Application of Smart Watch-Based Functional Evaluation for Upper Extremity Impairment: A Preliminary Study on Older Emirati Stroke Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol and Participants
2.2. Clinical Functional Evaluation
2.3. Smart Watch-Based Data Acquisition and Processing
2.4. Sensor-Based Parameters
2.5. Serial Follow-Up of Sensor-Based Parameters
2.6. Statistical Analysis
3. Results
3.1. Demographic Data
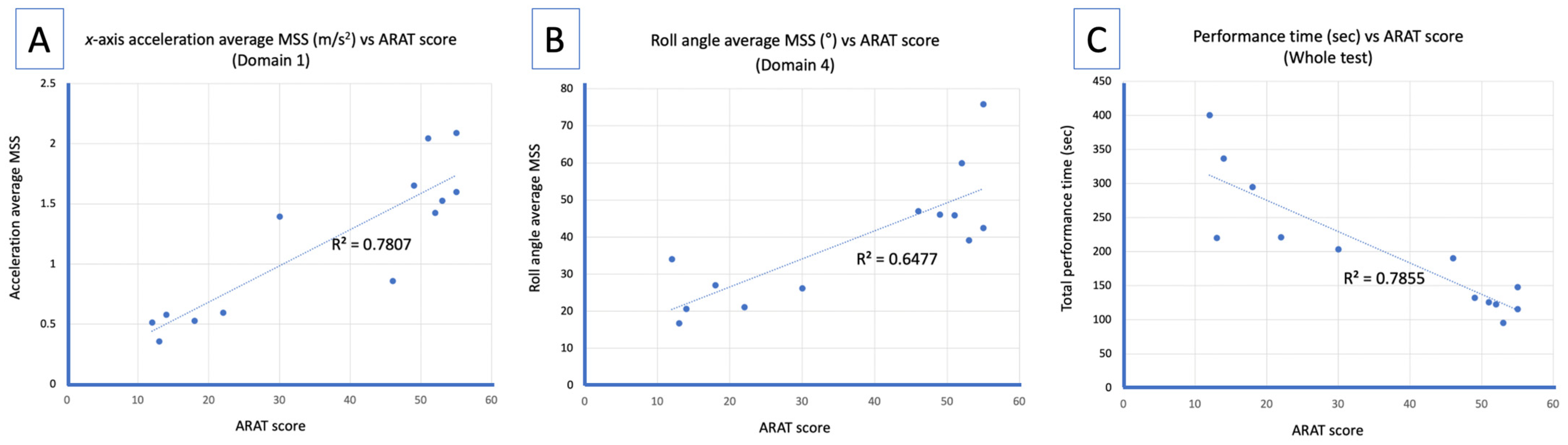
3.2. Correlation Analysis Between Average MSS and ARAT Score
3.3. Case-Series of Average MSS Before and After the Intervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IMU | Inertial Measurement Unit |
| ADL | Activities of Daily Living |
| ARAT | Action Research Arm Test |
| MBI | Modified Barthel Index |
| MSS | Motion Segment Size |
| MMSE | Mini-mental Status Exam |
| FMUE | Fugl-Meyer Upper Extremity |
References
- Rodgers, M.M.; Alon, G.; Pai, V.M.; Conroy, R.S. Wearable technologies for active living and rehabilitation: Current research challenges and future opportunities. J. Rehabil. Assist. Technol. Eng. 2019, 6, 2055668319839607. [Google Scholar] [PubMed]
- Liang, W.; Wang, F.; Fan, A.; Zhao, W.; Yao, W.; Yang, P. Extended Application of Inertial Measurement Units in Biomechanics: From Activity Recognition to Force Estimation. Sensors 2023, 23, 4229. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.-R.; Hwang, I.-W.; Kim, H.-J.; Yang, S.-H.; Lee, J.-M. Apple Watch 6 vs. Galaxy Watch 4: A Validity Study of Step-Count Estimation in Daily Activities. Sensors 2024, 24, 4658. [Google Scholar] [CrossRef] [PubMed]
- Smuck, M.; Muaremi, A.; Zheng, P.; Norden, J.; Sinha, A.; Hu, R.; Tomkins-Lane, C. Objective measurement of function following lumbar spinal stenosis decompression reveals improved functional capacity with stagnant real-life physical activity. Spine J. 2018, 18, 15–21. [Google Scholar]
- Dong, K.; Jiang, L.; Ding, B. Performances Investigation of Multi-Configuration Connections in Piezoelectric Energy Harvester. J. Vib. Eng. Technol. 2025, 13, 16. [Google Scholar] [CrossRef]
- Singh, B.; Chastin, S.; Miatke, A.; Curtis, R.; Dumuid, D.; Brinsley, J.; Ferguson, T.; Szeto, K.; Simpson, C.; Eglitis, E.; et al. Real-World Accuracy of Wearable Activity Trackers for Detecting Medical Conditions: Systematic Review and Meta-Analysis. JMIR mHealth uHealth 2024, 12, e56972. [Google Scholar]
- Masoumian Hosseini, M.; Hosseini, S.T.M.; Qayumi, K.; Hosseinzadeh, S.; Tabar, S.S.S. Smartwatches in healthcare medicine: Assistance and monitoring; A scoping review. BMC Med. Inform. Decis. Mak. 2023, 23, 248. [Google Scholar]
- Kristoffersson, A.; Linden, M. A Systematic Review of Wearable Sensors for Monitoring Physical Activity. Sensors 2022, 22, 573. [Google Scholar] [CrossRef]
- Shajari, S.; Kuruvinashetti, K.; Komeili, A.; Sundararaj, U. The Emergence of AI-Based Wearable Sensors for Digital Health Technology: A Review. Sensors 2023, 23, 9498. [Google Scholar] [CrossRef]
- Han, S.; Paul, R. Smartwatch gait coordination index: New measure for human gait utilizing smartwatch sensor. Medicine 2023, 102, e33267. [Google Scholar]
- Okita, S.; De Lucena, D.S.; Chan, V.; Reinkensmeyer, D.J. Measuring Movement Quality of the Stroke-Impaired Upper Extremity with a Wearable Sensor: Toward a Smoothness Metric for Home Rehabilitation Exercise Programs. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021; pp. 6691–6694. [Google Scholar]
- Cornec, G.; Lempereur, M.; Mensah-Gourmel, J.; Robertson, J.; Miramand, L.; Medee, B.; Bellaiche, S.; Gross, R.; Gracies, J.-M.; Remy-Neris, O.; et al. Measurement properties of movement smoothness metrics for upper limb reaching movements in people with moderate to severe subacute stroke. J. Neuroeng. Rehabil. 2024, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Cornella-Barba, G.; Okita, S.; Li, Z.; Reinkensmeyer, D.J. Real-Time Sensing of Upper Extremity Movement Diversity Using Kurtosis Implemented on a Smartwatch. Sensors 2024, 24, 5266. [Google Scholar] [CrossRef] [PubMed]
- Goubault, E.; Duval, C.; Martin, C.; Lebel, K. Innovative Detection and Segmentation of Mobility Activities in Patients Living with Parkinson’s Disease Using a Single Ankle-Positioned Smartwatch. Sensors 2024, 24, 5486. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.H.; Kim, Y.; Lee, K.-S.; Park, H.-S. Development and Clinical Evaluation of a Web-Based Upper Limb Home Rehabilitation System Using a Smartwatch and Machine Learning Model for Chronic Stroke Survivors: Prospective Comparative Study. JMIR mHealth uHealth 2020, 8, e17216. [Google Scholar] [CrossRef]
- Tripuraneni, K.R.; Foran, J.R.; Munson, N.R.; Racca, N.E.; Carothers, J.T. A Smartwatch Paired with A Mobile Application Provides Postoperative Self-Directed Rehabilitation Without Compromising Total Knee Arthroplasty Outcomes: A Randomized Controlled Trial. J. Arthroplast. 2021, 36, 3888–3893. [Google Scholar] [CrossRef]
- Demers, M.; Bishop, L.; Cain, A.; Saba, J.; Rowe, J.; Zondervan, D.K.; Winstein, C.J. Wearable Technology to Capture Arm Use of People with Stroke in Home and Community Settings: Feasibility and Early Insights on Motor Performance. Phys. Ther. 2024, 104, pzad172. [Google Scholar] [CrossRef]
- Straeten, F.A.; van Zyl, S.; Maus, B.; Bauer, J.; Raum, H.; Gross, C.C.; Bruchmann, S.; Landmeyer, N.C.; Faber, C.; Minnerup, J.; et al. EXERTION: A pilot trial on the effect of aerobic, smartwatch-controlled exercise on stroke recovery: Effects on motor function, structural repair, cognition, mental well-being, and the immune system. Neurol. Res. Pract. 2023, 5, 18. [Google Scholar] [CrossRef]
- Lawrie, S.; Dong, Y.; Steins, D.; Xia, Z.; Esser, P.; Sun, S.; Li, F.; Amor, J.D.; James, C.; Izadi, H.; et al. Evaluation of a smartwatch-based intervention providing feedback of daily activity within a research-naive stroke ward: A pilot randomised controlled trial. Pilot Feasibility Stud. 2018, 4, 157. [Google Scholar] [CrossRef]
- Alt Murphy, M.; Resteghini, C.; Feys, P.; Lamers, I. An overview of systematic reviews on upper extremity outcome measures after stroke. BMC Neurol. 2015, 15, 29. [Google Scholar] [CrossRef]
- Santisteban, L.; Térémetz, M.; Bleton, J.-P.; Baron, J.-C.; Maier, M.A.; Lindberg, P.G. Upper Limb Outcome Measures Used in Stroke Rehabilitation Studies: A Systematic Literature Review. PLoS ONE 2016, 11, e0154792. [Google Scholar] [CrossRef]
- Ohura, T.; Hase, K.; Nakajima, Y.; Nakayama, T. Validity and reliability of a performance evaluation tool based on the modified Barthel Index for stroke patients. BMC Med. Res. Methodol. 2017, 17, 131. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.S.; Lee, W.H.; Gil Seo, H.; Smuck, M.W.; Kim, S. Evaluation of Motion Segment Size as a New Sensor-based Functional Outcome Measure in Stroke Rehabilitation. J. Int. Med. Res. 2022, 50, 3000605221122750. [Google Scholar] [CrossRef]
- Nam, H.S.; Han, S.; Leigh, J.-H.; Bang, M.S. Smartwatch-based functional assessment for upper extremity impairment after musculoskeletal injuries: A pilot study. Hong Kong J. Occup. Ther. 2024, 37, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Kim, M.-Y.; Lee, J.-Y.; Jeon, Y.-J.; Kim, S.; Lee, S.; Seo, B.; Choi, Y. Effects of virtual reality-based rehabilitation on distal upper extremity function and health-related quality of life: A single-blinded, randomized controlled trial. J. Neuroeng. Rehabil. 2016, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Ko, M.-H.; Oh, S.-W.; Lee, J.-Y.; Ham, Y.; Yi, H.; Choi, Y.; Ha, D.; Shin, J.-H. Effects of virtual reality-based planar motion exercises on upper extremity function, range of motion, and health-related quality of life: A multicenter, single-blinded, randomized, controlled pilot study. J. Neuroeng. Rehabil. 2019, 16, 122. [Google Scholar] [CrossRef]
- Shin, S.; Lee, H.-J.; Chang, W.H.; Ko, S.H.; Shin, Y.-I.; Kim, Y.-H. A Smart Glove Digital System Promotes Restoration of Upper Limb Motor Function and Enhances Cortical Hemodynamic Changes in Subacute Stroke Patients with Mild to Moderate Weakness: A Randomized Controlled Trial. J. Clin. Med. 2022, 11, 7343. [Google Scholar] [CrossRef]
- Perneczky, R.; Wagenpfeil, S.; Komossa, K.; Grimmer, T.; Diehl, J.; Kurz, A. Mapping scores onto stages: Mini-mental state examination and clinical dementia rating. Am. J. Geriatr. Psychiatry 2006, 14, 139–144. [Google Scholar] [CrossRef]
- Platz, T.; Pinkowski, C.; van Wijck, F.; Kim, I.-H.; di Bella, P.; Johnson, G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: A multicentre study. Clin. Rehabil. 2005, 19, 404–411. [Google Scholar] [CrossRef]
- Small, S.; Khalid, S.; Dhiman, P.; Chan, S.; Jackson, D.; Doherty, A.; Price, A. Impact of Reduced Sampling Rate on Accelerometer-Based Physical Activity Monitoring and Machine Learning Activity Classification. J. Meas. Phys. Behav. 2021, 4, 298–310. [Google Scholar] [CrossRef]
- Dekker, J.; de Boer, M.; Ostelo, R. Minimal important change and difference in health outcome: An overview of approaches, concepts, and methods. Osteoarthr. Cartil. 2024, 32, 8–17. [Google Scholar] [CrossRef]
- Terwee, C.B.; Peipert, J.D.; Chapman, R.; Lai, J.-S.; Terluin, B.; Cella, D.; Griffiths, P.; Mokkink, L.B. Minimal important change (MIC): A conceptual clarification and systematic review of MIC estimates of PROMIS measures. Qual. Life Res. 2021, 30, 2729–2754. [Google Scholar] [CrossRef]
- Coster, W.J. Making the best match: Selecting outcome measures for clinical trials and outcome studies. Am. J. Occup. Ther. 2013, 67, 162–170. [Google Scholar] [CrossRef]
- Saes, M.; Refai, M.I.M.; van Kordelaar, J.; Scheltinga, B.L.; van Beijnum, B.-J.F.; Bussmann, J.B.J.; Buurke, J.H.; Veltink, P.H.; Meskers, C.G.M.; van Wegen, E.E.H.; et al. Smoothness metric during reach-to-grasp after stroke: Part 2. longitudinal association with motor impairment. J. Neuroeng. Rehabil. 2021, 18, 144. [Google Scholar] [CrossRef]
- Gulde, P.; Hermsdorfer, J. Smoothness Metrics in Complex Movement Tasks. Front. Neurol. 2018, 9, 615. [Google Scholar] [CrossRef] [PubMed]
- Maura, R.M.; Parra, S.R.; Stevens, R.E.; Weeks, D.L.; Wolbrecht, E.T.; Perry, J.C. Literature review of stroke assessment for upper-extremity physical function via EEG, EMG, kinematic, and kinetic measurements and their reliability. J. Neuroeng. Rehabil. 2023, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zhang, A.; Chen, C.; Wang, X.; Jiang, X.; Tao, L.; Fan, J.; Wu, X.; Dai, C.; Zhang, Y.; et al. Exploration of Human Activity Recognition Using a Single Sensor for Stroke Survivors and Able-Bodied People. Sensors 2021, 21, 799. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Oppenheim, T.; Tu-Chan, A.; Abrams, G.; Ganguly, K. Measuring Arm and Hand Joint Kinematics to Estimate Impairment During a Functional Reach and Grasp Task after Stroke. Neurorehabilit. Neural Repair 2023, 37, 409–417. [Google Scholar] [CrossRef]
- Bonfiglio, A.; Tacconi, D.; Bongers, R.M.; Farella, E. Effects of IMU sensor-to-segment calibration on clinical 3D elbow joint angles estimation. Front. Bioeng. Biotechnol. 2024, 12, 1385750. [Google Scholar] [CrossRef]
- Garner, B.A.; Pandy, M.G. A Kinematic Model of the Upper Limb Based on the Visible Human Project (VHP) Image Dataset. Comput. Methods Biomech. Biomed. Eng. 1999, 2, 107–124. [Google Scholar] [CrossRef]
- Turk, R.; Whitall, J.; Meagher, C.; Stokes, M.; Roberts, S.; Woodham, S.; Clatworthy, P.; Burridge, J. Task selection for a sensor-based, wearable, upper limb training device for stroke survivors: A multi-stage approach. Disabil. Rehabil. 2023, 45, 1480–1487. [Google Scholar] [CrossRef]
- Grattan, E.S.; Velozo, C.A.; Skidmore, E.R.; Page, S.J.; Woodbury, M.L. Interpreting Action Research Arm Test Assessment Scores to Plan Treatment. OTJR Occup. Ther. J. Res. 2019, 39, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Turolla, A.; Venneri, A.; Farina, D.; Cagnin, A.; Cheung, V.C.K. Rehabilitation Induced Neural Plasticity after Acquired Brain Injury. Neural Plast. 2018, 2018, 6565418. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.H.; Corbett, D. Plasticity during stroke recovery: From synapse to behaviour. Nat. Rev. Neurosci. 2009, 10, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Borschmann, K.N.; Hayward, K.S. Recovery of upper limb function is greatest early after stroke but does continue to improve during the chronic phase: A two-year, observational study. Physiotherapy 2020, 107, 216–223. [Google Scholar] [CrossRef]
- Li, S. Spasticity, Motor Recovery, and Neural Plasticity after Stroke. Front. Neurol. 2017, 8, 120. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Kwak, J.-M.; Ha, T.-H.; Sun, Y.; Kholinne, E.; Koh, K.-H.; Jeon, I.-H. Motion quality in rotator cuff tear using an inertial measurement unit: New parameters for dynamic motion assessment. J. Shoulder Elb. Surg. 2020, 29, 593–599. [Google Scholar] [CrossRef]
- Fuller, D.; Colwell, E.; Low, J.; Orychock, K.; Tobin, M.A.; Simango, B.; Buote, R.; Van Heerden, D.; Luan, H.; Cullen, K.; et al. Reliability and Validity of Commercially Available Wearable Devices for Measuring Steps, Energy Expenditure, and Heart Rate: Systematic Review. JMIR mHealth uHealth 2020, 8, e18694. [Google Scholar] [CrossRef]


| Subject No. | Age | Sex | Diagnosis | Days Since Onset | Hemiplegic Side | Initial Score | Follow-Up Score (After Intervention *) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ARAT | MBI | FMUE | ARAT | MBI | FMUE | ||||||
| 1 | 42 | M | Left pontine infarction | 28 | Right | 30 | 77 | 44 | 53 | 93 | 55 |
| 2 | 63 | F | Right basal ganglia, thalamic hemorrhage | 148 | Left | 14 | 44 | 29 | 18 | 48 | 38 |
| 3 | 65 | M | Left corona radiata, basal ganglia infarction | 185 | Right | 46 | 80 | 56 | |||
| 4 | 69 | M | Left corona radiata, basal ganglia infarction | 17 | Right | 12 | 17 | 16 | |||
| 5 | 45 | M | Left PCA infarction | 344 | Right | 49 | 63 | 52 | 55 | 66 | 62 |
| 6 | 52 | F | Right thalamic infarction | 16 | Left | 51 | 83 | 53 | 55 | 92 | 62 |
| 7 | 56 | F | Right MCA infarction | 86 | Left | 52 | 86 | 56 | |||
| 8 | 71 | F | Left thalamic hemorrhage | 25 | Right | 13 | 20 | 4 | |||
| 9 | 89 | M | Left middle cerebral artery territory infarction | 26 | Right | 22 | 16 | 31 | |||
| ARAT Domain | x | y | z | Roll | Pitch | Yaw | Performance Time |
|---|---|---|---|---|---|---|---|
| 1 | 0.894 ** (<0.001) | 0.743 ** (0.004) | 0.759 ** (0.003) | 0.077 (0.802) | 0.420 (0.175) | −0.357 (0.255) | −0.867 ** (<0.001) |
| 2 | 0.814 ** (<0.001) | 0.702 ** (0.008) | 0.696 ** (0.008) | 0.311 (0.301) | 0.364 (0.245) | 0.182 (0.572) | −0.757 ** (0.003) |
| 3 | 0.682 * (0.010) | 0.726 ** (0.005) | 0.834 ** (<0.001) | 0.393 (0.184) | 0.671 * (0.012) | 0.239 (0.431) | −0.520 (0.069) |
| 4 | 0.635 * (0.020) | 0.726 ** (0.005) | 0.801 ** (0.001) | 0.735 ** (0.004) | 0.715 ** (0.009) | 0.704 ** (0.007) | −0.713 ** (0.006) |
| Whole Test | 0.856 ** (<0.001) | 0.765 ** (0.002) | 0.842 ** (<0.001) | 0.754 ** (0.003) | 0.688 ** (0.009) | 0.465 (0.109) | −0.891 ** (<0.001) |
| Subject | ARAT Score | ∆ARAT Score | ∆FMUE Score | Domain 4 (Degrees) Average MSS | Total Time (s) | ||
|---|---|---|---|---|---|---|---|
| Roll | Pitch | Yaw | |||||
| 1 | 30→53 | +23 | +11 | 26.3→39.2 | 36.6→45.9 | 54.1→81.8 | 203→96.3 |
| 2 | 14→18 | +4 | +9 | 20.8→27.2 | 24.0→32.0 | 49.0→62.8 | 337→295 |
| 5 | 49→55 | +6 | +10 | 46.2→42.6 | 38.2→46.0 | 93.2→87.5 | 133→149 |
| 6 | 51→55 | +4 | +9 | 45.9→76.0 | 33.3→44.4 | 59.4→120 | 126→117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.H.; Kim, S.; Nam, H.S. Application of Smart Watch-Based Functional Evaluation for Upper Extremity Impairment: A Preliminary Study on Older Emirati Stroke Population. Sensors 2025, 25, 1554. https://doi.org/10.3390/s25051554
Kim YH, Kim S, Nam HS. Application of Smart Watch-Based Functional Evaluation for Upper Extremity Impairment: A Preliminary Study on Older Emirati Stroke Population. Sensors. 2025; 25(5):1554. https://doi.org/10.3390/s25051554
Chicago/Turabian StyleKim, Yeo Hyung, Sarah Kim, and Hyung Seok Nam. 2025. "Application of Smart Watch-Based Functional Evaluation for Upper Extremity Impairment: A Preliminary Study on Older Emirati Stroke Population" Sensors 25, no. 5: 1554. https://doi.org/10.3390/s25051554
APA StyleKim, Y. H., Kim, S., & Nam, H. S. (2025). Application of Smart Watch-Based Functional Evaluation for Upper Extremity Impairment: A Preliminary Study on Older Emirati Stroke Population. Sensors, 25(5), 1554. https://doi.org/10.3390/s25051554






