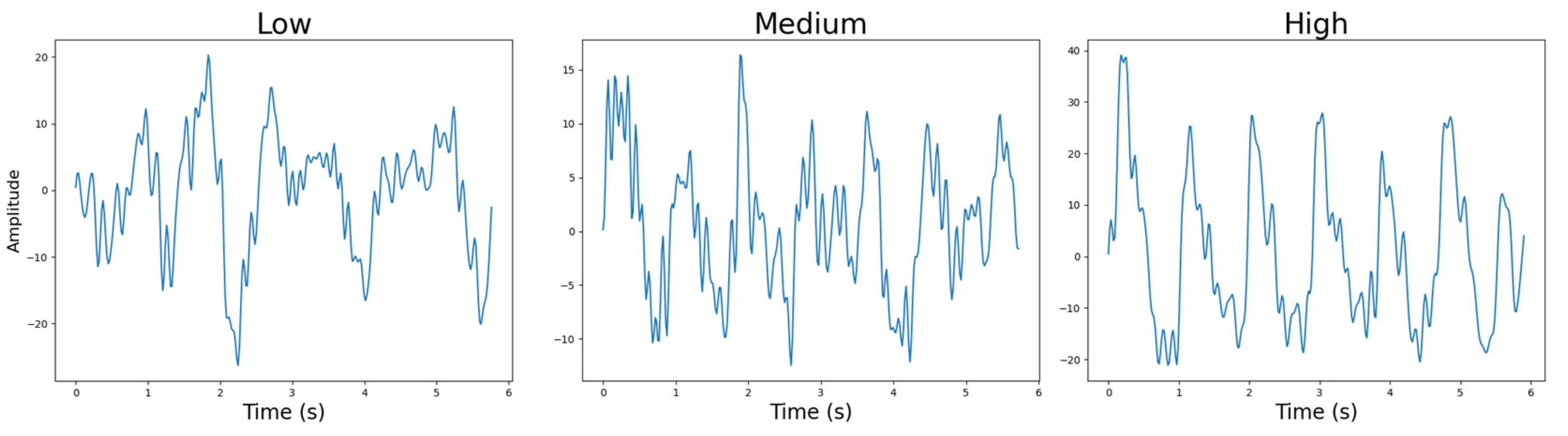
4.1.5. Sensor-to-Skin Contact Pressure
- Low pressure: The device can easily lose contact with the user’s skin;
- Medium pressure: The device stays flush with the user’s skin but can be moved from side to side;
- High pressure: The device does not lose contact with the user’s skin and cannot easily be moved from side to side.
4.1.6. Body Location
4.2. Ethical Approval
4.3. Participants
4.4. Evaluation Insights
4.4.1. Body Location
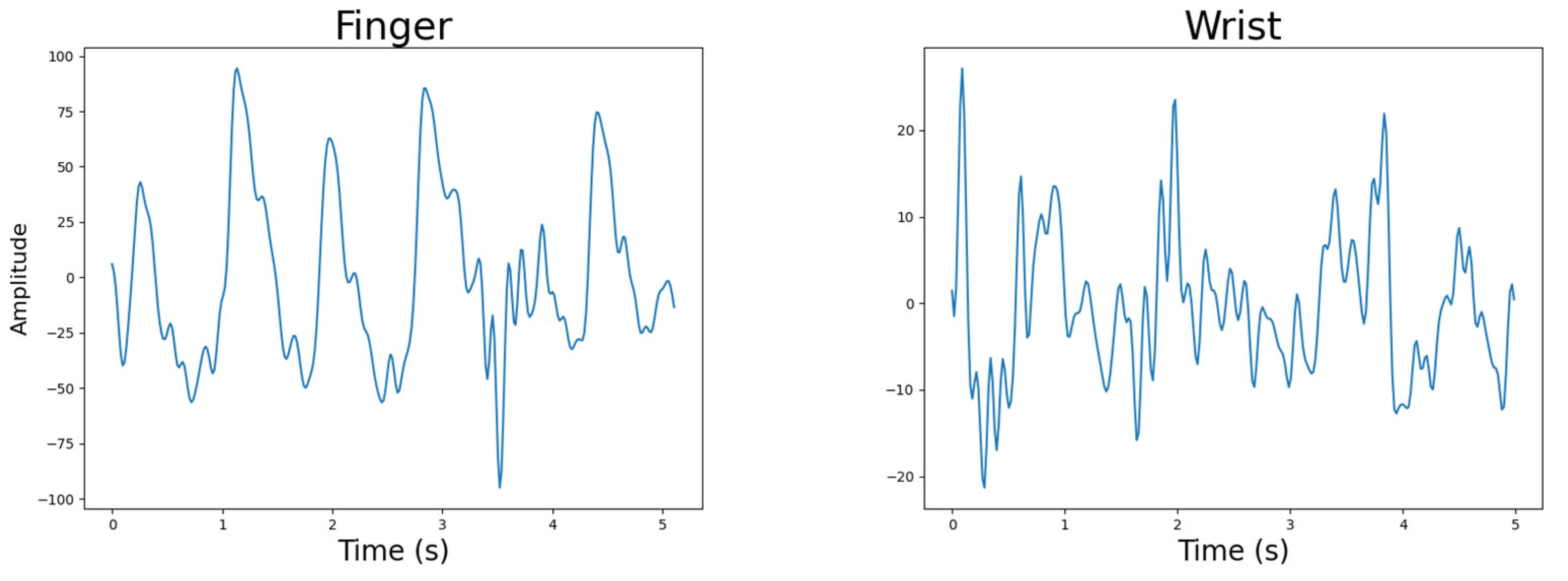
4.4.2. Light Source Wavelength
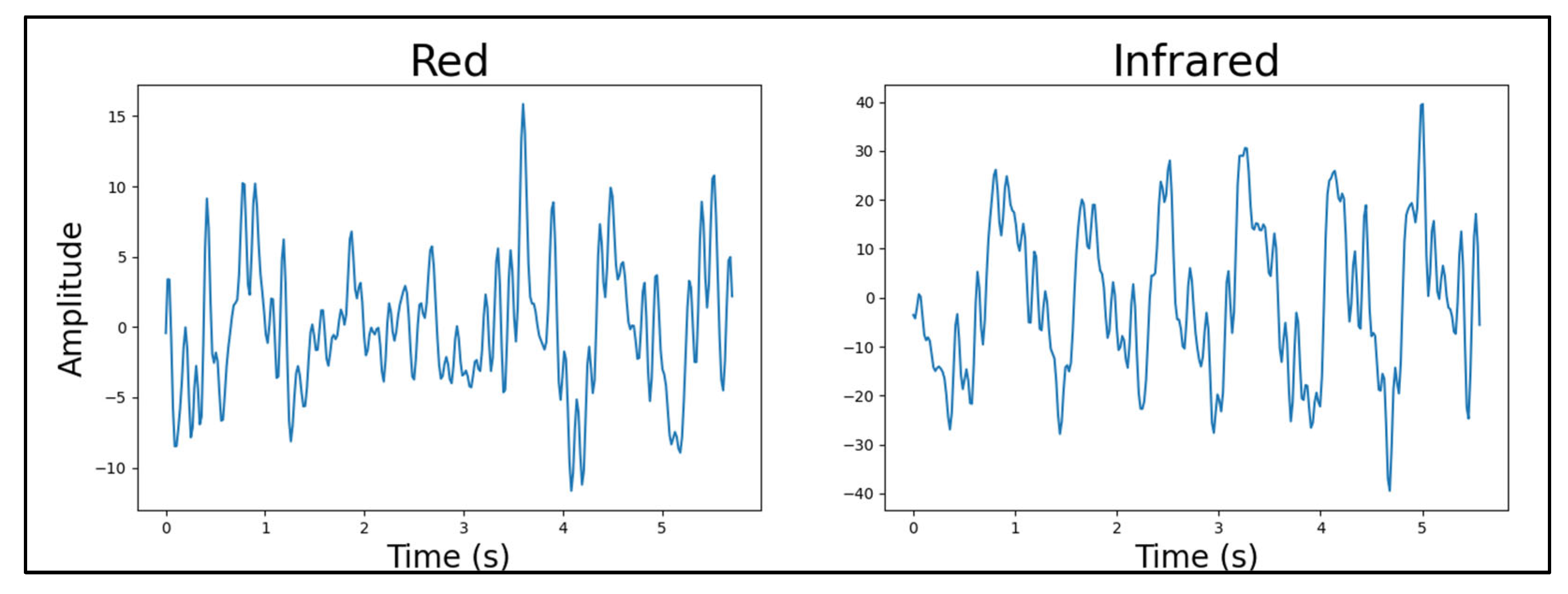
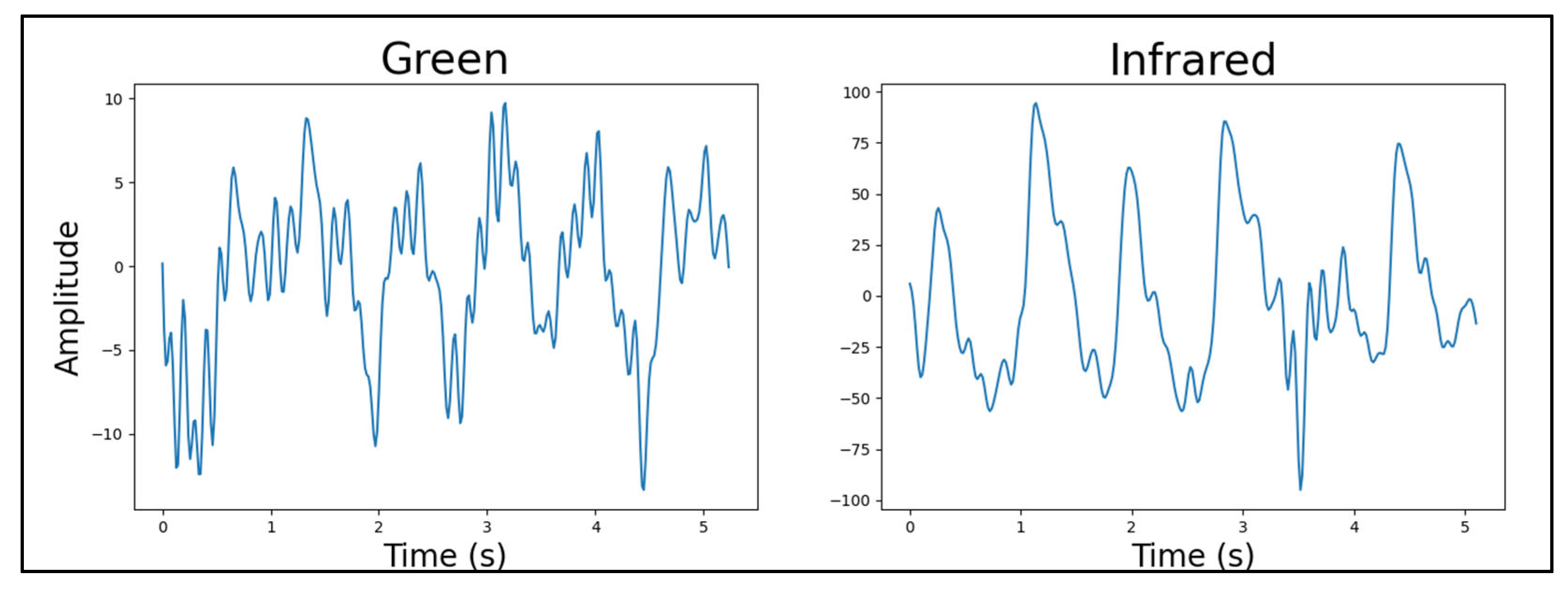
4.4.3. Number of Light Sources
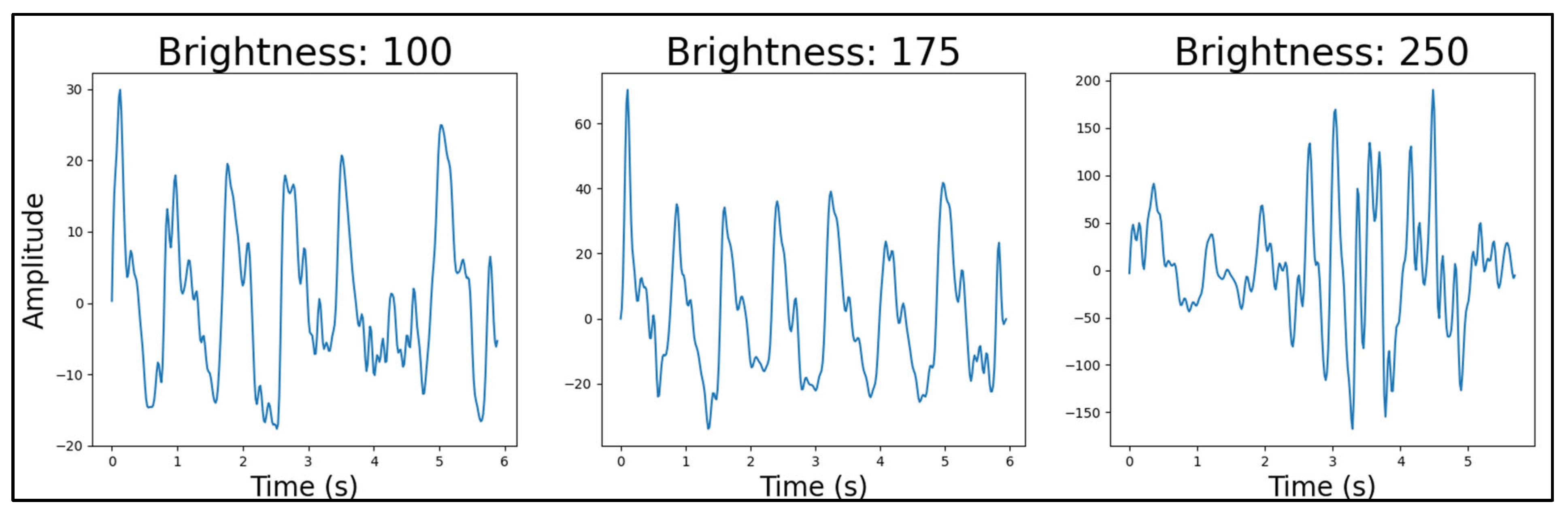
4.4.4. Light Source Brightness
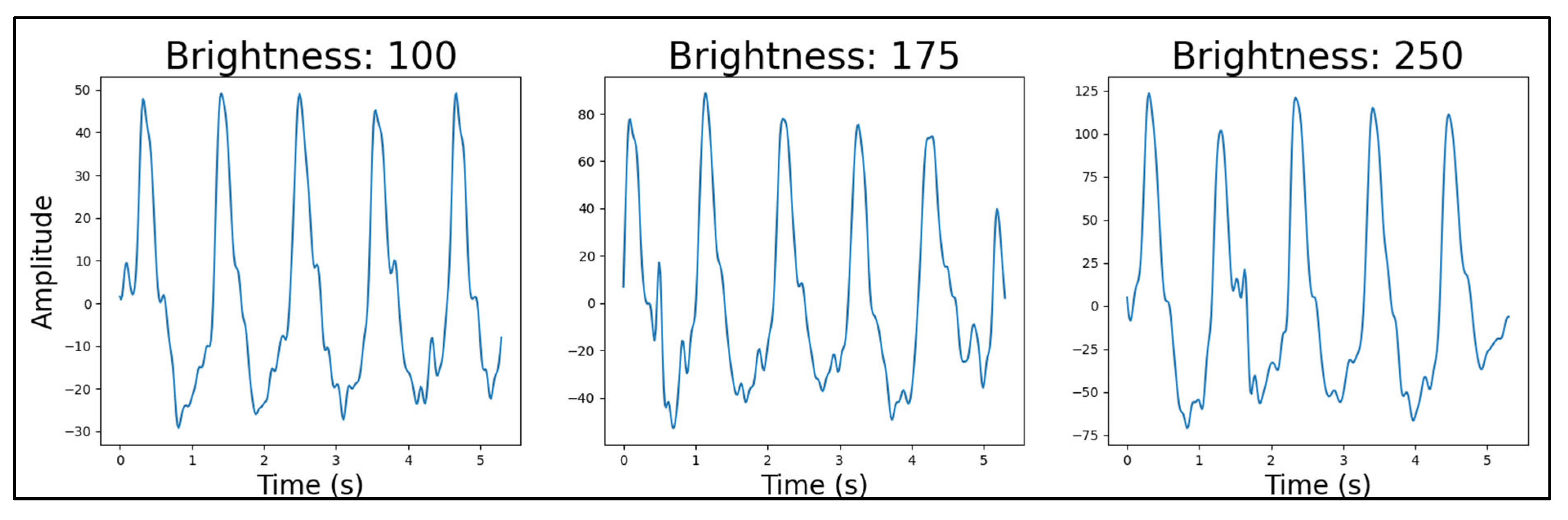
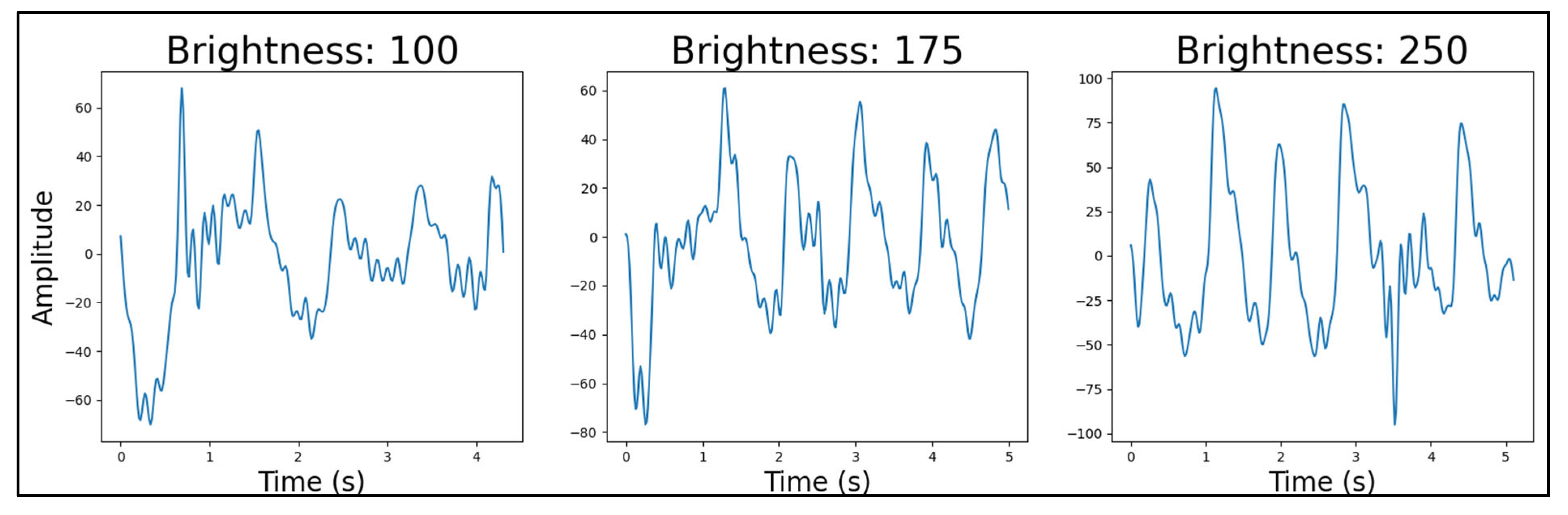
4.4.5. Photodetector Lens Shape
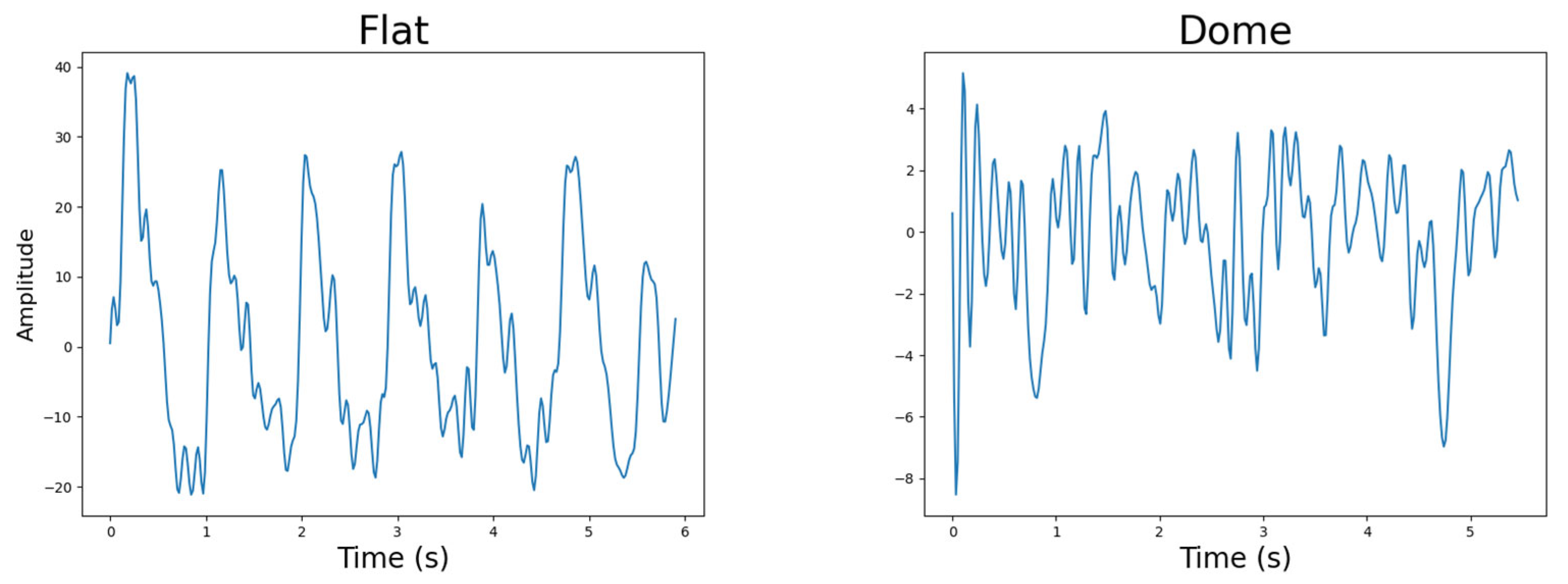
4.4.6. Sensor-to-Skin Contact Pressure
4.4.7. Skin Tone
4.5. Evaluation Conclusion
5. Participant Study Results
5.1. PPG Device Configuration
5.2. Bias to Skin Tone
6. Conclusions
6.1. Article Contributions
- A promising hardware configuration of a PPG device: This includes both the electronic circuit and printed circuit board (PCB) design, with full schematics published with this work;
- A dataset of the heart rate, blood oxygen saturation, blood pressure, and PPG data of 110 participants.
6.2. Future Work
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
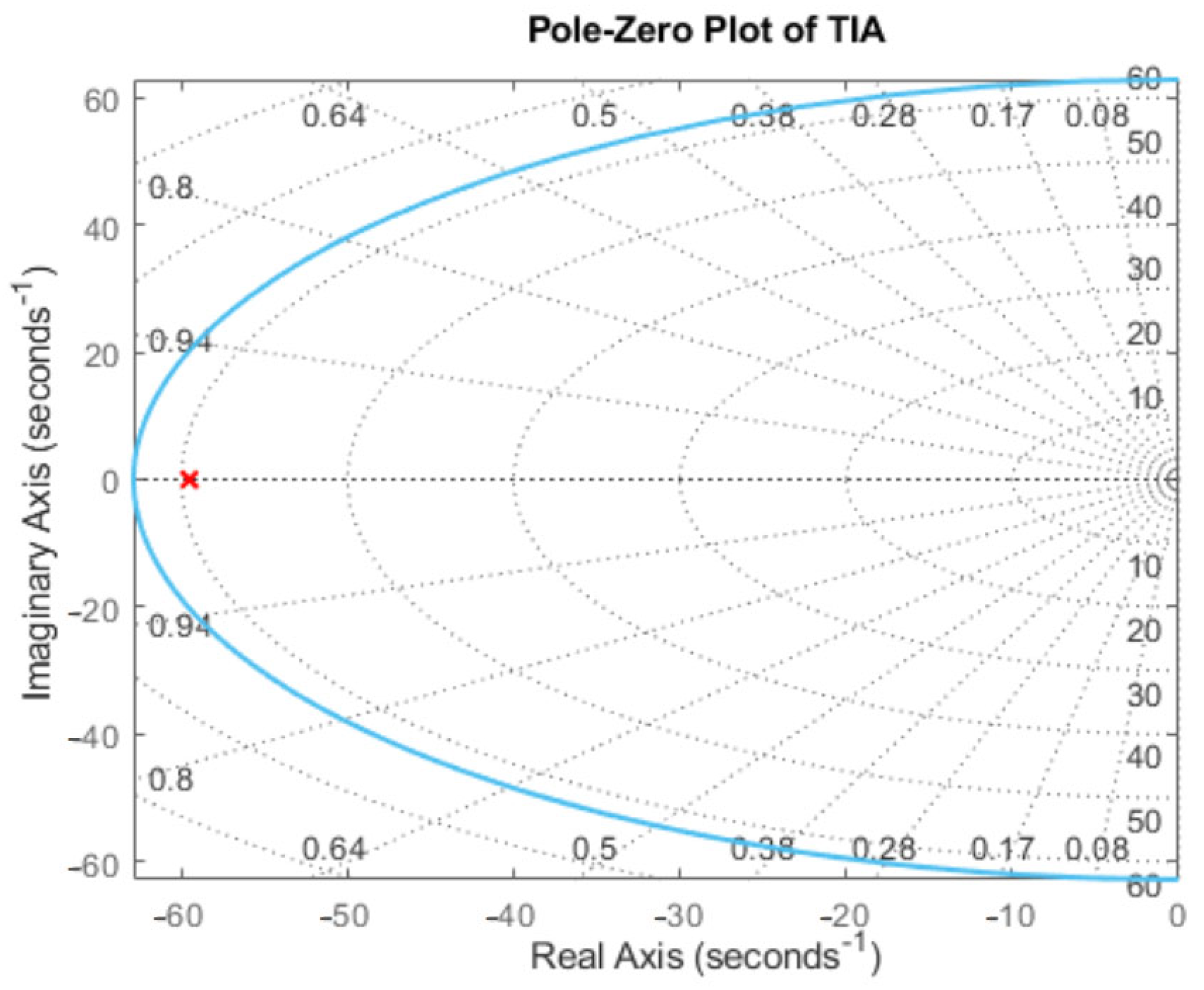
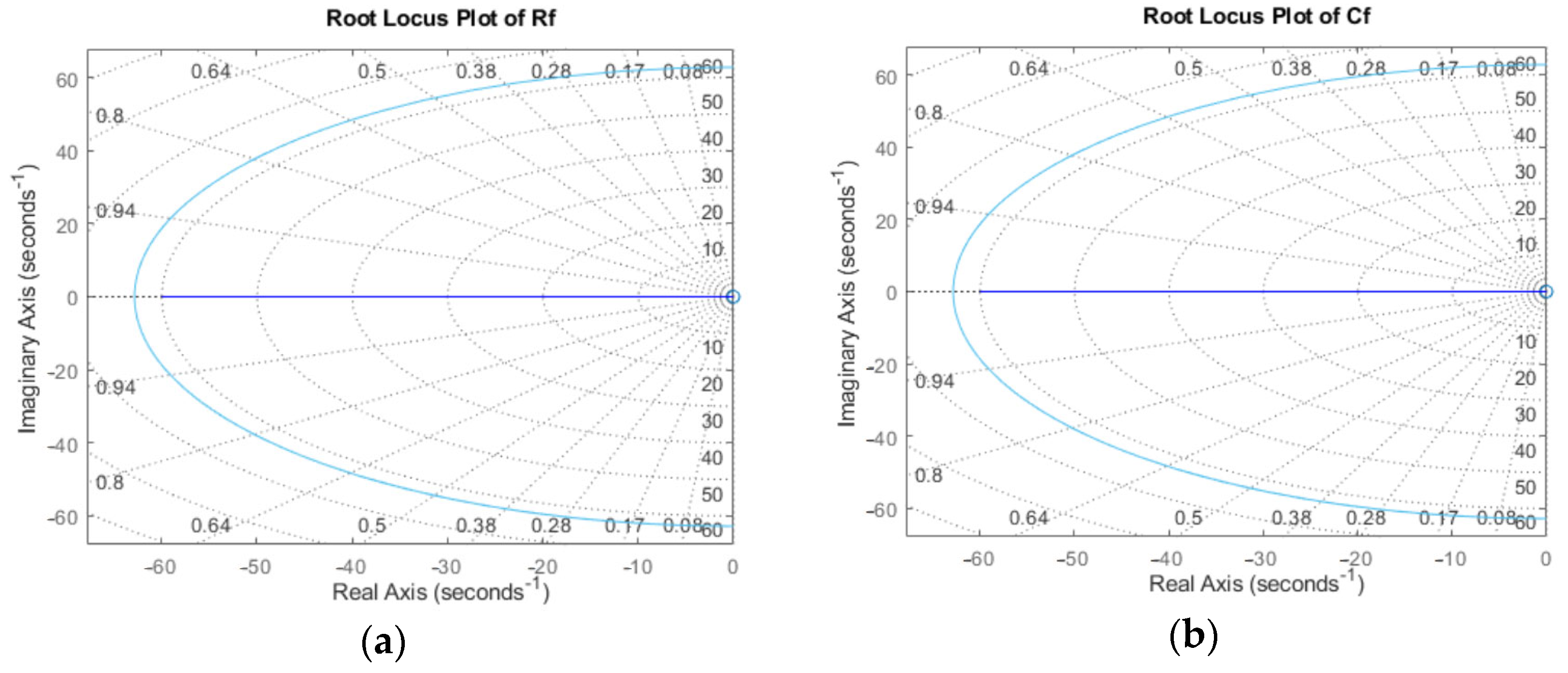
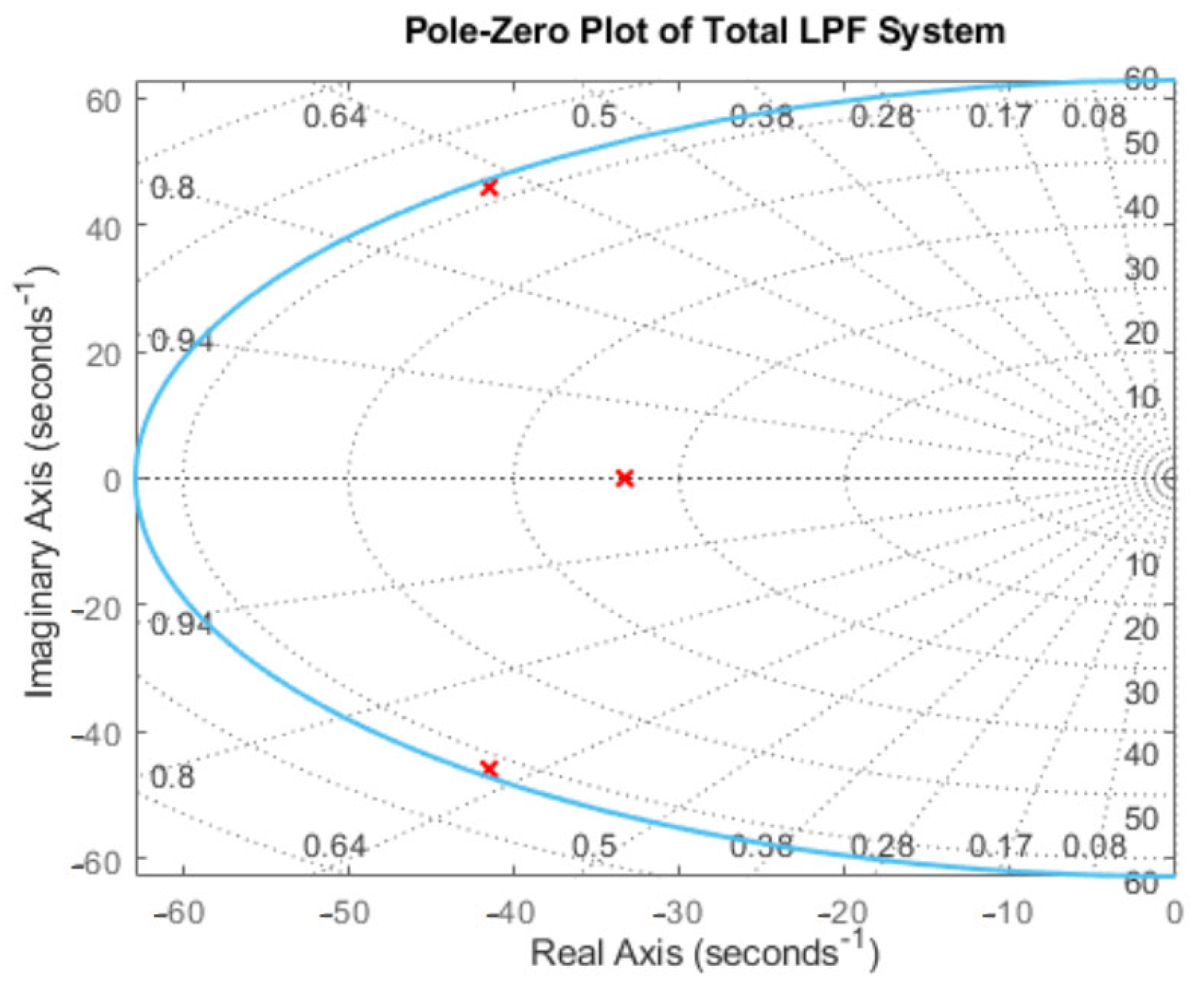
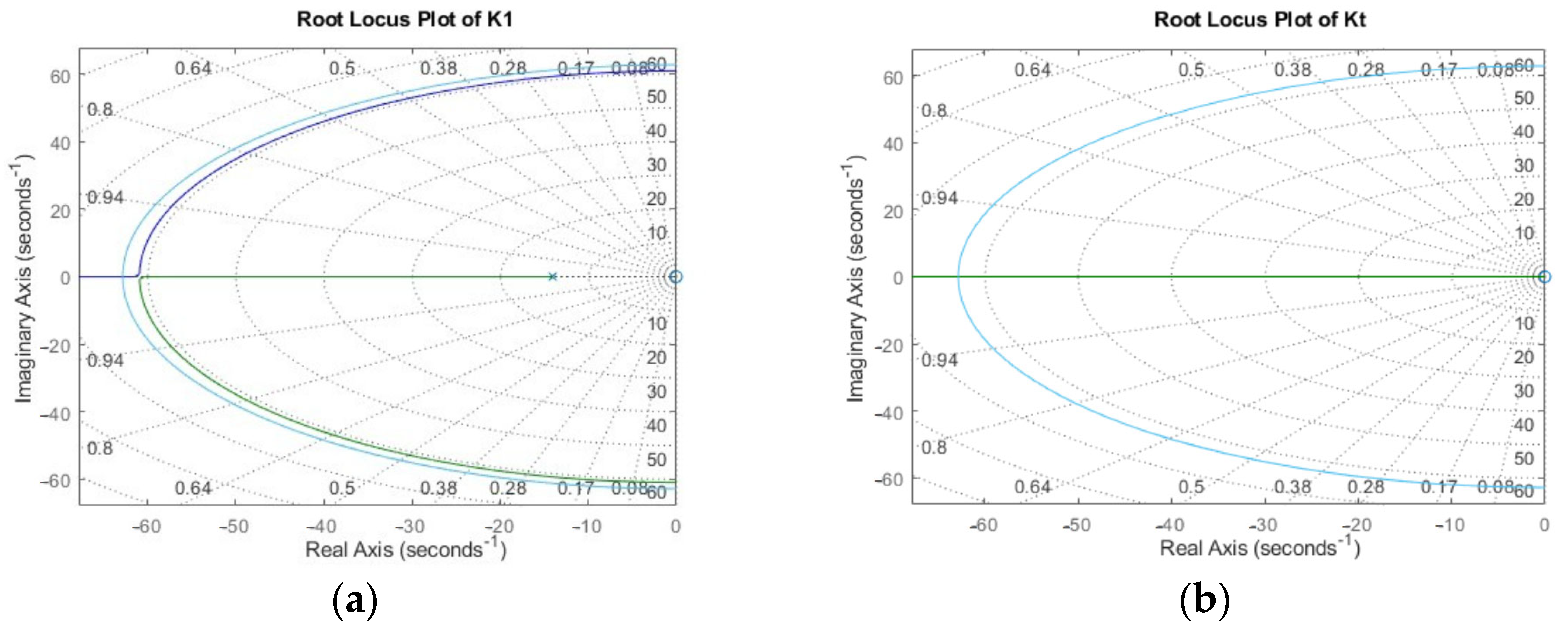
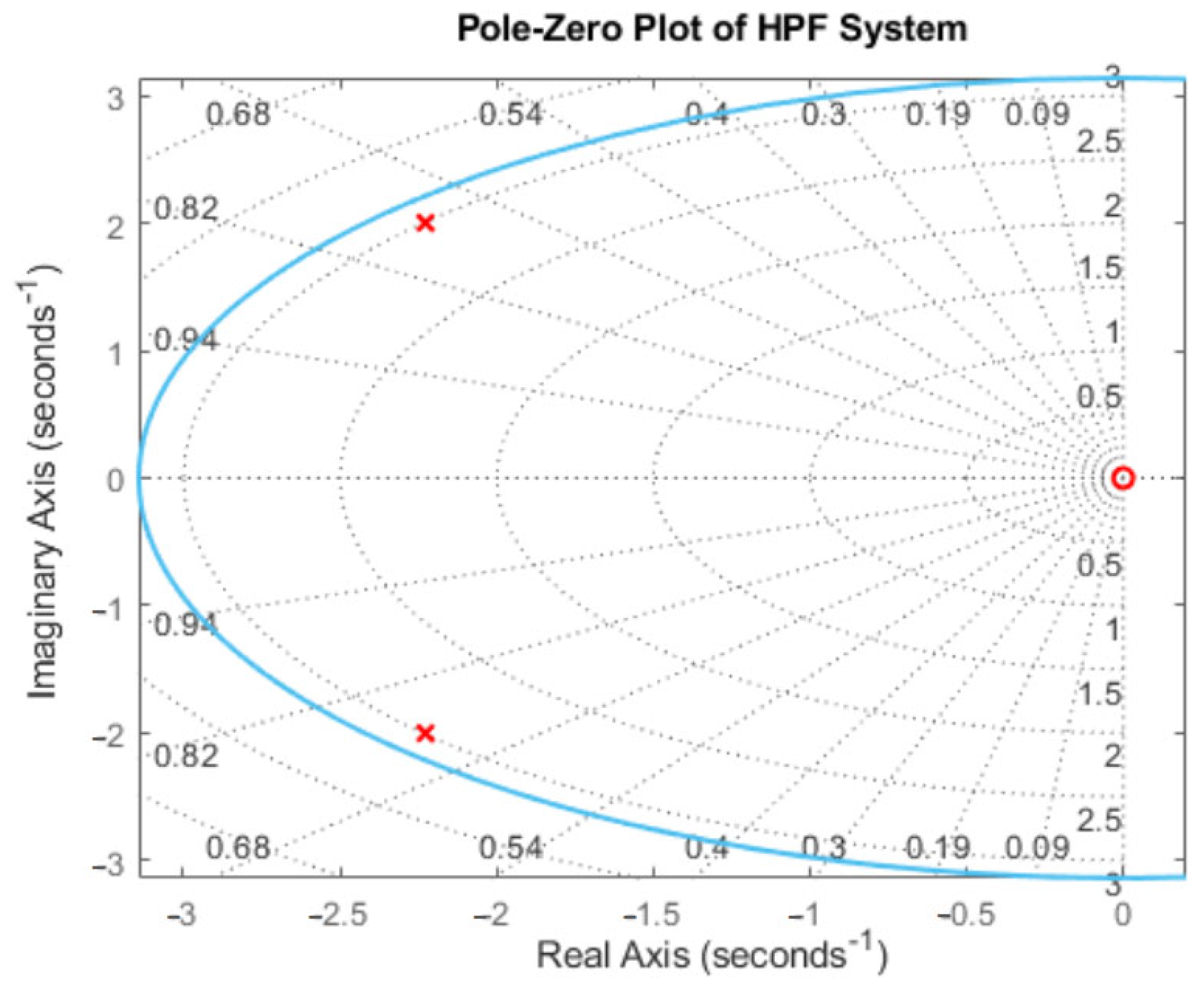
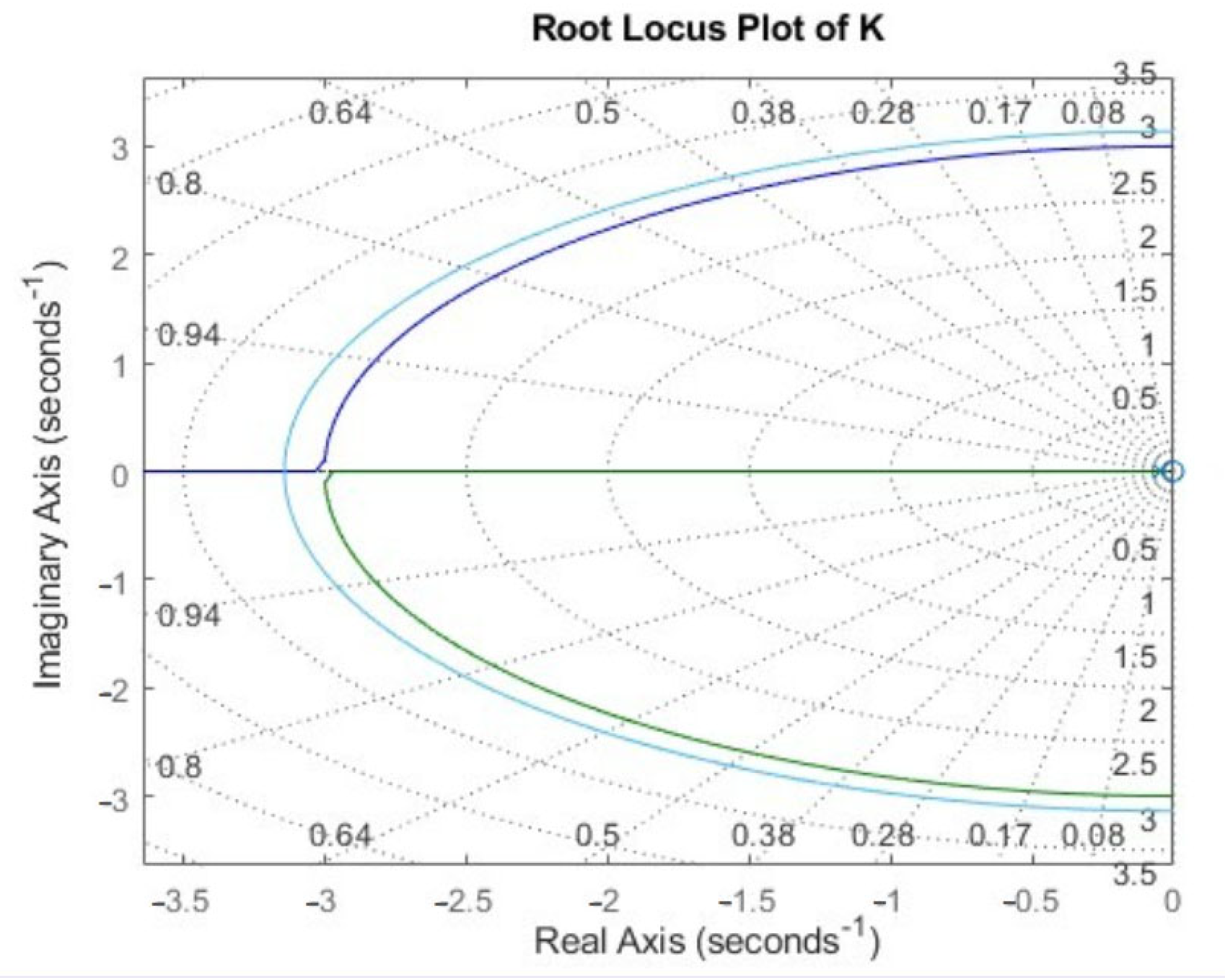
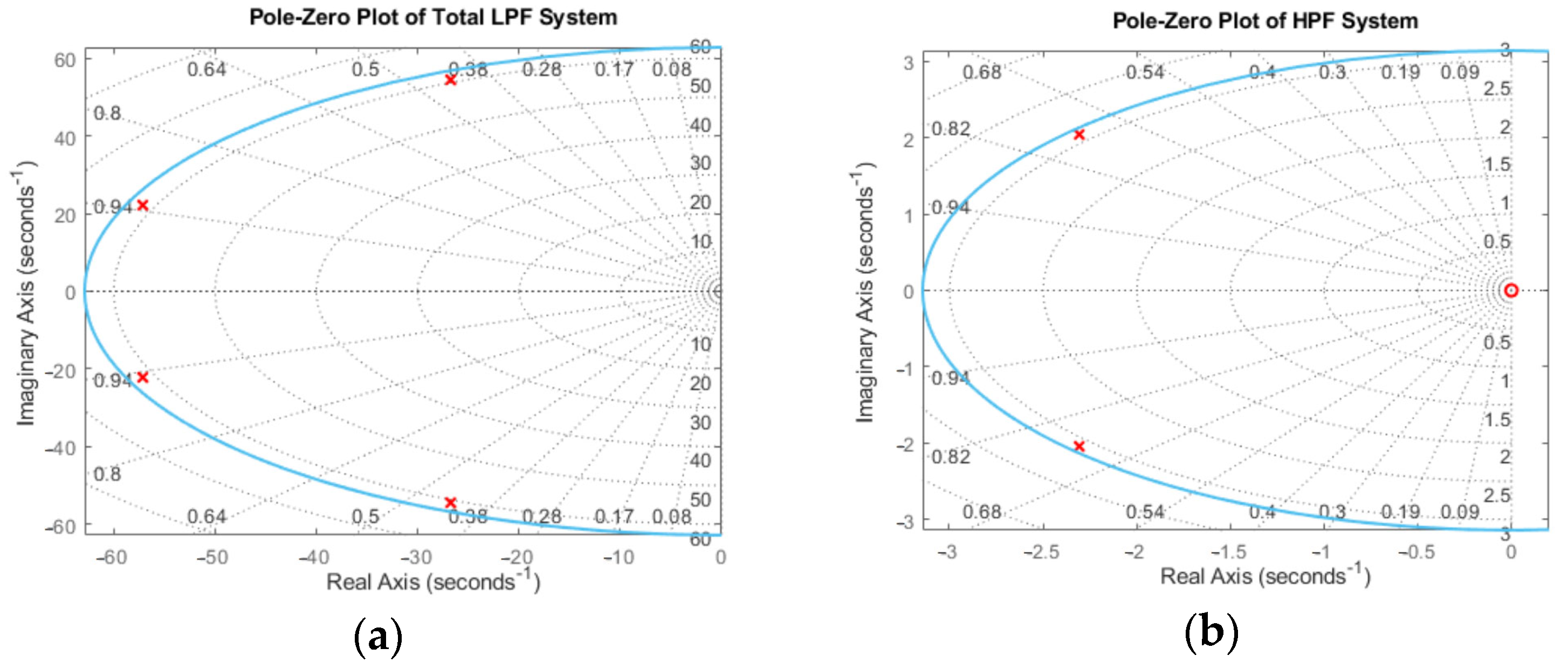
Appendix B
Appendix B.1. Experimental Conditions
Appendix B.2. Reference Devices
Appendix B.3. Methodology
- Four red LEDs, four infrared LEDs, and a flat photodetector lens;
- Four red LEDs, four infrared LEDs, and a dome-shaped photodetector lens;
- Four green LEDs, four infrared LEDs, and a flat photodetector lens;
- Four green LEDs, four infrared LEDs, and a dome-shaped photodetector lens.
- The participant was brought to the testing room and asked to sit at the desk;
- If the participant had long sleeves or any wrist accessories, they were asked to remove them from the area of measurement;
- The participant’s Monk Skin Tone was recorded by the researcher. This was performed by holding a Monk Skin Tone chart against the inside of the participant’s forearm;
- The participant was asked to sit still and not to speak, with their forearms resting on the tabletop in the supine position;
- The PPG devices were wired to a computer nearby via a USB hub and USB-to-TTL (serial) converter, each with an individual port for serial communication. This connection also provided power to the PPG devices;
- A PPG device was attached to each of the participants’ index fingers and wrists, with the red LED devices on the fingers and the green LED devices on the wrists;
- The reference pulse oximeter device was attached to the participant’s right middle finger;
- The contact pressures were set to low by adjusting the PPG devices’ straps;
- The lights in the room were turned off;
- The devices were turned on at the same time;
- For the two-and-a-half minutes of measurement, the researcher manually wrote down the participant’s heart rate as measured by the pulse oximeter;
- The measurements were stored on the computer using the serial terminal and on the researcher’s smartphone via the FTP server;
- The devices were turned off;
- Steps 8 to 13 were then repeated for both medium and high contact pressures;
- The PPG devices were removed from the participant’s wrists and fingers;
- A PPG device was attached to each of the participants’ index fingers and wrists, with the green LED devices on the fingers and the red LED devices on the wrists;
- Steps 8 to 15 were then repeated;
- The reference pulse oximeter device was removed from the participant’s right middle finger;
- The participant was thanked for their participation and shown out of the room;
- Each device’s surface was cleaned with 91% rubbing alcohol to remove any contaminants.
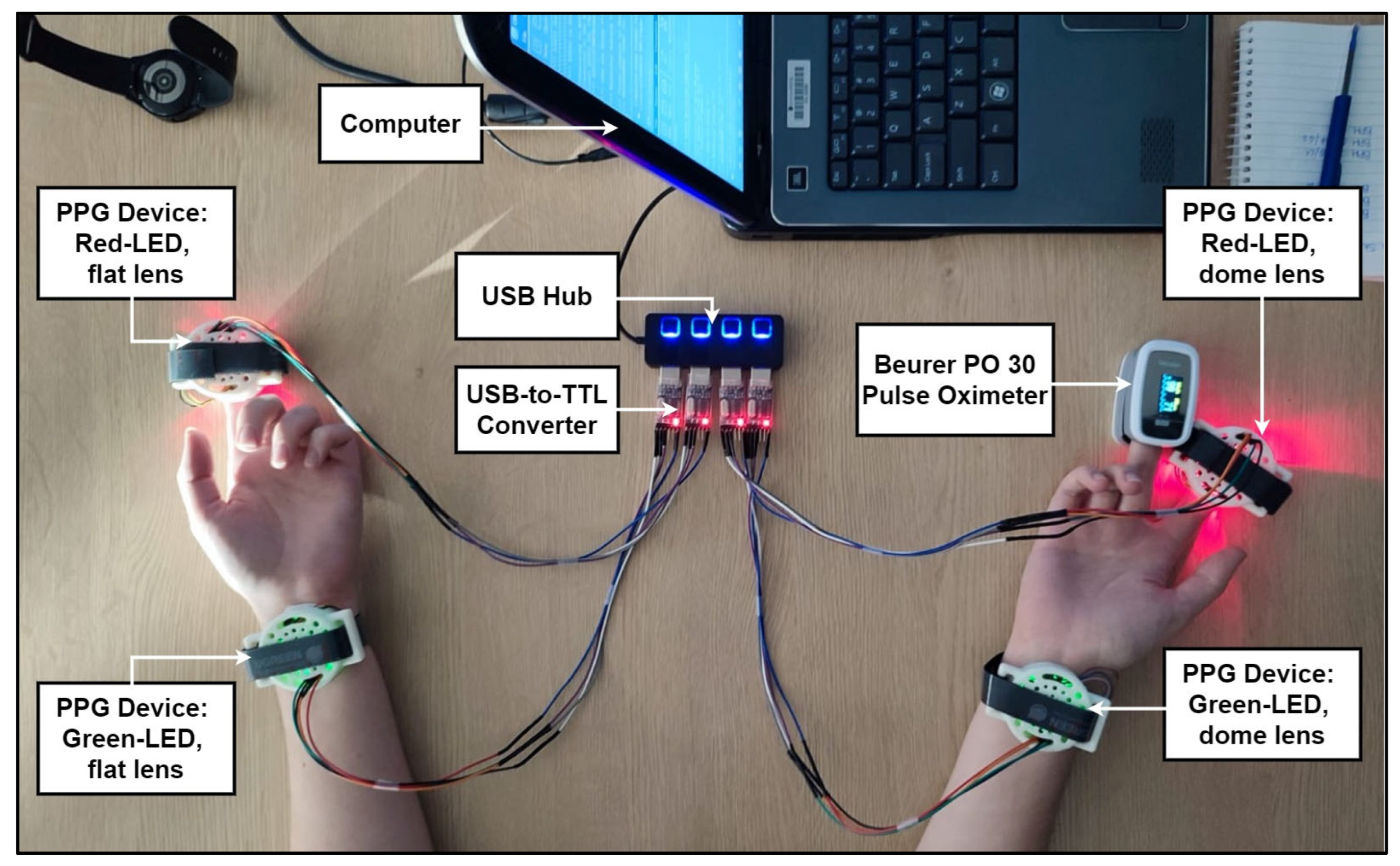
Appendix B.4. Performance Evaluation Metrics
| Quality Factor | Description |
|---|---|
| 1 | The largest peak is in the correct heart rate range. |
| 2 | One of the largest peaks is in the correct heart rate range, or the largest peak is within 0.1 Hz of the correct heart rate range. |
| 3 | One of the largest peaks is within 0.1 Hz of the heart rate range. |
| 4 | A peak is in or within 0.1 Hz of the heart rate range. |
| 5 | There are no notable peaks in or close to the heart rate range. |
| Quality Factor | Description |
|---|---|
| 1 | The signal exhibits clear systolic and diastolic peaks, by eye. |
| 2 | The signal exhibits clear systolic peaks, by eye. |
| 3 | The signal exhibits a somewhat PPG-like shape, with identifiable peaks and troughs, by eye. |
| 4 | The signal exhibits a periodic attribute that could be indicative of the PPG-shape, by eye. This could mean that there are evident troughs with no specific peak in between or vice versa. |
| 5 | The signal exhibits mostly noise and a PPG shape cannot be identified, by eye. |
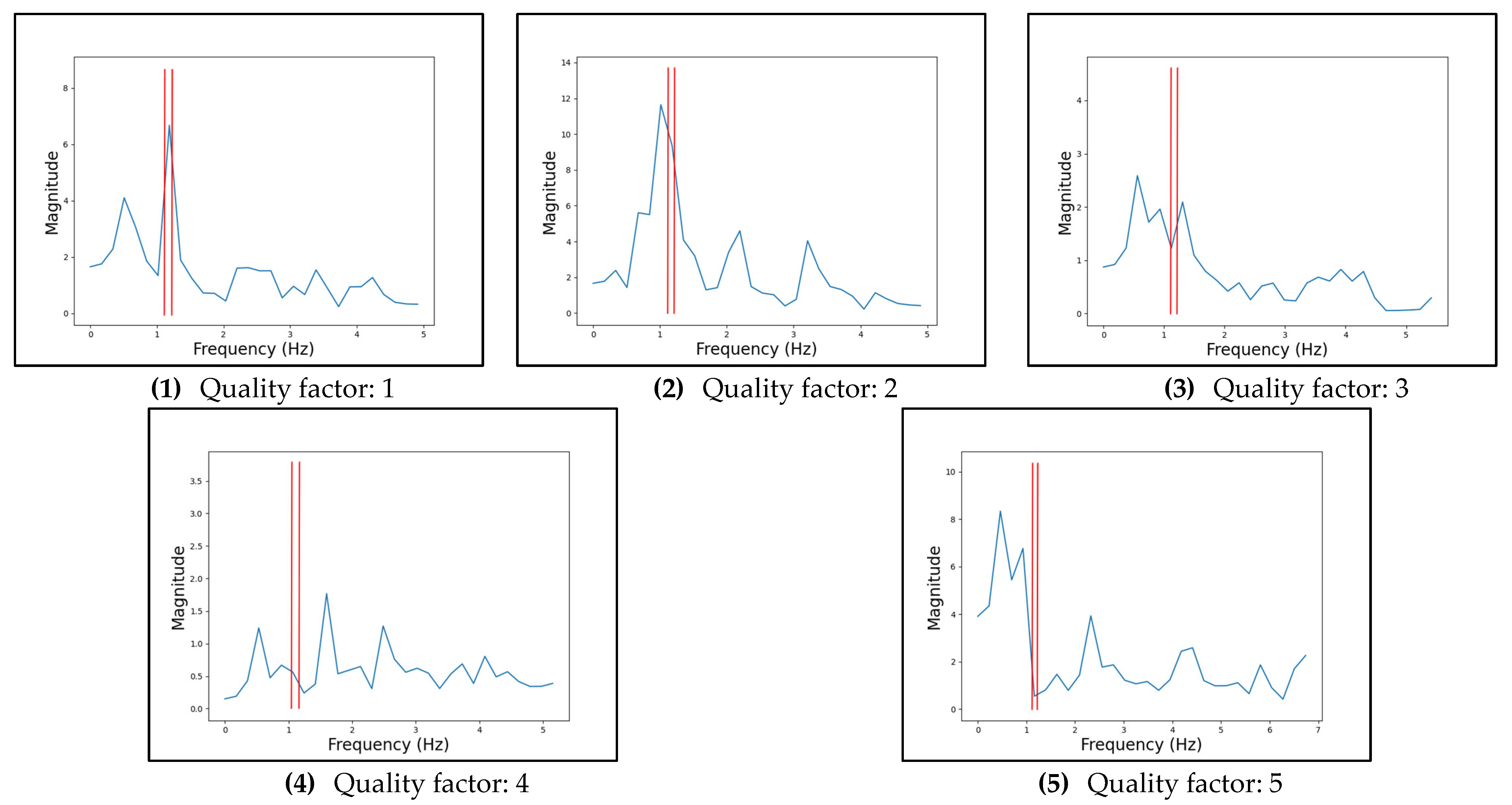
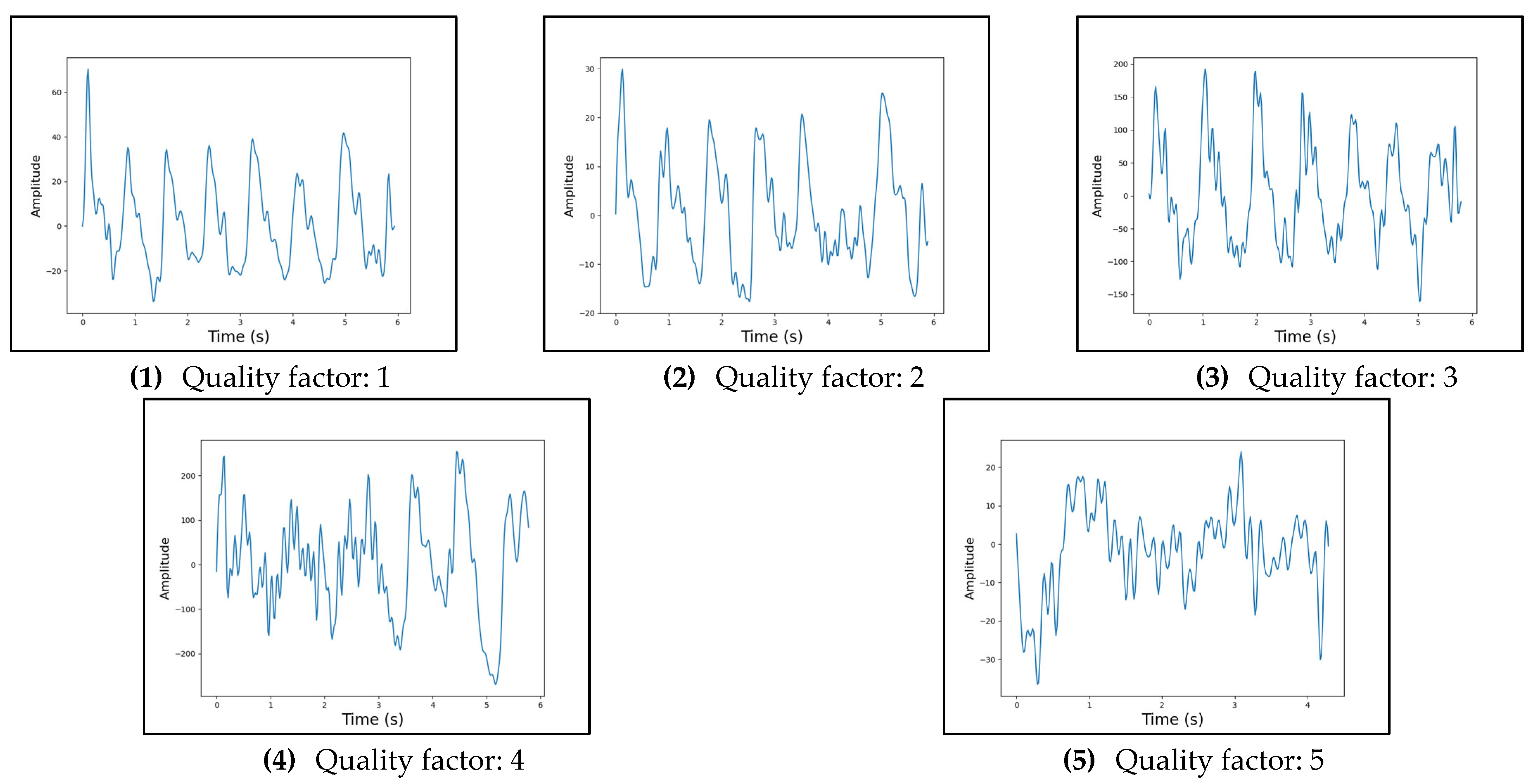
Appendix C
Appendix C.1. Experimental Conditions
Appendix C.2. Reference Devices
Appendix C.3. Methodology
- The participant was brought to the testing room and asked to sit at the desk;
- The consent form was explained by the researcher, and the participant was asked to sign in the relevant spaces;
- If the participant wore a jacket, jersey, or similar clothing piece, they were asked to remove it for the blood pressure measurement;
- The cuff of the reference blood pressure monitor was attached to the upper right arm of the participant and properly secured;
- The participant’s blood pressure was measured;
- The blood pressure monitor’s cuff was removed from the participant, and they were asked to sit in front of the USB hub where the PPG devices were connected;
- The PPG devices were connected to the participant’s index fingertips, with the green LED device connected to the left hand and the red LED device to the right;
- The sensor-to-skin contact pressures were set to high by adjusting the devices’ straps;
- The reference pulse oximeter device was attached to the participant’s right middle finger;
- The participant was asked to sit still and not speak, with their arms resting on the tabletop in the supine position;
- The lights in the room were turned off;
- The PPG devices were turned on at the same time;
- For the 1 min and 50 s of measurements, the researcher manually wrote down the heart rate and blood oxygen saturation measured by the pulse oximeter every 20 s, starting after 10 s of measurement;
- The measurements were stored on the computer using the serial terminal and on the researcher’s phone via the FTP server connected via Wi-Fi;
- The PPG devices were turned off;
- The PPG devices and pulse oximeter were removed from the participant;
- The lights in the room were turned on;
- The participant was thanked for their participation and shown out of the room;
- Each device’s surface was cleaned with 91% rubbing alcohol to remove any contaminants.
References
- WHO. WHO Reveals Leading Causes of Death and Disability Worldwide: 2000–2019. 9 December 2020. Available online: https://www.who.int/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019 (accessed on 3 March 2025).
- WHO. WHO Mortality Database. 2018. Available online: https://platform.who.int/mortality/themes/theme-details/MDB/all-causes (accessed on 3 March 2025).
- Department of Statistics South Africa. Mortality and Causes of Death in South Africa: Findings from Death Notification. 2018. Available online: https://www.statssa.gov.za/publications/P03093/P030932018.pdf (accessed on 3 March 2025).
- Allen, J. Photoplethysmography and Its Application in Clinical Physiological Measurement. Physiol. Meas. 2007, 28, R1. [Google Scholar] [CrossRef] [PubMed]
- Moyle, J.T. Pulse Oximetry. In How Pulse Oximetry Works; BMJ Books: London, UK, 2002; pp. 7–53. [Google Scholar]
- Regiug, F. Photoplethysmogram Signal Analysis for Detecting Vital Physiological Parameters: An Evaluating Study. In Proceedings of the 2016 International Symposium on Signal, Image, Video and Communications (ISIVC), Tunis, Tunisia, 21–23 November 2016. [Google Scholar]
- Ayers, T.M.; Paetsch, C.R. In-Ear Biometric Monitoring Using Photoplethysmography (ppg); Bose Corporation: Framingham, MA, USA, 2020. [Google Scholar]
- Kraudel, R. Optical Heart-Rate Sensors for Biometric Wearables. 2018. Available online: https://e2e.ti.com/blogs_/b/analogwire/posts/optical-heart-rate-sensors-for-biometric-wearables (accessed on 3 March 2025).
- ENaeini, E.K.; Azimi, I.; Rahmani, A.M.; Liljeberg, P.; Dutt, N. A Real-time PPG Quality Assessment Approach for Healthcare Internet-of-Things. Procedia Comput. Sci. 2019, 151, 551–558. [Google Scholar] [CrossRef]
- Fawzy, A.; Wu, T.D.; Wang, K.; Robinson, M.L.; Farha, J.; Bradke, A.; Golden, S.H.; Xu, Y.; Garibaldi, B.T. Racial and Ethnic Discrepancy in Pulse Oximetry and Delayed Identification of Treatment Eligibility Among Patients with COVID-19. JAMA Intern. Med. 2022, 182, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, E.R.; Ziegler, J.; Morley, K.; Rush, B.; Celi, L.A. Assessment of Racial and Ethnic Differences in Oxygen Supplementation Among Patients in the Intensive Care Unit. JAMA Intern. Med. 2022, 182, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Al-Halawani, R.; Charlton, P.H.; Qassem, M.; A Kyriacou, P. A review of the effect of skin pigmentation on pulse oximeter accuracy. Physiol. Meas. 2023, 44, 05TR01. [Google Scholar] [CrossRef] [PubMed]
- LeMoult, C. When It Comes to Darker Skin, Pulse Oximeters Fall Short. 2022. Available online: https://www.npr.org/sections/health-shots/2022/07/11/1110370384/when-it-comes-to-darker-skin-pulse-oximeters-fall-short (accessed on 3 March 2025).
- Michel, R. Innovators Develop Multi-Analyte Pulse Oximeters that Accurately Read Oxygen Levels in People with Darker Skin Pigmentation. 2023. Available online: https://www.darkdaily.com/2023/05/22/innovators-develop-multi-analyte-pulse-oximeters-that-accurately-read-oxygen-levels-in-people-with-darker-skin-pigmentation/ (accessed on 3 March 2025).
- Espressif Systems. ESP32PICOV3—Datasheet. 16 April 2020. Available online: https://www.espressif.com/sites/default/files/documentation/esp32-pico-v3_datasheet_en.pdf (accessed on 3 March 2025).
- Texas Instruments. Filter Design Tool. 2023. Available online: https://webench.ti.com/filter-design-tool/ (accessed on 3 March 2025).
- Onsemi. Surface-Mount Silicon Pin Photodiode QSB34GR, QSB34ZR, QSB34CGR, QSB34CZR. August 2023. Available online: https://www.onsemi.com/pdf/datasheet/qsb34-d.pdf (accessed on 3 March 2025).
- Texas Instruments. Transimpedance Amplifier Circuit; Texas Instruments: Dallas, TX, USA, 2018. [Google Scholar]
- Analog Devices. Low Input Bias Current Op Amps (≤100 pA). Available online: https://www.analog.com/en/product-category/low-input-bias-current-op-amps.html (accessed on 3 March 2025).
- Texas Instruments. OPAx170 36-V, Single-Supply, SOT553, Low-Power Operational Amplifiers Value Line Series. August 2018. Available online: https://www.ti.com/product/OPA170 (accessed on 3 March 2025).
- Chhabra, L.; Goel, N.; Prajapat, L.; Spodick, D.; Goyal, S. Mouse Heart Rate in a Human: Diagnostic Mystery of an Extreme Tachyarrhythmia. Indian Pacing Electrophysiol. J. 2012, 12, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Guinness World Records. Lowest Heart Rate. 2005. Available online: https://www.guinnessworldrecords.com/world-records/lowest-heart-rate (accessed on 3 March 2025).
- Meserve, C. What Is Skin Temperature and Why Should You Monitor It? 2021. Available online: https://www.whoop.com/us/en/thelocker/what-is-skin-temperature-and-why-should-you-monitor-it/ (accessed on 3 March 2025).
- Han, S.; Roh, D.; Park, J.; Shin, H. Design of Multi-Wavelength Optical Sensor Module for Depth-Dependent Photoplethysmography. Sensors 2019, 19, 5441. [Google Scholar] [CrossRef] [PubMed]
- SunLED. XZTNI54W. 7 December 2020. Available online: https://www.sunledusa.com/products/spec/XZTNI54W.pdf (accessed on 3 March 2025).
- SunLED. XZCDGK54W-1VF. 2 January 2020. Available online: https://www.sunledusa.com/products/spec/XZCDGK54W-1VF.pdf (accessed on 3 March 2025).
- SunLED. XZCM2CRK54WA-1VF. 21 May 2019. Available online: https://www.sunledusa.com/products/spec/XZCM2CRK54WA-1VF.pdf (accessed on 3 March 2025).
- Microchip. MCP6024. 2023. Available online: https://www.microchip.com/en-us/product/mcp6024 (accessed on 3 March 2025).
- Texas Instruments. ADS111x Ultra-Small, Low-Power, I2C-Compatible, 860-SPS, 16-Bit ADCs Wit Internal Reference, Oscillator, and Programmable Comparator. May 2009. Available online: https://www.ti.com/lit/ds/symlink/ads1114.pdf (accessed on 3 March 2025).
- Shenzhen Kinghelm Elec. KH5220-A36. 2023. Available online: https://jlcpcb.com/partdetail/Shenzhen_KinghelmElec-KH5220A36/C484543 (accessed on 3 March 2025).
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beamwidth on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M. Low-Level Laser (Light) Therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar] [PubMed]
- Ou-Yang, H.; Stamatas, G.; Kollias, N. Spectral Responses of Melanin to Ultraviolet A Irradiation. J. Investig. Dermatol. 2004, 122, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Shriram, R.; Sundhararajan, M.; Daimiwal, N. Effect of change in intensity of infrared LED on a photople-thysmogram. In Proceedings of the 2014 International Conference on Communication and Signal Processing, Chennai, India, 3–5 April 2014; pp. 1064–1067. [Google Scholar]
- Vishay. VEMD2503X01. 5 April 2013. Available online: https://www.vishay.com/docs/84163/vemd2503x01.pdf (accessed on 13 March 2025).
- Scardulla, F.; D’Acquisto, L.; Clombarini, R.; Hu, S.; Pasta, S.; Bellavia, D. A Study on the Effect of Contact Pressure During Physical Activity on Photoplethysmographic Heart Rate Measurements. Sensors 2020, 20, 5052. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.F.; Zhang, Y.T. The effect of contacting force on photoplethysmographic signals. Physiol. Meas. 2004, 25, 1323. [Google Scholar] [CrossRef] [PubMed]
- Complete Dissertation. Pearson’s Correlation Coefficient: A Comprehensive Overview. Available online: https://www.statisticssolutions.com/free-resources/directory-of-statistical-analyses/pearsons-correlation-coefficient/ (accessed on 3 March 2025).
- Sheldon, R.; Burke, J. Signal-to-Noise Ratio (S/N or SNR). Available online: https://www.techtarget.com/searchnetworking/definition/signal-to-noise-ratio (accessed on 3 March 2025).
- Bent, B.; Goldstein, B.A.; Kibbe, W.A.; Dunn, J.P. Investigating sources of inaccuracy in wearable optical heart rate sensors. Digit. Med. 2020, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Beurer. PO 30 Pulse Oximeter. 2018. Available online: https://hayesfirstaidsupplies.com/image/82150-USER-MANUAL-Beurer-PO30-SpO2-Monitor-Fingertip-Pulse-Oximeter.pdf (accessed on 3 March 2025).
- Aka, A. Source of 5.5 Hz Noise Showing up on ADC. 2018. Available online: https://electronics.stackexchange.com/questions/349182/source-of-5-5-hz-noise-showing-up-on-adc (accessed on 3 March 2025).
- Beurer. BM 28. 2023. Available online: https://res.cloudinary.com/beurer/raw/upload/v1592420995/product-and-content-live-uk/ef50fed72120ed36776d85f56c1b4ddff4733a5ecb99ac409ec12e97a411be9f.pdf (accessed on 3 March 2025).
| Category | Device 1 | Device 2 |
|---|---|---|
| Body Location | Index Fingertip | Index Fingertip |
| Light Source Wavelength | Red and Infrared | Green and Infrared |
| Number of Light Sources | Two Pairs for Red One Pair for Infrared | Two Pairs Green One Pair for Infrared |
| Light Source Brightness (Duty Cycle) | 175 for Red 250 for Infrared | 100 for Green 250 for Infrared |
| Photodetector Lens Shape | Flat | Flat |
| Sensor-to-Skin Contact Pressure | High | High |
| Device Configuration | Pearson Correlation Coefficient |
|---|---|
| Red LED PPG Device | 0.846 |
| Green LED PPG Device | 0.558 |
| Device Configuration | Average SNR (dB) |
|---|---|
| Red LED PPG Device | 10.723 |
| Green LED PPG Device | 7.165 |
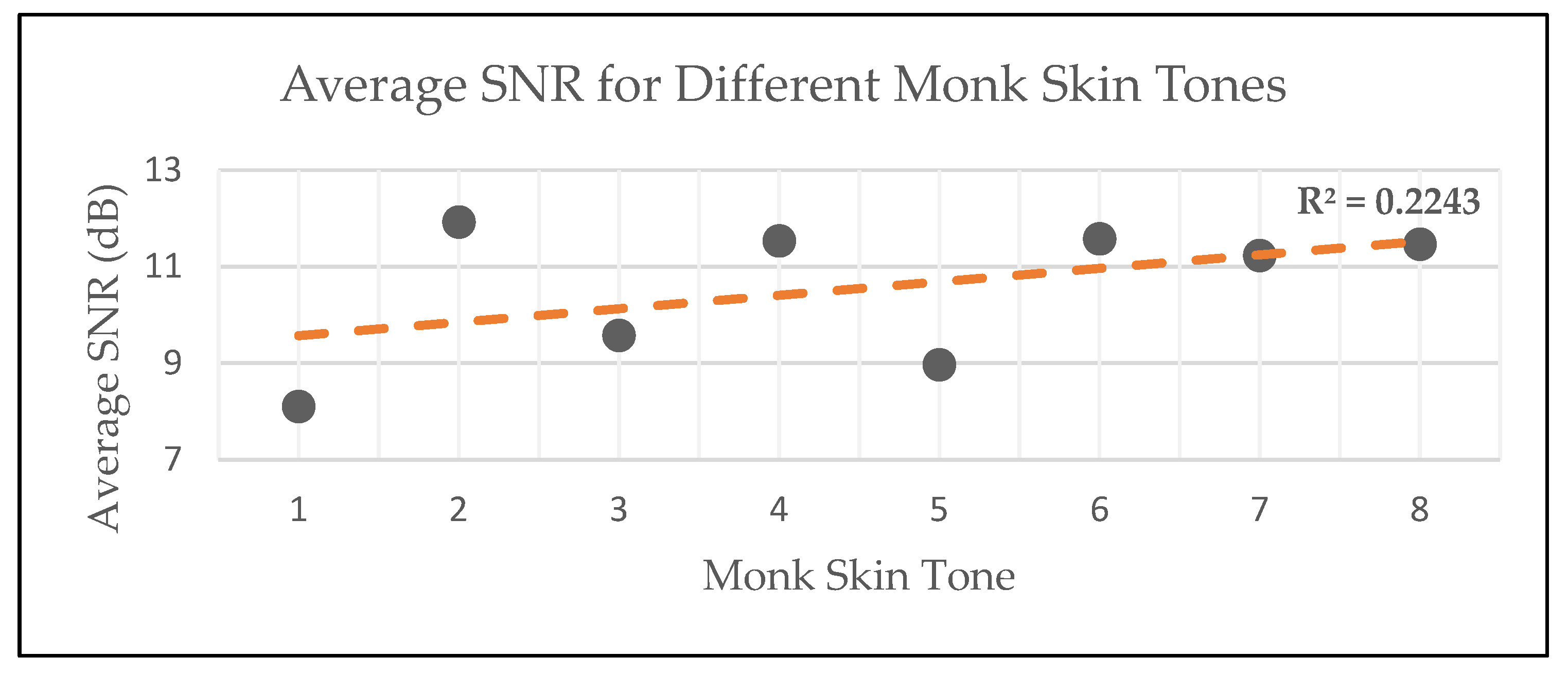
| Participants’ Monk Skin Tone | Number of Participants | Average SNR (dB) |
|---|---|---|
| 1 | 7 | 8.11 |
| 2 | 23 | 11.92 |
| 3 | 19 | 9.57 |
| 4 | 5 | 11.53 |
| 5 | 13 | 8.97 |
| 6 | 19 | 11.58 |
| 7 | 16 | 11.23 |
| 8 | 8 | 11.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appel, A.; Theart, R.P. Investigating the Design of a Photoplethysmography Device for Vital Sign Monitoring. Sensors 2025, 25, 1875. https://doi.org/10.3390/s25061875
Appel A, Theart RP. Investigating the Design of a Photoplethysmography Device for Vital Sign Monitoring. Sensors. 2025; 25(6):1875. https://doi.org/10.3390/s25061875
Chicago/Turabian StyleAppel, Anneri, and Rensu P. Theart. 2025. "Investigating the Design of a Photoplethysmography Device for Vital Sign Monitoring" Sensors 25, no. 6: 1875. https://doi.org/10.3390/s25061875
APA StyleAppel, A., & Theart, R. P. (2025). Investigating the Design of a Photoplethysmography Device for Vital Sign Monitoring. Sensors, 25(6), 1875. https://doi.org/10.3390/s25061875







