Electrodermal Activity Analysis at Different Body Locations
Abstract
1. Introduction
2. Materials and Methods
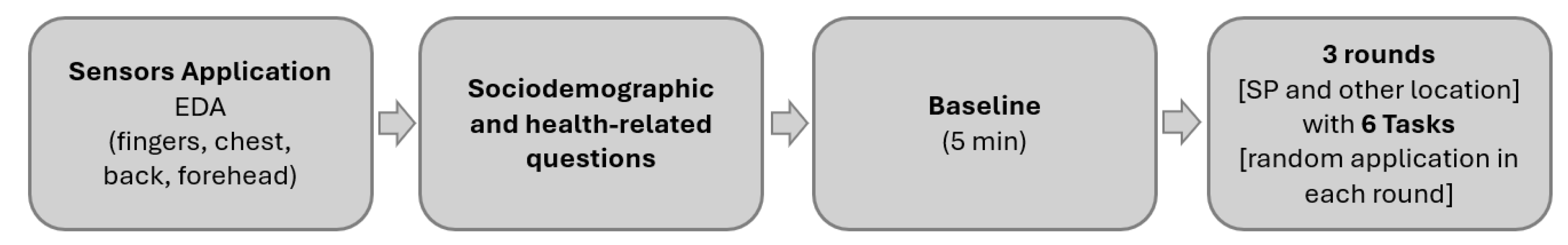
2.1. Data Collection
- On the hand (SP/gold standard): one of the electrodes was placed on the proximal phalange of the index finger and of the middle finger, on the non-dominant hand;
- On the anterior face of the torso (chest): the two electrodes were placed next to each other, on the Rectus Abdominis, at the sternum level;
- On the posterior face and superior part of the torso (back): the two electrodes were placed next to each other, on the inferior zone of the trapezius muscle;
- On the forehead: the two electrodes were placed next to each other, on the frontal area, approximately 2 cm above the procerus.
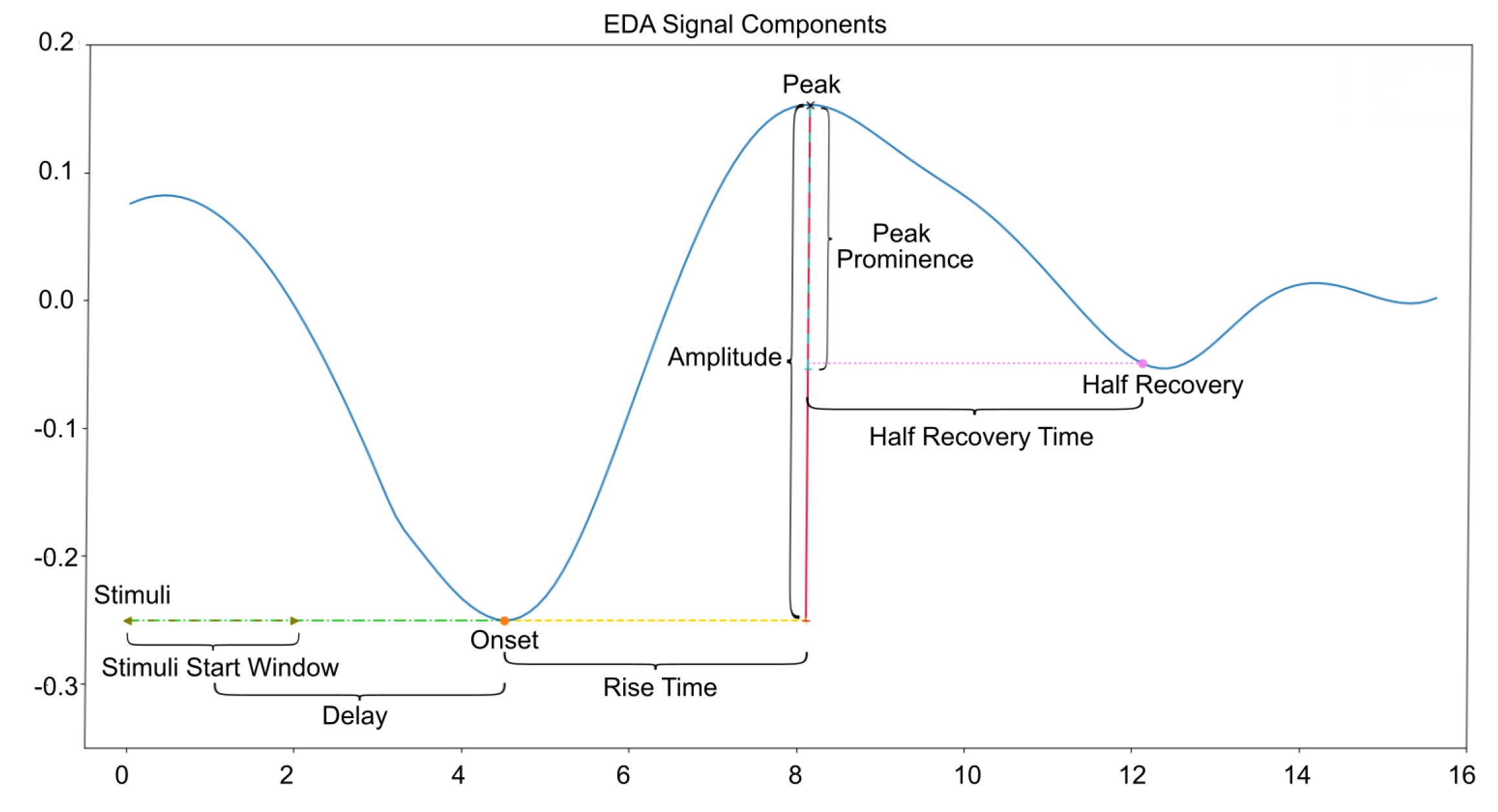
2.2. Data Processing
3. Results
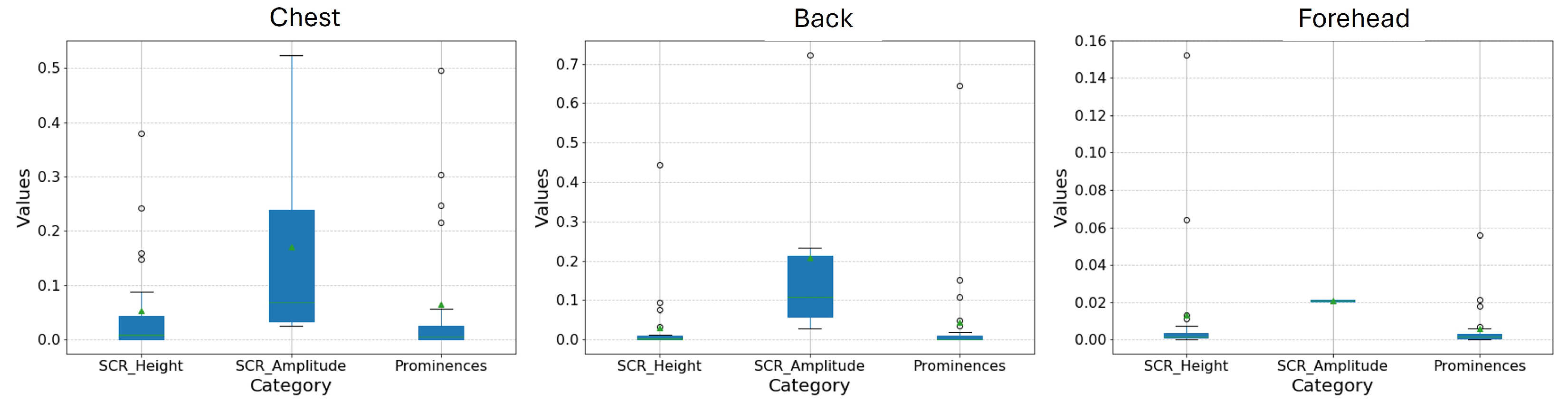
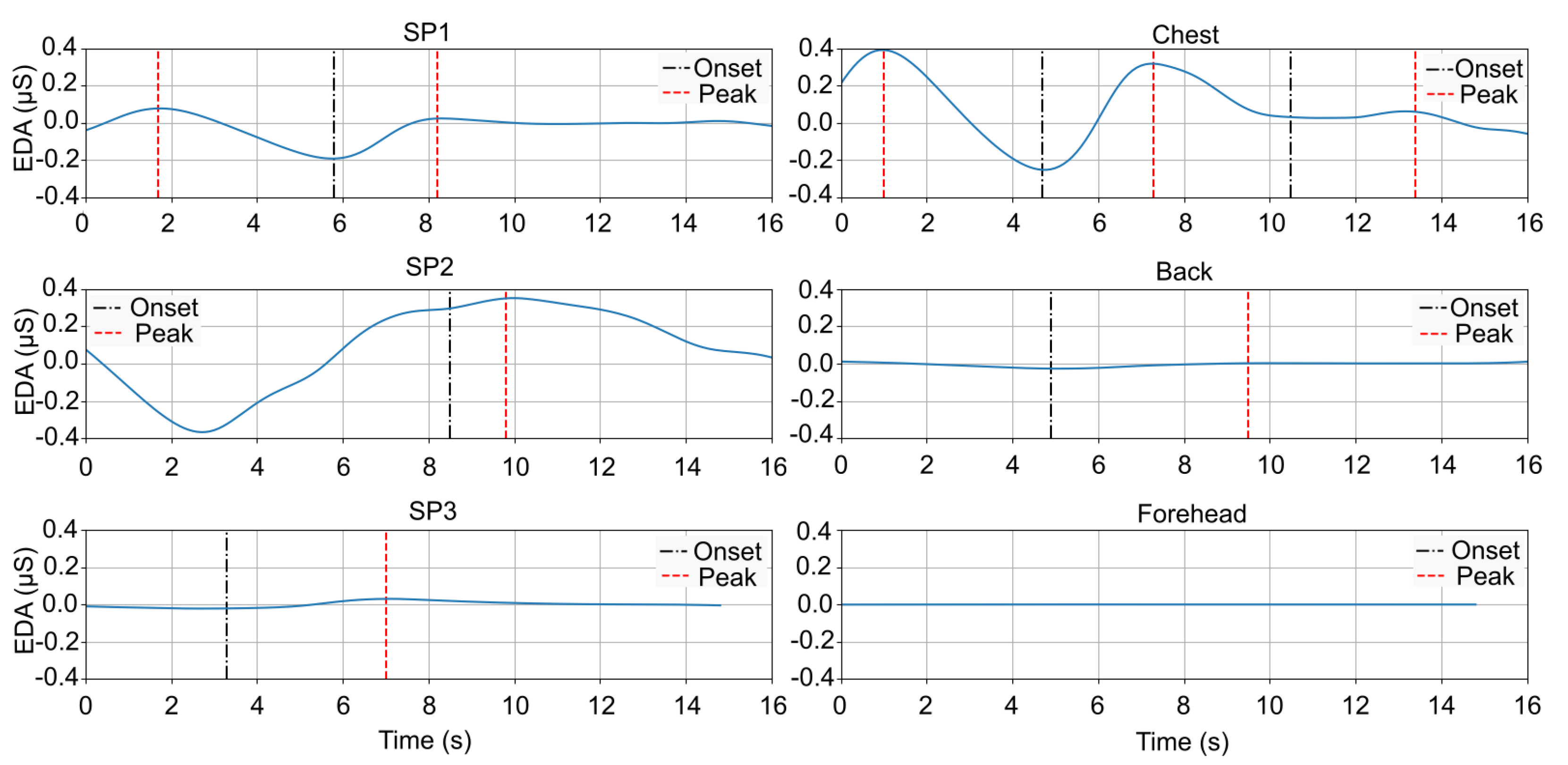
3.1. SCR Results by Location
3.2. Correlations with the Standard Position
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANS | Autonomic Nervous System |
| EDA | Electrodermal Activity |
| SCL | Skin Conductance Level |
| SCR | Skin Conductance Response |
| SP | Standard Position |
References
- Critchley, H.; Nagai, Y. Electrodermal Activity (EDA). In Encyclopedia of Behavioral Medicine; Gellman, M.D., Turner, J.R., Eds.; Springer: New York, NY, USA, 2013; pp. 666–669. [Google Scholar]
- Berne, R.; Levy, M. Fisiologia [Physiology]; Guanabara Koogan: Rio de Janeiro, Brazil, 1996. [Google Scholar]
- Bari, D.; Yacoob, H. Electrodermal Activity: Simultaneous Recordings; IntechOpen: London, UK, 2019. [Google Scholar]
- Posada-Quintero, H.F.; Florian, J.P.; Orjuela-Cañón, A.D.; Chon, K.H. Electrodermal Activity Is Sensitive to Cognitive Stress under Water. Front. Physiol. 2018, 8, 1128. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Mosa, A.H.; Machot, F.A.; Kyamakya, K. Emotion Recognition Involving Physiological and Speech Signals: A Comprehensive Review. In Recent Advances in Nonlinear Dynamics and Synchronization: With Selected Applications in Electrical Engineering, Neurocomputing, and Transportation; Kyamakya, K., Mathis, W., Stoop, R., Chedjou, J.C., Li, Z., Eds.; Studies in Systems, Decision and Control; Springer International Publishing: Cham, Switzerland, 2018; pp. 287–302. [Google Scholar]
- Braithwaite, J.; Watson, D.; Jones, R.; Rowe, M. A Guide for Analysing Electrodermal Activity (EDA) & Skin Conductance Responses (SCRs) for Psychological Experiments, 2nd ed.; Technical Report; University of Birmingham, Selective Attention&Awareness Laboratory (SAAL) Behavioural Brain Sciences Centre: Birmingham, UK, 2015. [Google Scholar]
- Vavrinsky, E.; Stopjakova, V.; Kopani, M.; Kosnacova, H. The Concept of Advanced Multi-Sensor Monitoring of Human Stress. Sensors 2021, 21, 3499. [Google Scholar] [CrossRef] [PubMed]
- Benedek, M.; Kaernbach, C. Decomposition of skin conductance data by means of nonnegative deconvolution. Psychophysiology 2010, 47, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.M.; Lang, P.J. Emotion and Motivation. In Handbook of Psychophysiology, 3rd ed.; Cambridge University Press: New York, NY, USA, 2007; pp. 581–607. [Google Scholar]
- Reinhardt, T.; Schmahl, C.; Wüst, S.; Bohus, M. Salivary cortisol, heart rate, electrodermal activity and subjective stress responses to the Mannheim Multicomponent Stress Test (MMST). Psychiatry Res. 2012, 198, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Boucsein, W. Electrodermal Activity; Springer: Boston, MA, USA, 2012. [Google Scholar]
- Wilke, K.; Martin, A.; Terstegen, L.; Biel, S.S. A short history of sweat gland biology. Int. J. Cosmet. Sci. 2007, 29, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J.; Hamlin, T.; Lantz, J.F.; Villavicencio, T.; Coles, J.; Beversdorf, D.Q. Examining the Association Between Electrodermal Activity and Problem Behavior in Severe Autism Spectrum Disorder: A Feasibility Study. Front. Psychiatry 2019, 10, 654. [Google Scholar] [CrossRef] [PubMed]
- Klimek, A.; Mannheim, I.; Schouten, G.; Wouters, E.J.M.; Peeters, M.W.H. Wearables measuring electrodermal activity to assess perceived stress in care: A scoping review. Acta Neuropsychiatr. 2023, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kappeler-Setz, C.; Gravenhorst, F.; Schumm, J.; Arnrich, B.; Tröster, G. Towards long term monitoring of electrodermal activity in daily life. Pers. Ubiquitous Comput. 2013, 17, 261–271. [Google Scholar] [CrossRef]
- Ferreira, A.F.; da Silva, H.P.; Alves, H.; Marques, N.; Fred, A. Feasibility of Electrodermal Activity and Photoplethysmography Data Acquisition at the Foot Using a Sock Form Factor. Sensors 2023, 23, 620. [Google Scholar] [CrossRef] [PubMed]
- van Dooren, M.; de Vries, J.J.G.G.J.; Janssen, J.H. Emotional sweating across the body: Comparing 16 different skin conductance measurement locations. Physiol. Behav. 2012, 106, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.F.H.; Schell, A.M.; Dawson, M.E. Lapses in skin conductance responding across anatomical sites: Comparison of fingers, feet, forehead, and wrist. Psychophysiology 2016, 53, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Kasos, K.; Kekecs, Z.; Csirmaz, L.; Zimonyi, S.; Vikor, F.; Kasos, E.; Veres, A.; Kotyuk, E.; Szekely, A. Bilateral comparison of traditional and alternate electrodermal measurement sites. Psychophysiology 2020, 57, e13645. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Kong, Y.; Posada-Quintero, H.F.; Chon, K.H. Comparison of Electrodermal Activity from Multiple Body Locations Based on Standard EDA Indices’ Quality and Robustness against Motion Artifact. Sensors 2022, 22, 3177. [Google Scholar] [CrossRef] [PubMed]
- Costardi, J.V.V.; Nampo, R.A.T.; Silva, G.L.; Ribeiro, M.A.F.; Stella, H.J.; Stella, M.B.; Malheiros, S.V.P. A review on alcohol: From the central action mechanism to chemical dependency. Rev. Assoc. Médica Bras. 2015, 61, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.A.; Smith, B.D. Caffeine and novelty: Effects on electrodermal activity and performance. Physiol. Behav. 1991, 49, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Boucsein, W.; Fowles, D.C.; Grimnes, S.; Ben-Shakhar, G.; Roth, W.T.; Dawson, M.E.; Filion, D.L. Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures. Publication recommendations for electrodermal measurements. Psychophysiology 2012, 49, 1017–1034. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, C.; Gomes, M.; Cardoso, H.; Ferreira, N.; Vigário, R.; Quintão, C.; Fonseca, M. An Integrated System Combining Virtual Reality with a Glove with Biosensors for Neuropathic Pain: A Concept Validation. In Advances in Human Factors and Systems Interaction. AHFE 2018. Advances in Intelligent Systems and Computing; Nunes, I.L., Ed.; Springer: Cham, Switzerland, 2019; pp. 274–284. [Google Scholar] [CrossRef]
- Makowski, D.; Pham, T.; Lau, Z.J.; Brammer, J.C.; Lespinasse, F.; Pham, H.; Schölzel, C.; Chen, S.H.A. NeuroKit2: A Python toolbox for neurophysiological signal processing. Behav. Res. Methods 2021, 53, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Walach, H. Electrodermal activity (EDA): State-of-the-art measurement and techniques for parapsychological purposes. J. Parapsychol. 2000, 64, 139–163. [Google Scholar]
- Chao, C.C.J. Correlation, Pearson. In The SAGE Encyclopedia of Communication Research Methods; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2017; pp. 267–270. [Google Scholar]
- Scarpina, F.; Tagini, S. The Stroop Color and Word Test. Front. Psychol. 2017, 8, 557. [Google Scholar] [CrossRef] [PubMed]

| Location | SCR Height | SCR Amplitude | SCR Prominence |
|---|---|---|---|
| SP | 0.19 (0.31) | 0.34 (0.48) | 0.26 (0.43) |
| Chest | 0.05 (0.10) | 0.17 (0.18) | 0.07 (0.13) |
| SP | 0.33 (0.44) | 0.75 (0.99) | 0.40 (0.52) |
| Back | 0.03 (0.09) | 0.21 (0.24) | 0.04 (0.13) |
| SP | 0.06 (0.06) | 0.10 (0.09) | 0.07 (0.08) |
| Forehead | 0.01 (0.03) | 0.02 (0.00) | 0.01 (0.01) |
| Location | Task | Tonic | Phasic |
|---|---|---|---|
| Chest | Hot | 0.526 | 0.357 |
| Sandpaper | 0.401 | 0.285 | |
| Pin | 0.551 | 0.244 | |
| Cold | 0.069 | 0.192 | |
| Cotton | 0.359 | 0.234 | |
| Breath | 0.041 | 0.253 | |
| Back | Hot | 0.462 | 0.143 |
| Sandpaper | 0.190 | 0.114 | |
| Pin | 0.054 | 0.084 | |
| Cold | 0.105 | 0.243 | |
| Cotton | 0.026 | 0.068 | |
| Breath | 0.219 | 0.167 | |
| Forehead | Hot | 0.501 | 0.110 |
| Sandpaper | 0.105 | 0.116 | |
| Pin | −0.316 | 0.211 | |
| Cold | −0.175 | −0.057 | |
| Cotton | −0.222 | 0.087 | |
| Breath | 0.080 | 0.122 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamboa, P.; Varandas, R.; Mrotzeck, K.; Plácido da Silva, H.; Quaresma, C. Electrodermal Activity Analysis at Different Body Locations. Sensors 2025, 25, 1762. https://doi.org/10.3390/s25061762
Gamboa P, Varandas R, Mrotzeck K, Plácido da Silva H, Quaresma C. Electrodermal Activity Analysis at Different Body Locations. Sensors. 2025; 25(6):1762. https://doi.org/10.3390/s25061762
Chicago/Turabian StyleGamboa, Patricia, Rui Varandas, Katrin Mrotzeck, Hugo Plácido da Silva, and Cláudia Quaresma. 2025. "Electrodermal Activity Analysis at Different Body Locations" Sensors 25, no. 6: 1762. https://doi.org/10.3390/s25061762
APA StyleGamboa, P., Varandas, R., Mrotzeck, K., Plácido da Silva, H., & Quaresma, C. (2025). Electrodermal Activity Analysis at Different Body Locations. Sensors, 25(6), 1762. https://doi.org/10.3390/s25061762












