Fast Detection of Uric Acid in Urine for Early Diagnosis Using THz Polarized Waves
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
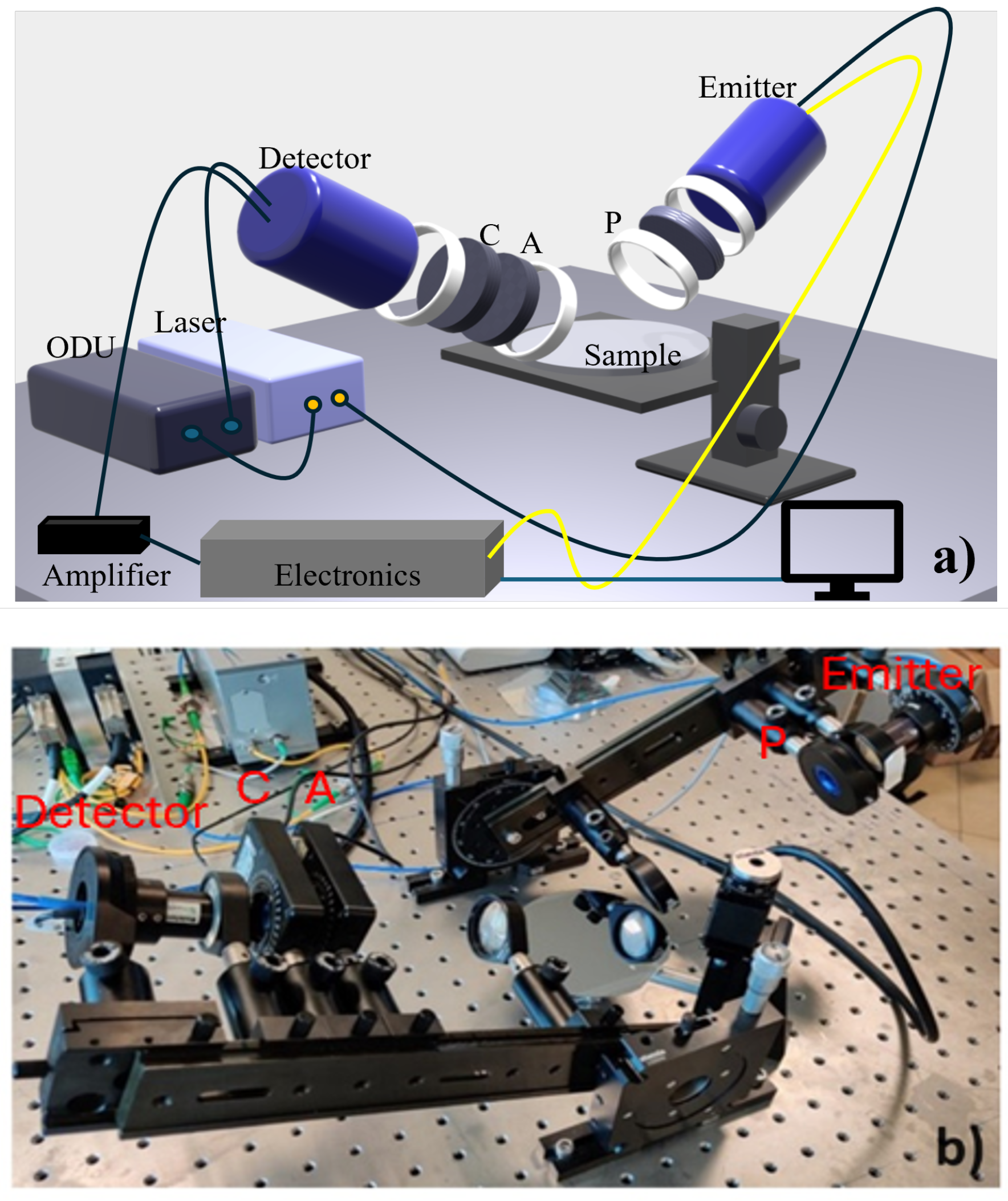
2.2. Experimental Setup
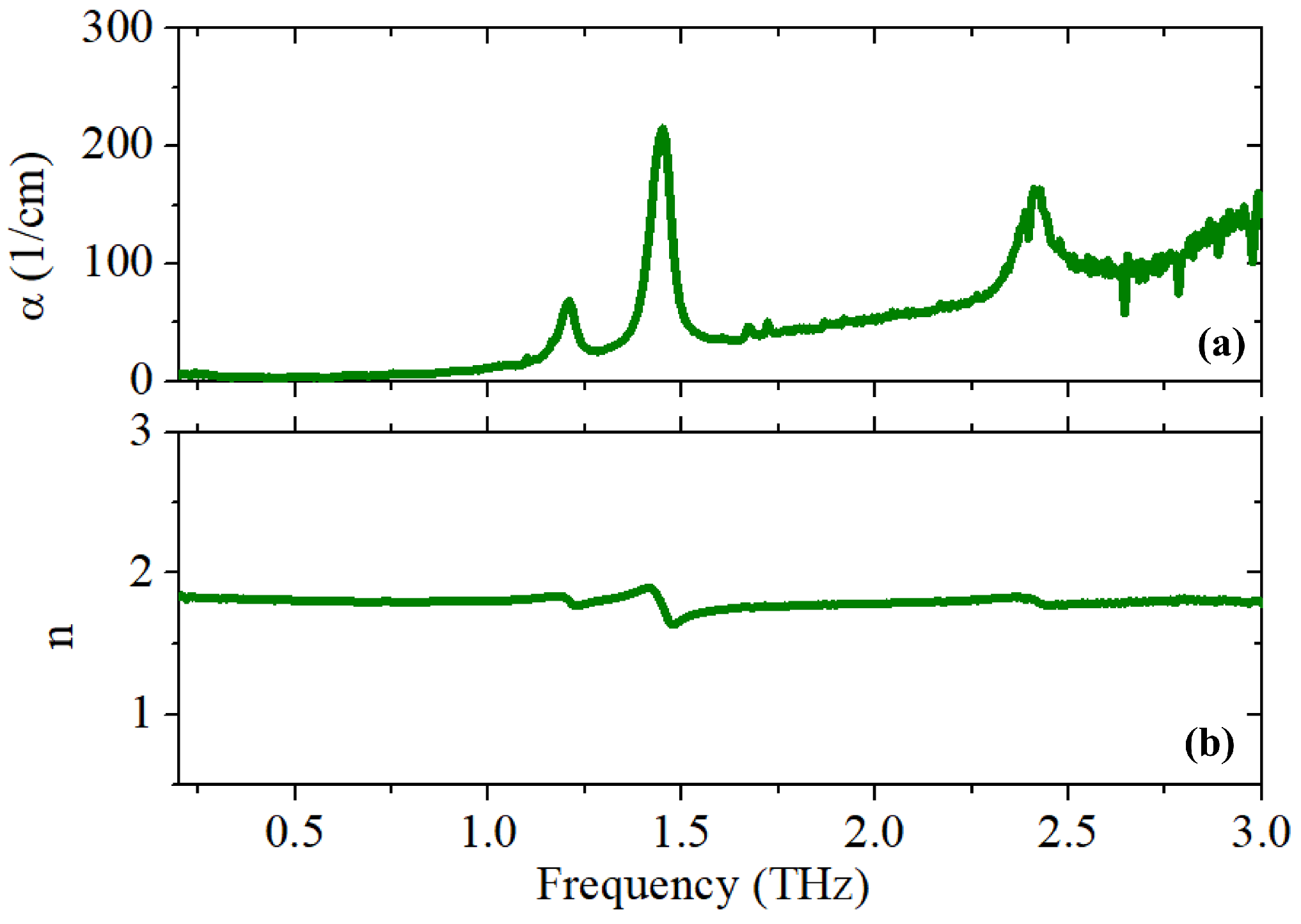
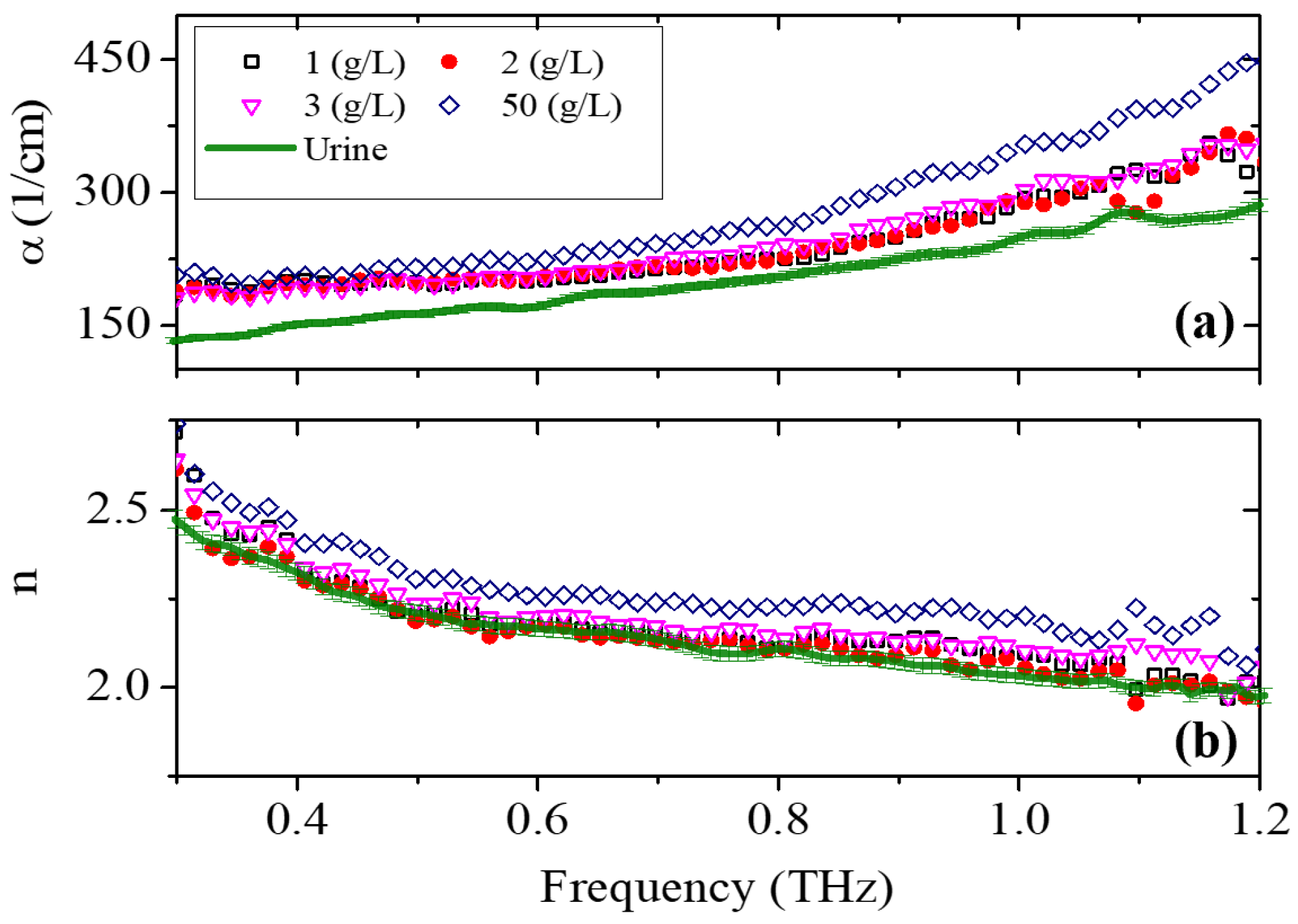
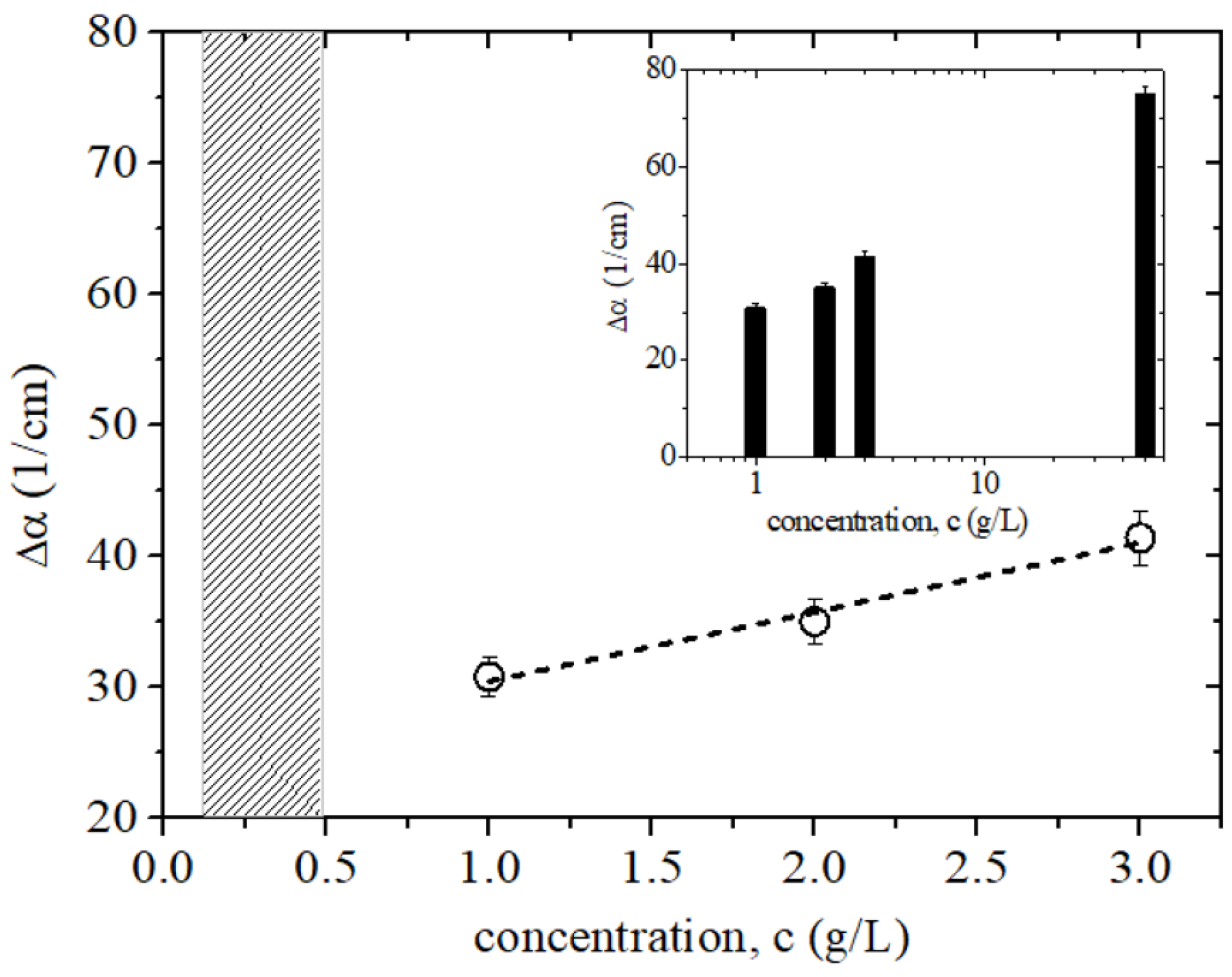
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heydorn, K.C.; Pehrsson, P.R.; Wu, X.; Dwyer, J.T.; Ershow, A.G.; Wambogo, E.; Gahche, J.; Hord, N.; Hays, F.; Garrett, T.J.; et al. USDA/NIH-ODS Special Interest Databases for Dietary Supplements and Foods: Bridging Data Gaps for Dietary Health. Curr. Dev. Nutr. 2024, 8, 102872. [Google Scholar] [CrossRef]
- Abina, A.; Korošec, T.; Puc, U.; Jazbinšek, M.; Zidanšek, A. Urinary metabolic biomarker profiling for cancer diagnosis by terahertz spectroscopy: Review and perspective. Photonics 2023, 10, 1051. [Google Scholar] [CrossRef]
- Adomako, E.; Moe, O.W. Uric acid and urate in urolithiasis: The innocent bystander, instigator, and perpetrator. Semin. Nephrol. 2020, 40, 564–573. [Google Scholar] [CrossRef]
- Feig, D.I.; Kang, D.H.; Johnson, R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008, 359, 1811–1821. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Andres-Hernando, A.; Kuwabara, M. Uric acid and hypertension. Hypertens. Res. 2020, 43, 832–834. [Google Scholar] [CrossRef] [PubMed]
- Madero, M.; Sarnak, M.J.; Wang, X.; Greene, T.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; Levey, A.S.; Menon, V. Uric acid and long-term outcomes in CKD. Am. J. Kidney Dis. 2009, 53, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Hegerty, K.; Jaure, A.; Scholes-Robertson, N.; Howard, K.; Ju, A.; Evangelidis, N.; Wolley, M.; Baumgart, A.; Johnson, D.W.; Hawley, C.M.; et al. Australian Workshops on patients’ perspectives on hemodialysis and incremental start. Kidney Int. Rep. 2023, 8, 478–488. [Google Scholar] [CrossRef]
- Lam, S.; MacAulay, C.; Palcic, B. Detection and localization of early lung cancer by imaging techniques. Chest 1993, 103, 12S–14S. [Google Scholar] [CrossRef]
- Allison, J.E.; Sakoda, L.C.; Levin, T.R.; Tucker, J.P.; Tekawa, I.S.; Cuff, T.; Pauly, M.P.; Shlager, L.; Palitz, A.M.; Zhao, W.K.; et al. Screening for colorectal neoplasms with new fecal occult blood tests: Update on performance characteristics. J. Natl. Cancer Inst. 2007, 99, 1462–1470. [Google Scholar] [CrossRef]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Beresford, M.W. Urinary biomarkers in childhood lupus nephritis. Clin. Immunol. 2017, 185, 21–31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lakowicz, J. Principles of Fluorescence Spectroscopy; University of Maryland School of Medicine Baltimore: Baltimore, ML, USA, 2006; Volume 132. [Google Scholar]
- Wang, Q.; Wen, X.; Kong, J. Recent progress on uric acid detection: A review. Crit. Rev. Anal. Chem. 2020, 50, 359–375. [Google Scholar] [CrossRef]
- Tian, Y.; Fan, X.; Chen, K.; Chen, X.; Peng, W.; Wang, L.; Wang, F. Optical biomarker analysis for renal cell carcinoma obtained from preoperative and postoperative patients using ATR-FTIR spectroscopy. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2024, 318, 124426. [Google Scholar] [CrossRef] [PubMed]
- Markelz, A.; Roitberg, A.; Heilweil, E.J. Pulsed terahertz spectroscopy of DNA, bovine serum albumin and collagen between 0.1 and 2.0 THz. Chem. Phys. Lett. 2000, 320, 42–48. [Google Scholar] [CrossRef]
- Zhou, J.W.; Zheng, X.B.; Liu, H.S.; Wen, B.Y.; Kou, Y.C.; Zhang, L.; Song, J.J.; Zhang, Y.J.; Li, J.F. Reliable quantitative detection of uric acid in urine by surface-enhanced Raman spectroscopy with endogenous internal standard. Biosens. Bioelectron. 2024, 251, 116101. [Google Scholar] [CrossRef] [PubMed]
- Koral, C.; Mazaheri, Z.; Papari, G.P.; Andreone, A.; Drebot, I.; Giove, D.; Masullo, M.R.; Mettivier, G.; Opromolla, M.; Paparo, D.; et al. Multi-pass free electron laser assisted spectral and imaging applications in the terahertz/far-IR range using the future superconducting electron source BriXSinO. Front. Phys. 2022, 10, 725901. [Google Scholar] [CrossRef]
- Mazaheri, Z.; Koral, C.; Andreone, A.; Marino, A. Terahertz time-domain ellipsometry: Tutorial. JOSA A 2022, 39, 1420–1433. [Google Scholar] [CrossRef]
- Papari, G.P.; Koral, C.; Andreone, A. Encoded-enhancement of THZ metasurface figure of merit for label-free sensing. Sensors 2019, 19, 2544. [Google Scholar] [CrossRef]
- Wu, M.; He, X.; Lu, G.; Geng, Z.; Zhang, Y. Multi-mode non-diffraction vortex beams enabled by polarization-frequency multiplexing transmissive terahertz metasurfaces. J. Appl. Phys. 2024, 136. [Google Scholar] [CrossRef]
- Sequeira-Antunes, B.; Ferreira, H.A. Urinary biomarkers and point-of-care urinalysis devices for early diagnosis and management of disease: A review. Biomedicines 2023, 11, 1051. [Google Scholar] [CrossRef]
- Hwang, C.; Lee, W.J.; Kim, S.D.; Park, S.; Kim, J.H. Recent advances in biosensor technologies for point-of-care urinalysis. Biosensors 2022, 12, 1020. [Google Scholar] [CrossRef]
- Mazaheri, Z.; Papari, G.P.; Andreone, A. Dielectric Response of Different Alcohols in Water-Rich Binary Mixtures from THz Ellipsometry. Int. J. Mol. Sci. 2024, 25, 4240. [Google Scholar] [CrossRef]
- Mazaheri, Z.; Koral, C.; Andreone, A. Accurate THz ellipsometry using calibration in time domain. Sci. Rep. 2022, 12, 7342. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Mao, P.; Peng, P.; Yan, S.; Zang, Z.; Yao, C. Terahertz spectra of proteinuria and non-proteinuria. Front. Bioeng. Biotechnol. 2023, 11, 1119694. [Google Scholar] [CrossRef]
- Emaminejad, H.; Mir, A.; Farmani, A. Design and simulation of a novel tunable terahertz biosensor based on metamaterials for simultaneous monitoring of blood and urine components. Plasmonics 2021, 16, 1537–1548. [Google Scholar] [CrossRef]
- Yu, W.; Shi, J.; Huang, G.; Zhou, J.; Zhan, X.; Guo, Z.; Tian, H.; Xie, F.; Yang, X.; Fu, W. THz-ATR spectroscopy integrated with species recognition based on multi-classifier voting for automated clinical microbial identification. Biosensors 2022, 12, 378. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, W.; Han, M.; Yang, Z.; Liu, J.; Wang, K. Terahertz ellipsometry based on the long-distance diffraction-free beam. Opt. Lasers Eng. 2024, 173, 107859. [Google Scholar] [CrossRef]
- Ngai, K. Interpretation of the GHz to THz dielectric relaxation dynamics of water in the framework of the coupling model. J. Mol. Liq. 2018, 253, 113–118. [Google Scholar] [CrossRef]
- Cai, Z.; Zhu, C.; Chen, G.; Wu, Y.; Gu, J.; Ma, C.; Gao, H.; Li, L.; Guo, S. Study on intermolecular hydrogen bond of uric acid water-clusters. Chem. Phys. Lett. 2023, 818, 140424. [Google Scholar] [CrossRef]
- Fujiwara, H. Spectroscopic Ellipsometry: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Banhegyi, G. Numerical analysis of complex dielectric mixture formulae. Colloid Polym. Sci. 1988, 266, 11–28. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Q.; Li, Z.; Hu, F. Intermolecular weak interactions of crystalline purine and uric acid investigated by terahertz spectroscopy and theoretical calculation. J. Lumin. 2020, 223, 117198. [Google Scholar] [CrossRef]
- Upadhya, P.; Shen, Y.; Davies, A.; Linfield, E. Terahertz time-domain spectroscopy of glucose and uric acid. J. Biol. Phys. 2003, 29, 117–121. [Google Scholar] [CrossRef]
- Wu, X.; Tao, R.; Zhang, T.; Liu, X.; Wang, J.; Zhang, Z.; Zhao, X.; Yang, P. Biomedical applications of terahertz spectra in clinical and molecular pathology of human glioma. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2023, 285, 121933. [Google Scholar] [CrossRef]
- Cataldo, A.; Cino, L.; Distante, C.; Maietta, G.; Masciullo, A.; Mazzeo, P.L.; Schiavoni, R. Integrating microwave reflectometry and deep learning imaging for in-vivo skin cancer diagnostics. Measurement 2024, 235, 114911. [Google Scholar] [CrossRef]
- Liu, X.; Gan, L.; Yang, B. Millimeter-wave free-space dielectric characterization. Measurement 2021, 179, 109472. [Google Scholar] [CrossRef]
- Burtis, C.A.; Ashwood, E.R. Tietz textbook of clinical chemistry. Philadelphia 1999, 37, 1136. [Google Scholar]
- Coe, F.L.; Coe, F. Uric acid and calcium oxalate nephrolithiasis. Kidney Int. 1983, 24, 392–403. [Google Scholar] [CrossRef]
- Armenta-Castro, A.; Núñez-Soto, M.T.; Rodriguez-Aguillón, K.O.; Aguayo-Acosta, A.; Oyervides-Muñoz, M.A.; Snyder, S.A.; Barceló, D.; Saththasivam, J.; Lawler, J.; Sosa-Hernández, J.E.; et al. Urine biomarkers for Alzheimer’s disease: A new opportunity for wastewater-based epidemiology? Environ. Int. 2024, 184, 108462. [Google Scholar] [CrossRef]

| Method | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Chemical | Based on chemical reactions, such as enzymatic or colorimetric assays |
|
|
| Optical | UV-Vis absorption, fluorescence, or Raman spectroscopy |
|
|
| THz-TDS | Investigate the complex refractive index (dispersion and absorption) in the THz range |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazaheri, Z.; Federico, G.; Koral, C.; Papari, G.P.; Ullatil, L.; Russo, P.; Andreone, A. Fast Detection of Uric Acid in Urine for Early Diagnosis Using THz Polarized Waves. Sensors 2025, 25, 1004. https://doi.org/10.3390/s25041004
Mazaheri Z, Federico G, Koral C, Papari GP, Ullatil L, Russo P, Andreone A. Fast Detection of Uric Acid in Urine for Early Diagnosis Using THz Polarized Waves. Sensors. 2025; 25(4):1004. https://doi.org/10.3390/s25041004
Chicago/Turabian StyleMazaheri, Zahra, Giorgia Federico, Can Koral, Gian Paolo Papari, Lakshmi Ullatil, Paolo Russo, and Antonello Andreone. 2025. "Fast Detection of Uric Acid in Urine for Early Diagnosis Using THz Polarized Waves" Sensors 25, no. 4: 1004. https://doi.org/10.3390/s25041004
APA StyleMazaheri, Z., Federico, G., Koral, C., Papari, G. P., Ullatil, L., Russo, P., & Andreone, A. (2025). Fast Detection of Uric Acid in Urine for Early Diagnosis Using THz Polarized Waves. Sensors, 25(4), 1004. https://doi.org/10.3390/s25041004








