High-Sensitivity Goos-Hänchen Shift Sensing via Surface Plasmon Resonance and Beam Displacement Amplification
Abstract
1. Introduction
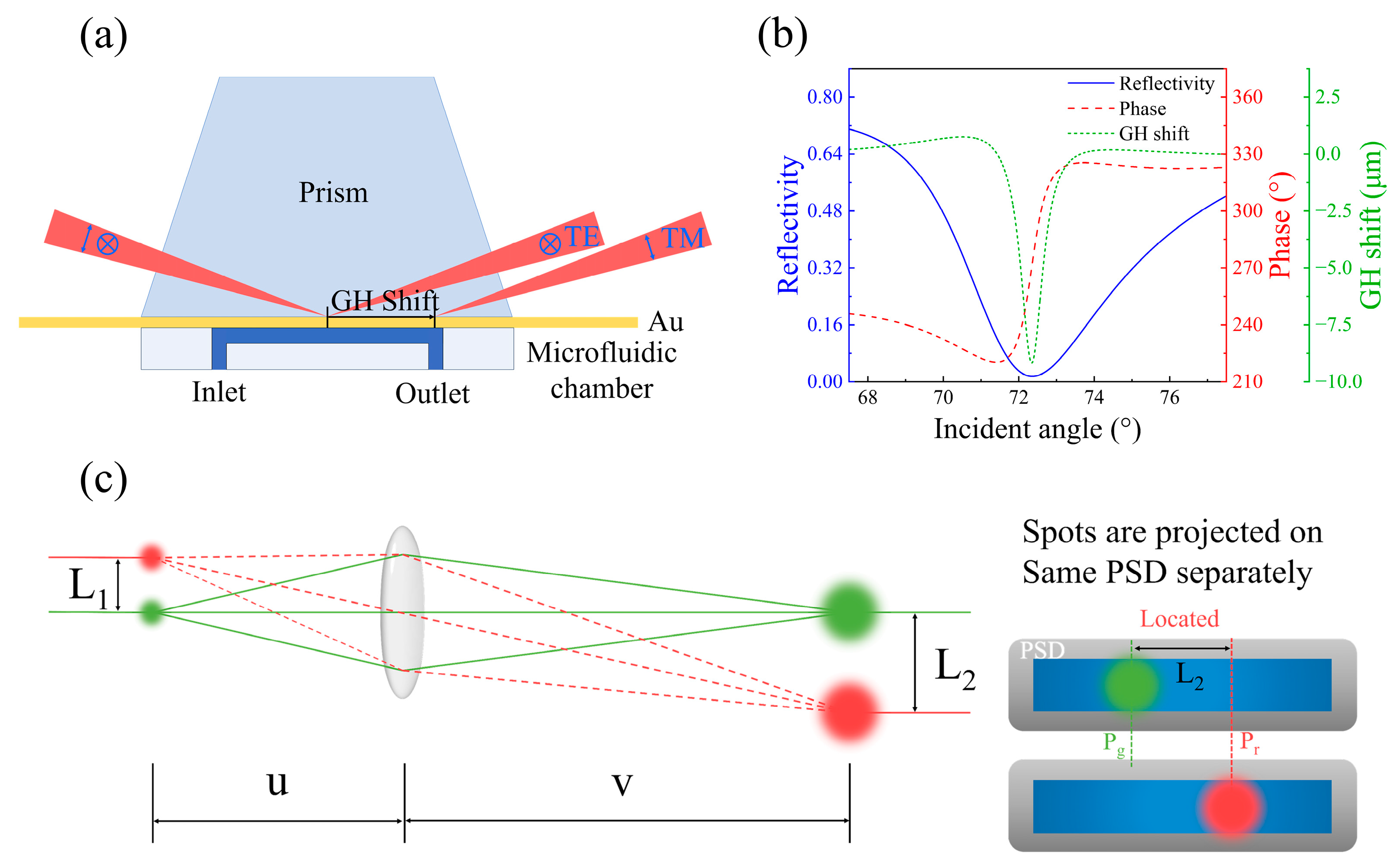
2. Method and Experiment
3. Results and Discussion
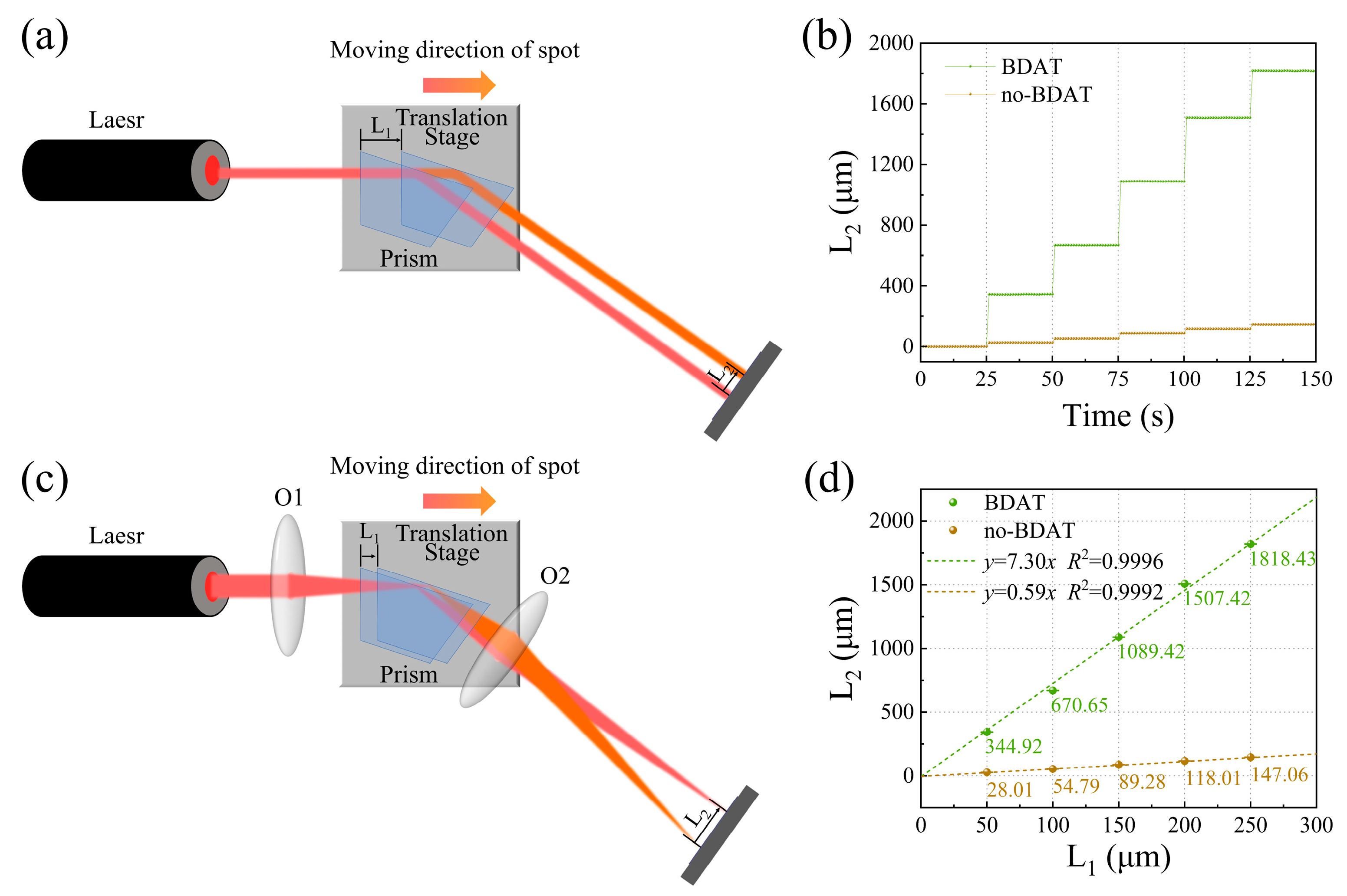
3.1. Evaluation of Amplification Effect and Stability of BDAT
3.2. Application of BDAT in GH Shift Sensing
3.3. Comparative Analysis of GH Shift and Reflectivity for Low-Concentration Sensing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kausaite-Minkstimiene, A.; Popov, A.; Ramanaviciene, A. Ultra-sensitive SPR immunosensors: A comprehensive review of labeling and interface modification using nanostructures. TrAC Trends Anal. Chem. 2024, 170, 117468. [Google Scholar] [CrossRef]
- Olaru, A.; Bala, C.; Jaffrezic-Renault, N.; Aboul-Enein, H.Y. Surface plasmon resonance (SPR) biosensors in pharmaceutical analysis. Crit. Rev. Anal. Chem. 2015, 45, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.S.; Raghuwanshi, S.K.; Kumar, S. Recent advances in two-dimensional materials-based Kretschmann configuration for SPR sensors: A review. IEEE Sens. J. 2021, 22, 1069–1080. [Google Scholar] [CrossRef]
- Pandey, P.S.; Raghuwanshi, S.K.; Shadab, A.; Ansari, M.T.I.; Tiwari, U.K.; Kumar, S. SPR based biosensing chip for COVID-19 diagnosis—A review. IEEE Sens. J. 2022, 22, 13800–13810. [Google Scholar] [CrossRef]
- Das, S.; Devireddy, R.; Gartia, M.R. Surface plasmon resonance (SPR) sensor for cancer biomarker detection. Biosensors 2023, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Raghuwanshi, S.K.; Kumar, S. Recent advances in carbon nanomaterials based SPR sensor for biomolecules and gas detection—A review. IEEE Sens. J. 2022, 22, 15661–15672. [Google Scholar] [CrossRef]
- Wood, R.W. On a remarkable case of uneven distribution of light in a diffraction grating spectrum. Philos. Mag. 1902, 4, 396–402. [Google Scholar] [CrossRef]
- Fano, U. The theory of anomalous diffraction gratings and of quasi-stationary waves on metallic surfaces (Sommerfeld’s waves). JOSA 1941, 31, 213–222. [Google Scholar] [CrossRef]
- Turbadar, T. Complete absorption of light by thin metal films. Proc. Phys. Soc. 1959, 73, 40. [Google Scholar] [CrossRef]
- Otto, A. Excitation of nonradiative surface plasma waves in silver by the method of frustrated total reflection. Z. Phys. A 1968, 216, 398–410. [Google Scholar] [CrossRef]
- Kretschmann, E.; Raether, H. Radiative decay of non radiative surface plasmons excited by light. Z. Naturforsch. A 1968, 23, 2135–2136. [Google Scholar] [CrossRef]
- Liedberg, B.; Nylander, C.; Lunström, I. Surface plasmon resonance for gas detection and biosensing. Sens. Actuat. 1983, 4, 299–304. [Google Scholar] [CrossRef]
- Šípová, H.; Homola, J. Surface plasmon resonance sensing of nucleic acids: A review. Anal. Chim. Acta 2013, 773, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Singh, P. SPR Biosensors: Historical Perspectives and Current Challenges. Sens. Actuators B Chem. 2016, 229, 110–130. [Google Scholar] [CrossRef]
- Souto, D.E.P.; Volpe, J.; Goncalves, C.D.C.; Ramos, C.H.I.; Kubota, L.T. A brief review on the strategy of developing SPR-based biosensors for application to the diagnosis of neglected tropical diseases. Talanta 2019, 205, 120122. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Baillargeat, D.; Ho, H.-P.; Yong, K.-T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, S.A.; Zhang, J.; Agrawal, G.P. Goos-Hänchen shift at a temporal boundary. Phys. Rev. A 2022, 106, L061501. [Google Scholar] [CrossRef]
- Yan, Y.; Zha, M.; Liu, J.; Tu, J.; Liu, Z. Tunable giant Goos-Hanchen shift in Au-ReS2-graphene heterostructure. Opt. Lett. 2024, 49, 3484–3487. [Google Scholar] [CrossRef] [PubMed]
- Goos, F.; Hänchen, H. Ein neuer und fundamentaler Versuch zur Totalreflexion. Ann. Phys. 1947, 436, 333–346. [Google Scholar] [CrossRef]
- Yin, X.; Hesselink, L.; Liu, Z.; Fang, N.; Zhang, X. Large positive and negative lateral optical beam displacements due to surface plasmon resonance. Appl. Phys. Lett. 2004, 85, 372–374. [Google Scholar] [CrossRef]
- You, Q.; Shan, Y.; Gan, S.; Zhao, Y.; Dai, X.; Xiang, Y. Giant and controllable Goos-Hanchen shifts based on surface plasmon resonance with graphene-MoS2 heterostructure. Opt. Mater. Express 2018, 8, 3036–3048. [Google Scholar] [CrossRef]
- Luo, M.; Wu, F. Refractive-index sensing based on large negative Goos-Hänchen shifts of a wavy dielectric grating. Phys. Rev. Appl. 2024, 22, 014050. [Google Scholar] [CrossRef]
- Yin, X.; Hesselink, L. Goos-Hanchen shift surface plasmon resonance sensor. Appl. Phys. Lett. 2006, 89. [Google Scholar] [CrossRef]
- Zeng, S.; Liang, G.; Gheno, A.; Vedraine, S.; Ratier, B.; Ho, H.-P.; Yu, N. Plasmonic Metasensors Based on 2D Hybrid Atomically Thin Perovskite Nanomaterials. Nanomaterials 2020, 10, 1289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, S.; Crunteanu, A.; Xie, Z.; Humbert, G.; Ma, L.; Wei, Y.; Brunel, A.; Bessette, B.; Orlianges, J.-C.; et al. Targeted Sub-Attomole Cancer Biomarker Detection Based on Phase Singularity 2D Nanomaterial-Enhanced Plasmonic Biosensor. Nano-Micro Lett. 2021, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shan, X.; Patel, U.; Huang, X.; Lu, J.; Li, J.; Tao, N. Label-free imaging, detection, and mass measurement of single viruses by surface plasmon resonance. Proc. Natl. Acad. Sci. USA 2010, 107, 16028–16032. [Google Scholar] [CrossRef]
- Karki, B.; Uniyal, A.; Srivastava, G.; Pal, A. Black phosphorous and cytop nanofilm-based long-range SPR sensor with enhanced quality factor. J. Sens. 2023, 2023, 2102915. [Google Scholar] [CrossRef]
- Nelson, B.P.; Frutos, A.G.; Brockman, J.M.; Corn, R.M. Near-infrared surface plasmon resonance measurements of ultrathin films. 1. Angle shift and SPR imaging experiments. Anal. Chem. 1999, 71, 3928–3934. [Google Scholar] [CrossRef]
- Kazuma, E.; Tatsuma, T. Localized surface plasmon resonance sensors based on wavelength-tunable spectral dips. Nanoscale 2014, 6, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.K. Wavelength modulation based surface plasmon resonance sensor for detection of cardiac marker proteins troponin I and troponin T. Sens. Actuator A Phys. 2021, 332, 113104. [Google Scholar] [CrossRef]
- Xu, G.; Tazawa, M.; Jin, P.; Nakao, S.; Yoshimura, K. Wavelength tuning of surface plasmon resonance using dielectric layers on silver island films. Appl. Phys. Lett. 2003, 82, 3811–3813. [Google Scholar] [CrossRef]
- Kravets, V.G.; Schedin, F.; Jalil, R.; Britnell, L.; Gorbachev, R.V.; Ansell, D.; Thackray, B.; Novoselov, K.S.; Geim, A.K.; Kabashin, A.V.; et al. Singular phase nano-optics in plasmonic metamaterials for label-free single-molecule detection. Nat. Mater. 2013, 12, 304–309. [Google Scholar] [CrossRef]
- Huang, Y.H.; Ho, H.P.; Wu, S.Y.; Kong, S.K. Detecting Phase Shifts in Surface Plasmon Resonance: A Review. Adv. Opt. Technol. 2012, 2012, 41957. [Google Scholar] [CrossRef]
- Chen, H.; Tong, J.; Wang, Y.; Jiang, L. Graphene enhanced surface plasmon resonance sensing based on Goos-Hänchen shift. In Proceedings of the Young Scientists Forum 2017, Shanghai, China, 24–26 November 2017. [Google Scholar]
- Han, L.; Hu, Z.; Pan, J.; Huang, T.; Luo, D. High-Sensitivity Goos-Hanchen Shifts Sensor Based on BlueP-TMDCs-Graphene Heterostructure. Sensors 2020, 20, 3605. [Google Scholar] [CrossRef] [PubMed]
- Zakirov, N.; Zhu, S.; Bruyant, A.; Lerondel, G.; Bachelot, R.; Zeng, S. Sensitivity Enhancement of Hybrid Two-Dimensional Nanomaterials-Based Surface Plasmon Resonance Biosensor. Biosensors 2022, 12, 810. [Google Scholar] [CrossRef]
- Hedhly, M.; Wang, Y.; Zeng, S.; Ouerghi, F.; Zhou, J.; Humbert, G. Highly Sensitive Plasmonic Waveguide Biosensor Based on Phase Singularity-Enhanced Goos-Hanchen Shift. Biosensors 2022, 12, 457. [Google Scholar] [CrossRef]
- Borg, K.N.; Jaffiol, R.; Ho, Y.P.; Zeng, S. Enhanced biosensing of tumor necrosis factor-alpha based on aptamer-functionalized surface plasmon resonance substrate and Goos-Hanchen shift. Analyst 2024, 149, 3017–3025. [Google Scholar] [CrossRef]
- Li, D.; Su, B.; Wen, R.; Hu, Z.; Huo, C.; Yan, X.; Kong, X.-T.; Liu, Z.; Tian, J. Abnormal Spatial Shifts in Graphene Measured via the Beam Displacement Amplification Technique: Implications for Sensors Based on the Goos–Hänchen Effect. ACS Appl. Nano Mater. 2021, 4, 13477–13485. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Liu, X.; Panzner, J.M.; Wesdemiotis, C.; Pang, Y. A binuclear Zn(II)-Zn(II) complex from a 2-hydroxybenzohydrazide-derived Schiff base for selective detection of pyrophosphate. Dalton Trans. 2014, 43, 14142–14146. [Google Scholar] [CrossRef] [PubMed]
- Li, C.F. Negative lateral shift of a light beam transmitted through a dielectric slab and interaction of boundary effects. Phys. Rev. Lett. 2003, 91, 133903. [Google Scholar] [CrossRef]
- Feng, F.; Si, G.; Min, C.; Yuan, X.; Somekh, M. On-chip plasmonic spin-Hall nanograting for simultaneously detecting phase and polarization singularities. Light Sci. Appl. 2020, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Malassis, L.; Masse, P.; Treguer-Delapierre, M.; Mornet, S.; Weisbecker, P.; Barois, P.; Simovski, C.R.; Kravets, V.G.; Grigorenko, A.N. Topological Darkness in Self-Assembled Plasmonic Metamaterials. Adv. Mater. 2014, 26, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Yesilkoy, F.; Terborg, R.A.; Pello, J.; Belushkin, A.A.; Jahani, Y.; Pruneri, V.; Altug, H. Phase-sensitive plasmonic biosensor using a portable and large field-of-view interferometric microarray imager. Light Sci. Appl. 2018, 7, 17152. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, W.; Chen, S.; Ling, X.; Shu, W.; Lu, H.; Wen, S.; Yin, X. Transitional Goos-Hanchen effect due to the topological phase transitions. Opt. Express 2018, 26, 23705–23713. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Guo, J.; Wang, Q.; Xiang, Y.; Wen, S. Electrically controlled Goos-Hanchen shift of a light beam reflected from the metal-insulator-semiconductor structure. Opt. Express 2013, 21, 10430–10439. [Google Scholar] [CrossRef]
- Lee, S.; Li, L. Rapid super-resolution imaging of sub-surface nanostructures beyond diffraction limit by high refractive index microsphere optical nanoscopy. Opt. Commun. 2015, 334, 253–257. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Xu, E.; Zhang, X.; Tian, J.; Liu, Z. High-Sensitivity Goos-Hänchen Shift Sensing via Surface Plasmon Resonance and Beam Displacement Amplification. Sensors 2025, 25, 1329. https://doi.org/10.3390/s25051329
Li Q, Xu E, Zhang X, Tian J, Liu Z. High-Sensitivity Goos-Hänchen Shift Sensing via Surface Plasmon Resonance and Beam Displacement Amplification. Sensors. 2025; 25(5):1329. https://doi.org/10.3390/s25051329
Chicago/Turabian StyleLi, Qian, Enze Xu, Xiaoliang Zhang, Jianguo Tian, and Zhibo Liu. 2025. "High-Sensitivity Goos-Hänchen Shift Sensing via Surface Plasmon Resonance and Beam Displacement Amplification" Sensors 25, no. 5: 1329. https://doi.org/10.3390/s25051329
APA StyleLi, Q., Xu, E., Zhang, X., Tian, J., & Liu, Z. (2025). High-Sensitivity Goos-Hänchen Shift Sensing via Surface Plasmon Resonance and Beam Displacement Amplification. Sensors, 25(5), 1329. https://doi.org/10.3390/s25051329





