An Enhanced Bimetallic Optical Fiber SPR Biosensor Using Graphene Oxide for the Label-Free and Sensitive Detection of Human IgG
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ag@Au/GO Fiber Optic SPR Sensors
2.3. Ag@Au/GO Sensor Antibody Fixation and Antigen–Antibody Immunoassay
2.4. Evaluation of Specificity and Selectivity of Antigen–Antibody Immunoassays and Sensor Stability Testing
3. Results and Discussion
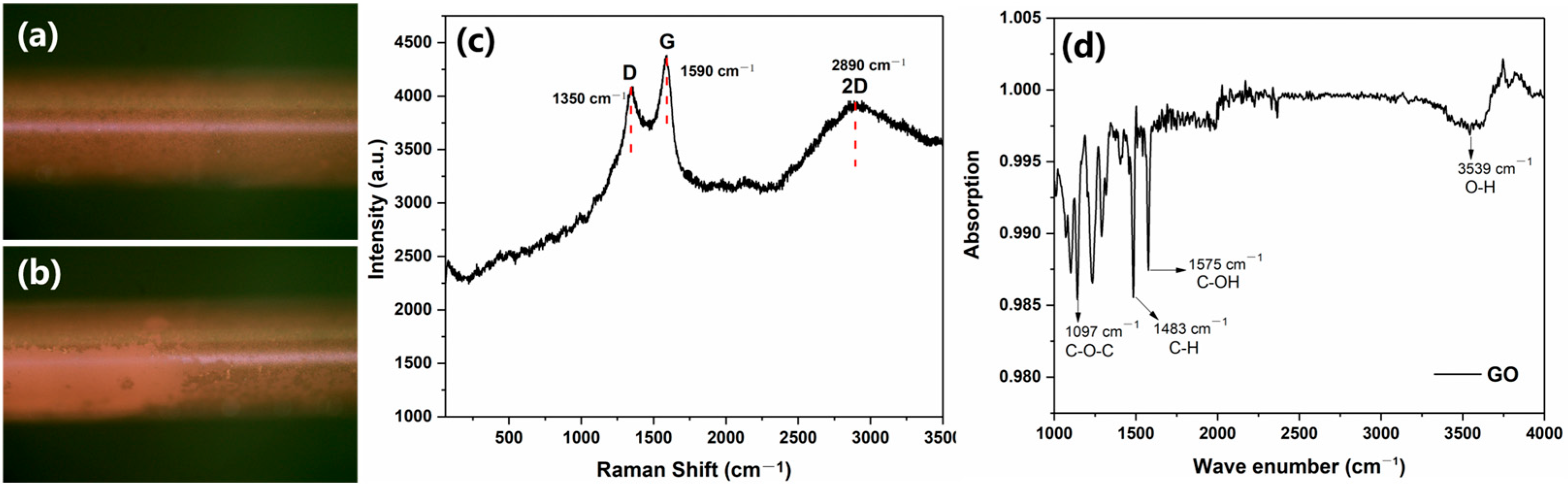
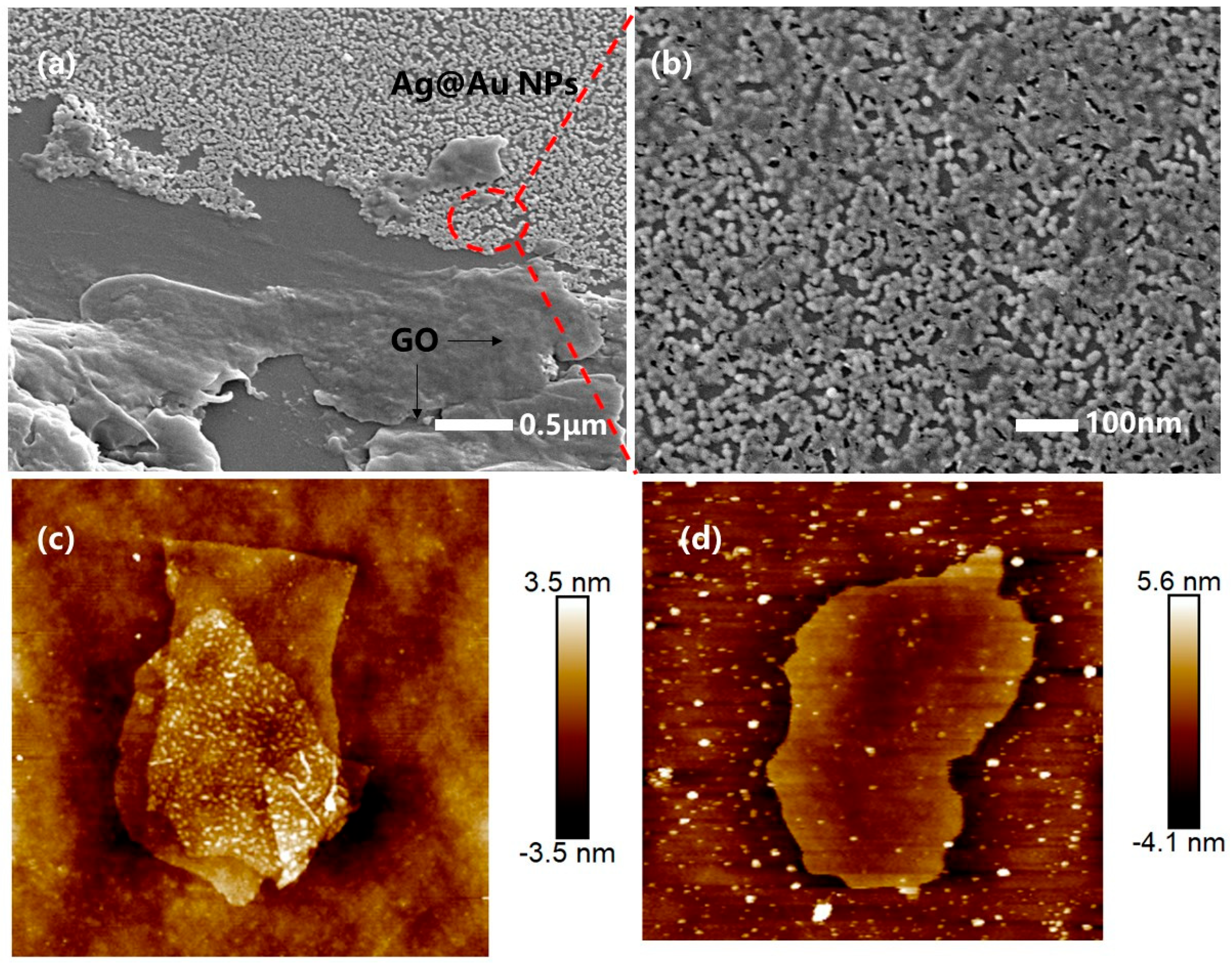
3.1. Characterization of Fiber Optic SPR Sensors for GO Functionalization
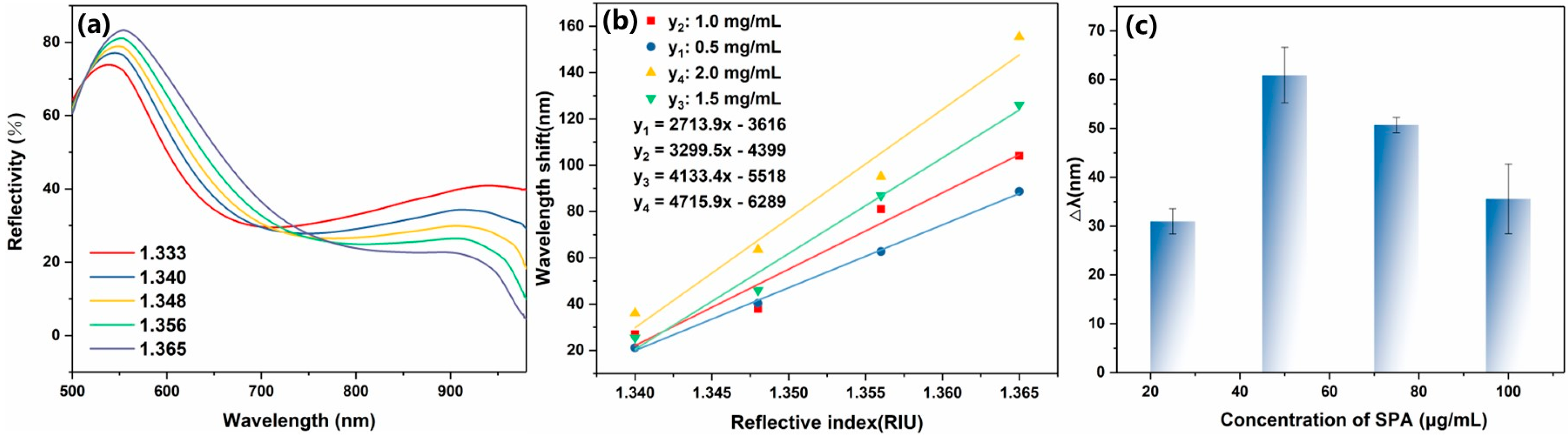
3.2. Ag@Au Sensor GO/SPA Modification and SPR Characterization Tests
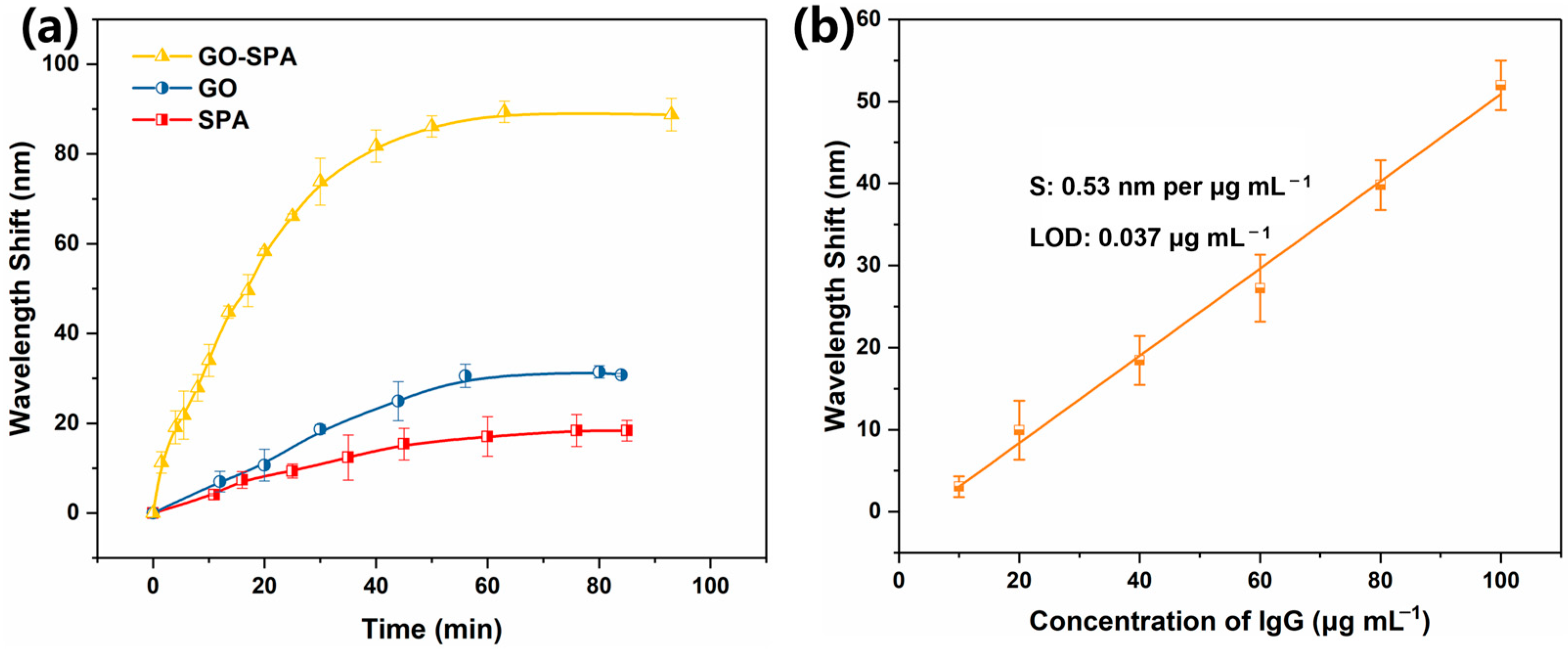
3.3. Ag@Au/GO/SPA Sensor Antibody Fixation and Antigen Detection
3.4. Sensor Detection Specificity, Selectivity, and Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larsen, M.D.; de Graaf, E.L.; Sonneveld, M.E.; Plomp, H.R.; Nouta, J.; Hoepel, W.; Chen, H.-J.; Linty, F.; Visser, R.; Brinkhaus, M.; et al. Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science 2021, 371, eabc8378. [Google Scholar] [CrossRef] [PubMed]
- Momeni, F.; Ahmadi, V.; Babashamsi, M. All fiber Mach-Zehnder interferometer immunosensor based on rabbit anti-human IgG and protein a for human IgG detection. Measurement 2024, 236, 115099. [Google Scholar] [CrossRef]
- Klein, S.; Pekosz, A.; Park, H.-S.; Ursin, R.; Shapiro, J.; Benner, S.; Littlefield, K.; Kumar, S.; Naik, H.M.; Betenbaugh, M.; et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J. Clin. Investig. 2020, 130, 6141–6150. [Google Scholar] [CrossRef]
- Liu, W.; Liu, L.; Kou, G.; Zheng, Y.; Ding, Y.; Ni, W.; Wang, Q.; Tan, L.; Wu, W.; Tang, S.; et al. Evaluation of Nucleocapsid and Spike Protein-Based Enzyme-Linked Immunosorbent Assays for Detecting Antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020, 58, e00461-20. [Google Scholar] [CrossRef]
- Fukuyama, M.; Nakamura, A.; Nishiyama, K.; Imai, A.; Tokeshi, M.; Shigemura, K.; Hibara, A. Noncompetitive Fluorescence Polarization Immunoassay for Protein Determination. Anal. Chem. 2020, 92, 14393–14397. [Google Scholar] [CrossRef]
- Liang, A.; Tang, S.; Liu, M.; Yi, Y.; Xie, B.; Hou, H.; Luo, A. A molecularly imprinted electrochemical sensor with tunable electrosynthesized Cu-MOFs modification for ultrasensitive detection of human IgG. Bioelectrochemistry 2022, 146, 108154. [Google Scholar] [CrossRef]
- Liang, A.A.; Hou, B.H.; Tang, C.S.; Sun, D.L.; Luo, E.A. An advanced molecularly imprinted electrochemical sensor for the highly sensitive and selective detection and determination of Human IgG. Bioelectrochemistry 2021, 137, 107671. [Google Scholar] [CrossRef]
- Gumilar, G.; Chowdhury, S.; Shukri, G.; Patah, A.; Nugraha, N.; Henzie, J.; Anshori, I.; Kaneti, Y.V.; Yuliarto, B. The revelation of glucose adsorption mechanisms on hierarchical metal-organic frameworks using a surface plasmon resonance sensor. J. Mater. Chem. B 2023, 11, 4428–4444. [Google Scholar] [CrossRef]
- Yuan, Y.; Peng, X.; Weng, X.; He, J.; Liao, C.; Wang, Y.; Liu, L.; Zeng, S.; Song, J.; Qu, J. Two-dimensional nanomaterials as enhanced surface plasmon resonance sensing platforms: Design perspectives and illustrative applications. Biosens. Bioelectron. 2023, 241, 115672. [Google Scholar] [CrossRef]
- Vandezande, W.; Dillen, A.; Lammertyn, J.; Roeffaers, M.B.J. FO-SPR Model for Full-Spectrum Signal Analysis of Back-reflecting FO-SPR Sensors to Monitor MOF Deposition. ACS Sens. 2024, 9, 2110–2121. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, B.-T. Surface plasmon resonance biosensor based on graphene oxide/silver coated polymer cladding silica fiber. Sens. Actuators B 2018, 275, 332–338. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, C.; Xia, B.; Wang, N.; Chen, X.; Jing, X.; Chen, M.; Xu, X. An enhanced SPR optical fiber biosensor using Ti3C2TxMXene/AuNPs for label-free and sensitive detection of human IgG. Nanoscale 2024, 16, 18477–18487. [Google Scholar] [CrossRef] [PubMed]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chang, H.; Li, P.; Liu, R.; Xue, Q. Fabrication and characterization of an ultrasensitive humidity sensor based on metal oxide/graphene hybrid nanocomposite. Sens. Actuators B 2016, 225, 233–240. [Google Scholar] [CrossRef]
- Ahn, Y.; Jeong, Y.; Lee, Y. Improved Thermal Oxidation Stability of Solution-Processable Silver Nanowire Transparent Electrode by Reduced Graphene Oxide. ACS Appl. Mater. Interfaces 2012, 4, 6410–6414. [Google Scholar] [CrossRef]
- Kumar, H.; Sharma, R.; Yadav, A.; Kumari, R. Recent advancement made in the field of reduced graphene oxide-based nanocomposites used in the energy storage devices: A review. J. Energy Storage 2021, 33, 102032. [Google Scholar] [CrossRef]
- Li, Z.; Xie, R.; Sun, G.; Liu, X.; Xin, H.; Chen, Y.; Chen, S.; Rao, L.; Yan, B.; Wang, K.; et al. Ultrasensitive detection of SCCA employing a graphene oxide integrated microfiber ring laser biosensor. Biosens. Bioelectron. 2025, 267, 116772. [Google Scholar] [CrossRef]
- Keyvani, A.H.; Mohammadnejad, P.; Pazoki-Toroudi, H.; Gilabert, I.P.; Chu, T.; Manshian, B.B.; Soenen, S.J.; Sohrabi, B. Advancements in Cancer Treatment: Harnessing the Synergistic Potential of Graphene-Based Nanomaterials in Combination Therapy. ACS Appl. Mater. Interfaces 2025, 17, 2756–2790. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Yang, X. Probing the formation mechanisms of reactive oxygen species in graphene oxide-catalyzed ozone advanced oxidation processes. Carbon 2025, 233, 119831. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Wang, Q. Gold nanoparticles (AuNPs) and graphene oxide heterostructures with gold film coupling for an enhanced sensitivity surface plasmon resonance (SPR) fiber sensor. Instrum. Sci. Technol. 2022, 50, 530–542. [Google Scholar] [CrossRef]
- Islam, M.A.; Masson, J.-F. Plasmonic Biosensors for Health Monitoring: Inflammation Biomarker Detection. ACS Sens. 2025, 10, 577–601. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fu, Y.; Yang, R.; Guo, J.; Guo, J. Surface plasmonic biosensors: Principles, designs and applications. Analyst 2023, 148, 6146–6160. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yin, H.T.; Zhao, Z.; Cui, M.; Huang, R.L.; Su, R.X. An Au-Ag@Au fiber surface plasmon resonance sensor for highly sensitive detection of fluoroquinolone residues. Analyst 2025, 150, 877–886. [Google Scholar] [CrossRef]
- Qian, Y.Z.; Wang, Q. A U-Shaped Long-Range Surface Plasmon Resonance (LRSPR) Biosensor With Low Detection Limit. IEEE Sens. J. 2024, 24, 23818–23825. [Google Scholar] [CrossRef]
- Shi, S.; Wang, L.; Su, R.; Liu, B.; Huang, R.; Qi, W.; He, Z. A polydopamine-modified optical fiber SPR biosensor using electroless-plated gold films for immunoassays. Biosens. Bioelectron. 2015, 74, 454–460. [Google Scholar] [CrossRef]
- Yan, S.; Pu, S.; Zhang, Y.; Yuan, M.; Zhang, C. Sensing properties of graphene-oxide-functionalized single-mode-no-core-single-mode fiber structure. Results Phys. 2021, 25, 104310. [Google Scholar] [CrossRef]
- Azeman, N.H.; Bakar, M.H.A.; Nazri, N.A.A.; Mobarak, N.N.; Khushaini, M.A.A.; Aziz, T.H.T.A.; Zain, A.R.M.; Bakar, A.A.A. Carboxymethyl chitosan/graphene oxide/silver nanotriangles nanohybrid as the sensing materials for the enhancement of ammonia localized surface plasmon resonance sensor. Opt. Laser Technol. 2022, 148, 107789. [Google Scholar] [CrossRef]
- Shahriari, S.; Sastry, M.; Panjikar, S.; Raman, R.K.S. Graphene and Graphene Oxide as a Support for Biomolecules in the Development of Biosensors. Nanotechnol. Sci. Appl. 2021, 14, 197–220. [Google Scholar] [CrossRef]
- Wang, Q.; Jing, J.-Y.; Wang, B.-T. Highly Sensitive SPR Biosensor Based on Graphene Oxide and Staphylococcal Protein A Co-Modified TFBG for Human IgG Detection. IEEE Trans. Instrum. Meas. 2019, 68, 3350–3357. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, B. Sensitivity enhanced SPR immunosensor based on graphene oxide and SPA co-modified photonic crystal fiber. Opt. Laser Technol. 2018, 107, 210–215. [Google Scholar] [CrossRef]


| Analyte | Modified Materials for Sensors | RI Sensitivity (nm/RIU) | Analyte Detection Sensitivity (nm/μg/mL) | LOD (μg/mL) | Ref. |
|---|---|---|---|---|---|
| Human IgG | GO/AgNPs | 3311.47 | 0.4985 | 0.04 | [11] |
| Human IgG | Ti3C2Tx MXene/AuNPs | 2804.5 | 1.7046 | 0.17 | [12] |
| Human IgG | Carbon nanotubes | 5948.57 | 3.272 | 0.006 | [24] |
| Human IgG | GO/SPA | 4649.8 | / | 0.01 | [30] |
| Analyte | Double-layer Au NPs/GO | 3436.2 | / | / | [25] |
| Analyte | Au-Ag@AuNPs | 3512 | / | / | [23] |
| Human IgG | AuNPs | 2054 (1.333–1.359) 3980 (1.359–1.386) | 0.41 | 0.9 | [20] |
| Human IgG | Ag@AuNPs/GO | 4715.9 | 0.53 | 0.037 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Yin, H.; Cui, M.; Huang, R.; Su, R. An Enhanced Bimetallic Optical Fiber SPR Biosensor Using Graphene Oxide for the Label-Free and Sensitive Detection of Human IgG. Sensors 2025, 25, 1630. https://doi.org/10.3390/s25051630
Xu Q, Yin H, Cui M, Huang R, Su R. An Enhanced Bimetallic Optical Fiber SPR Biosensor Using Graphene Oxide for the Label-Free and Sensitive Detection of Human IgG. Sensors. 2025; 25(5):1630. https://doi.org/10.3390/s25051630
Chicago/Turabian StyleXu, Qiang, Huiting Yin, Mei Cui, Renliang Huang, and Rongxin Su. 2025. "An Enhanced Bimetallic Optical Fiber SPR Biosensor Using Graphene Oxide for the Label-Free and Sensitive Detection of Human IgG" Sensors 25, no. 5: 1630. https://doi.org/10.3390/s25051630
APA StyleXu, Q., Yin, H., Cui, M., Huang, R., & Su, R. (2025). An Enhanced Bimetallic Optical Fiber SPR Biosensor Using Graphene Oxide for the Label-Free and Sensitive Detection of Human IgG. Sensors, 25(5), 1630. https://doi.org/10.3390/s25051630







