OA−ICOS−Based Oxygen and Carbon Dioxide Sensors for Field Applications in Gas Reflux Chicken Coops
Abstract
1. Introduction
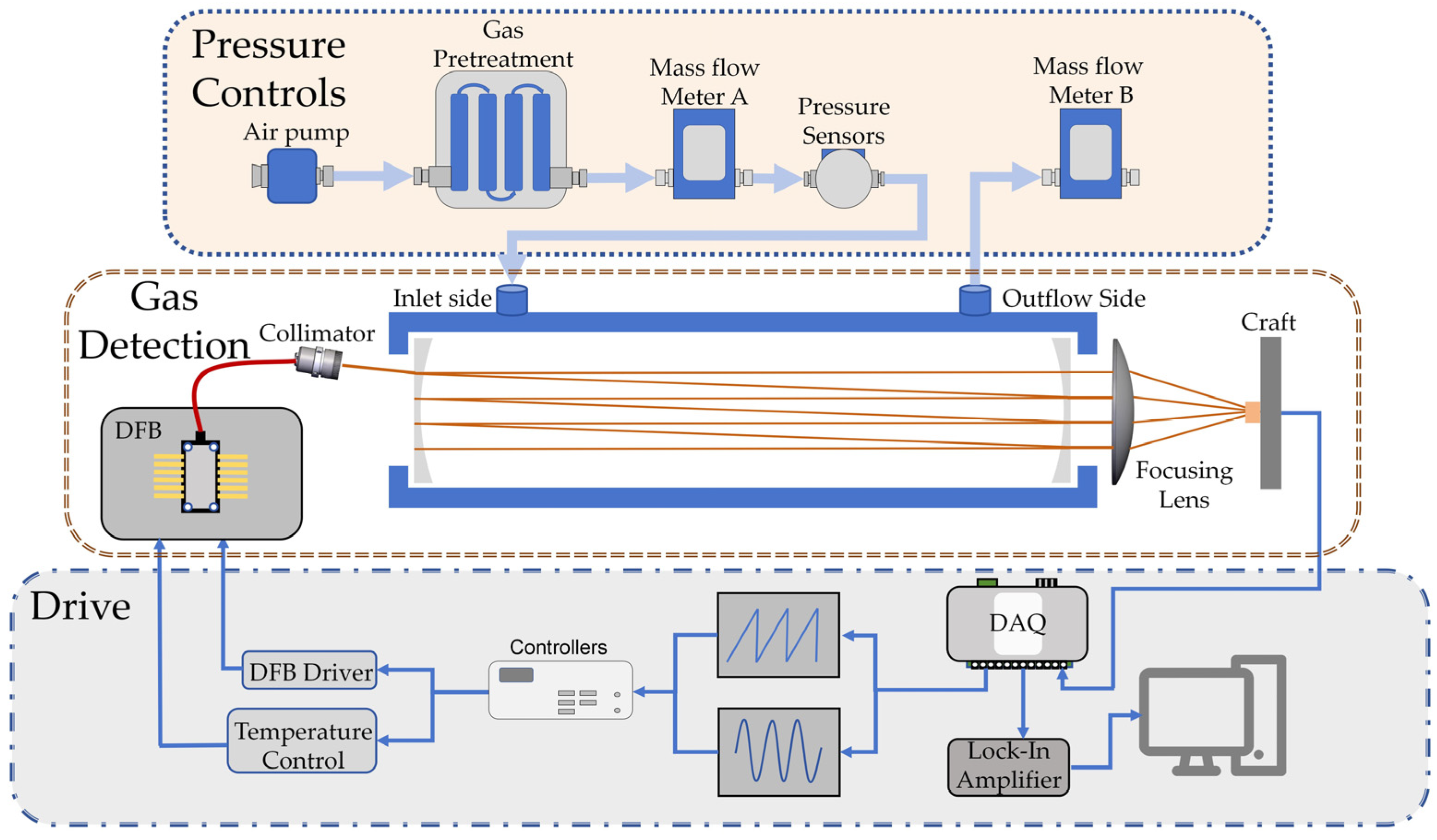
2. Materials and Methods
2.1. Sensor Solutions
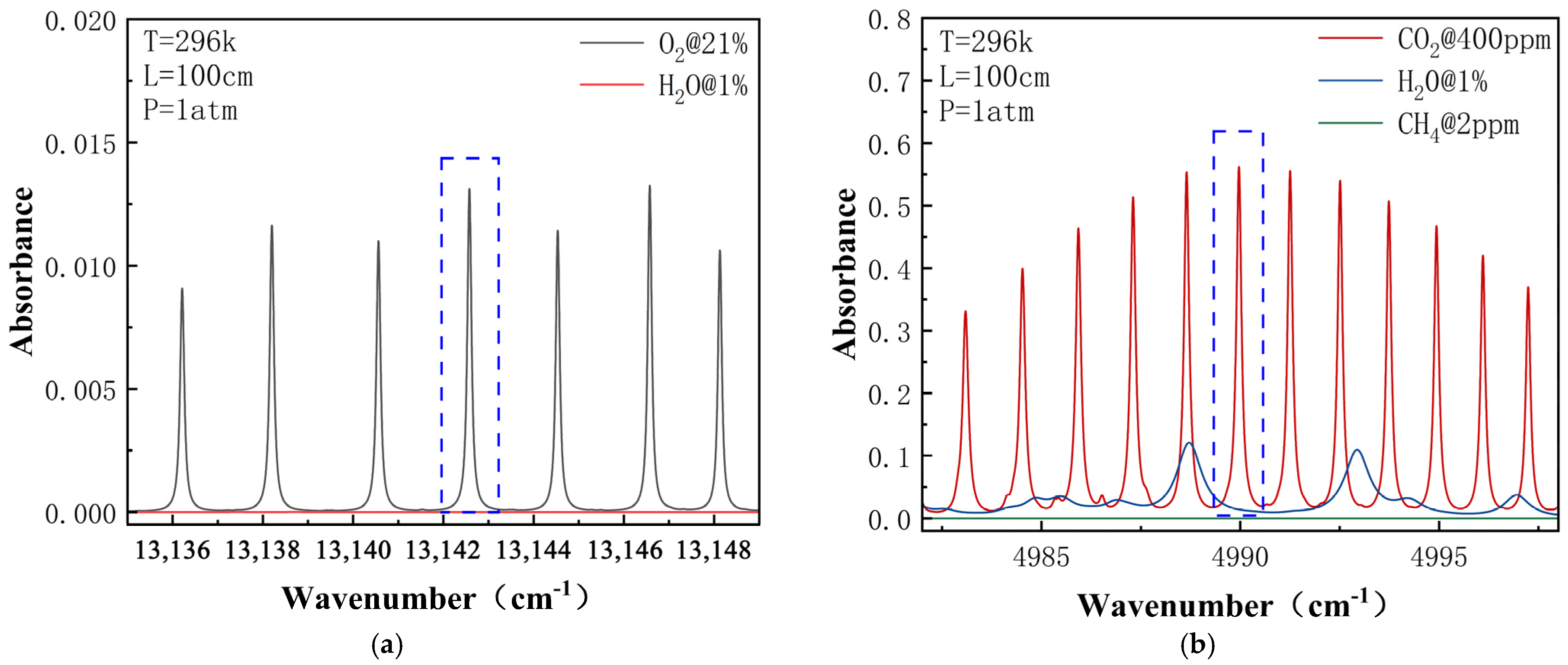
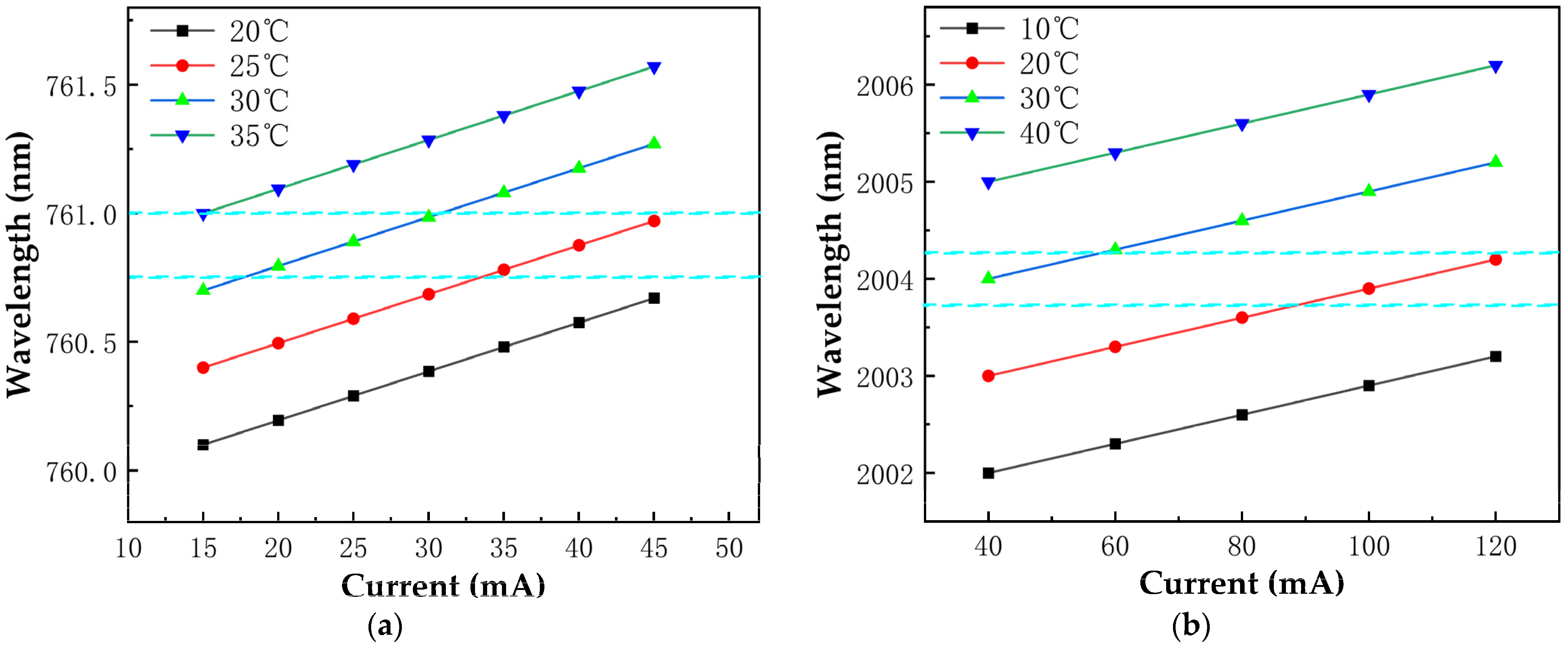
2.2. Absorption Spectra and Modulation
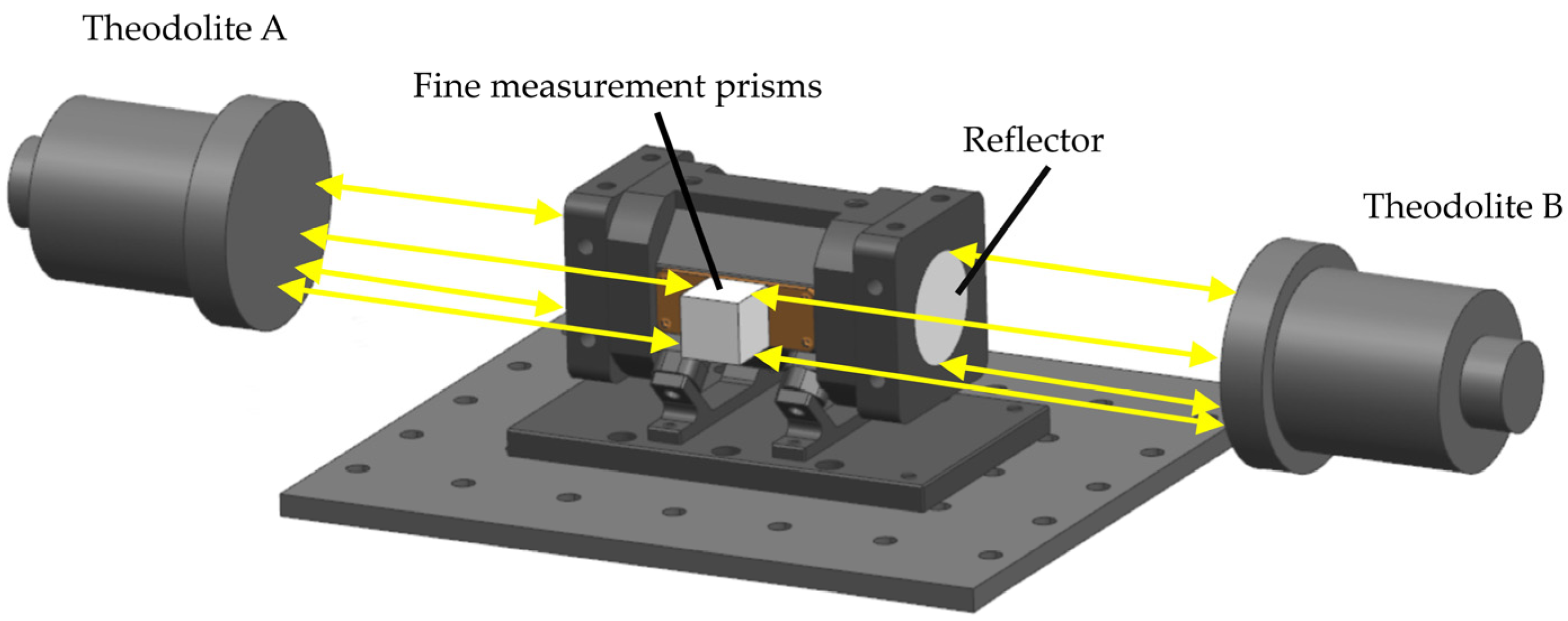
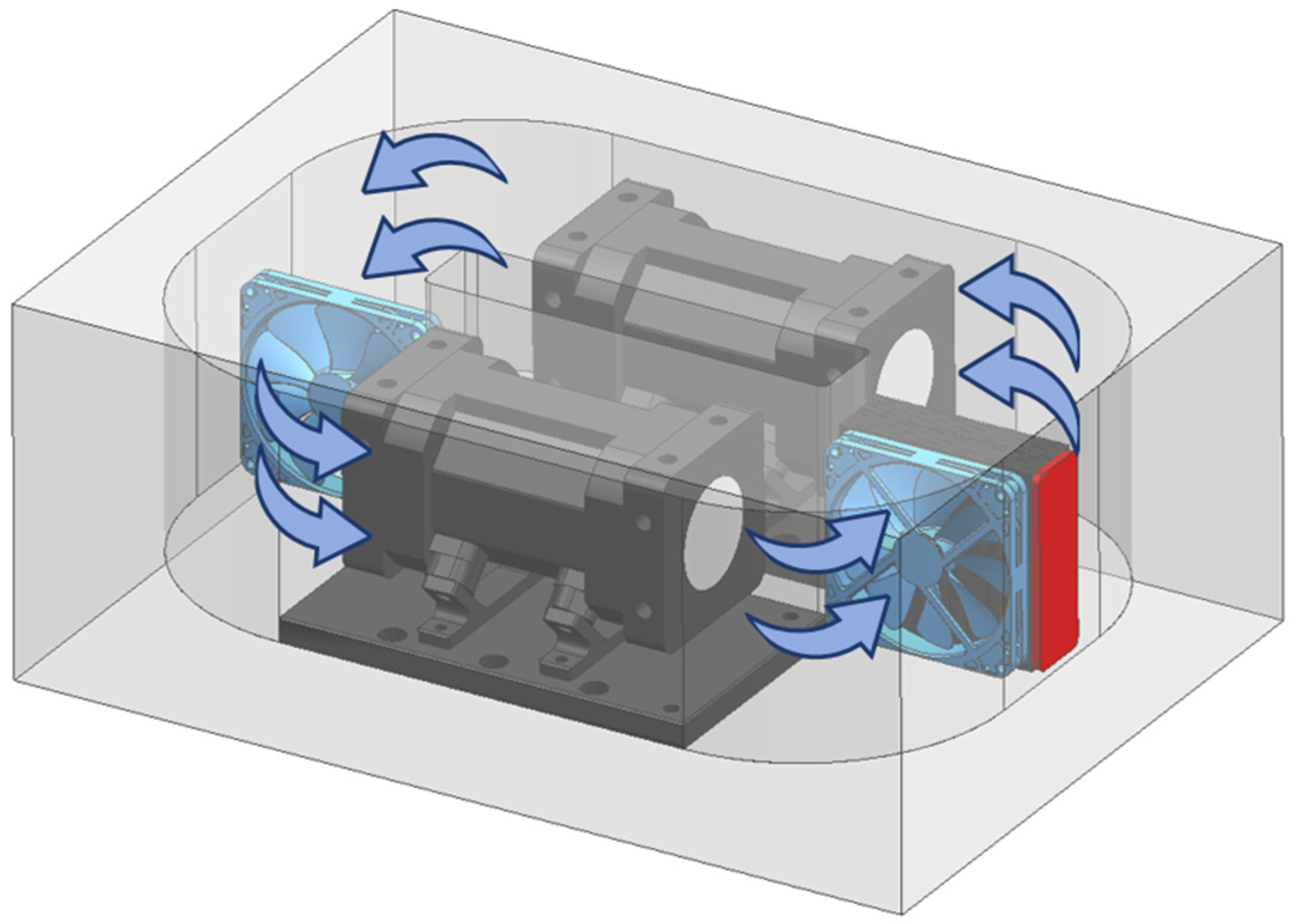
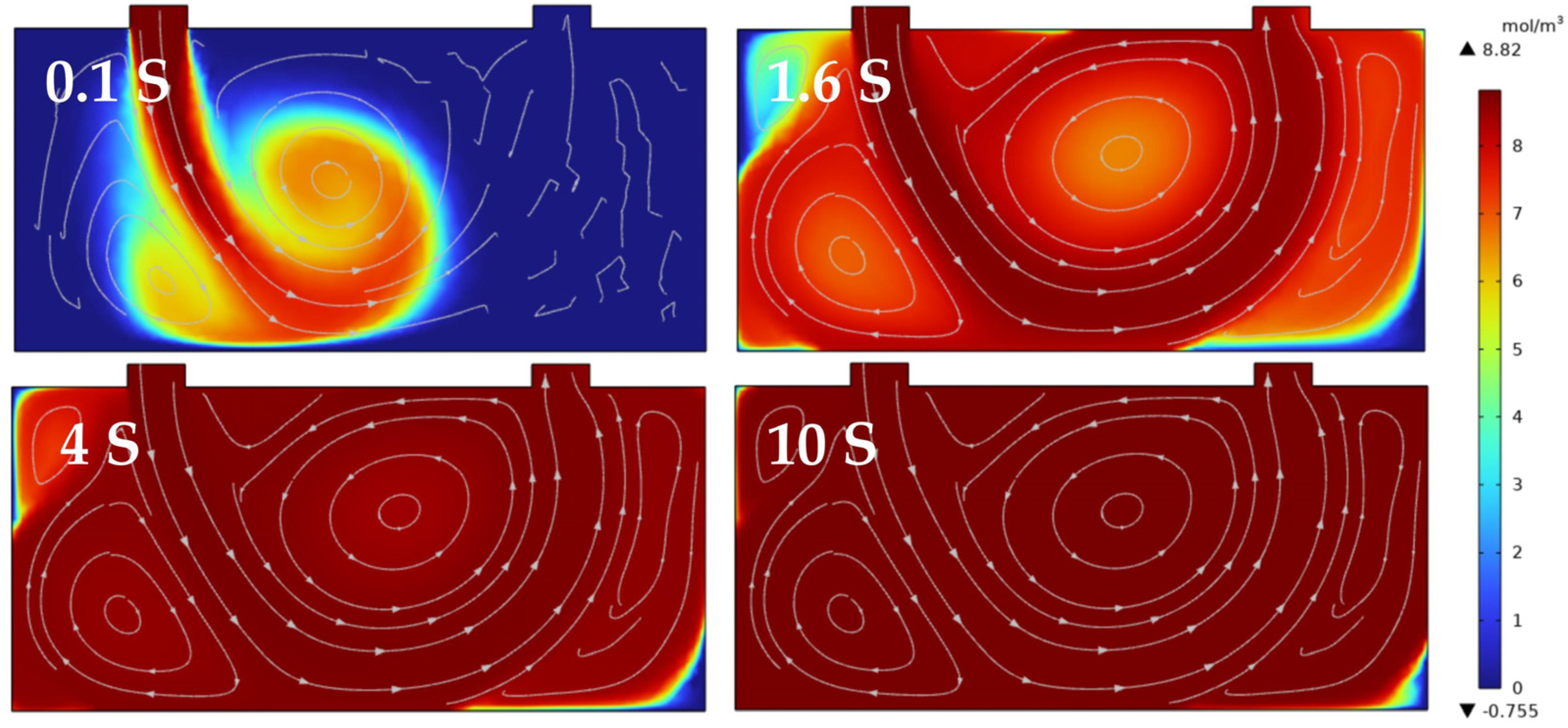
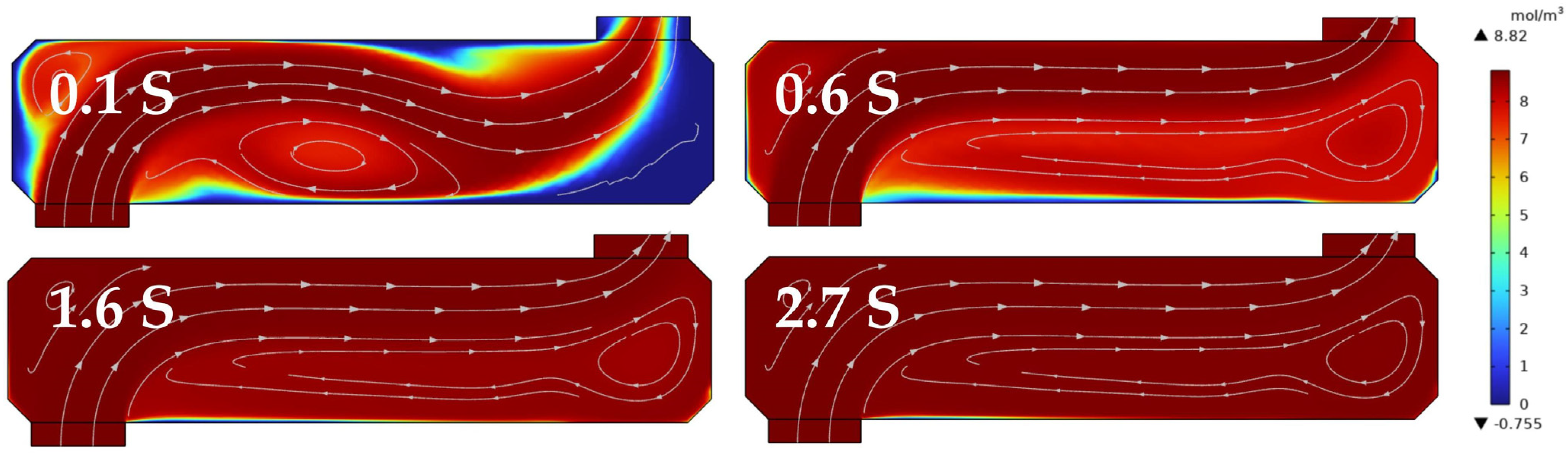
2.3. Light Path Analysis and Integrating Cavity Setting
2.4. Temperature and Pressure Control
3. Results
3.1. Gas Preparation
3.2. Calibration and Data Fitting
3.3. Allan Variance Estimation
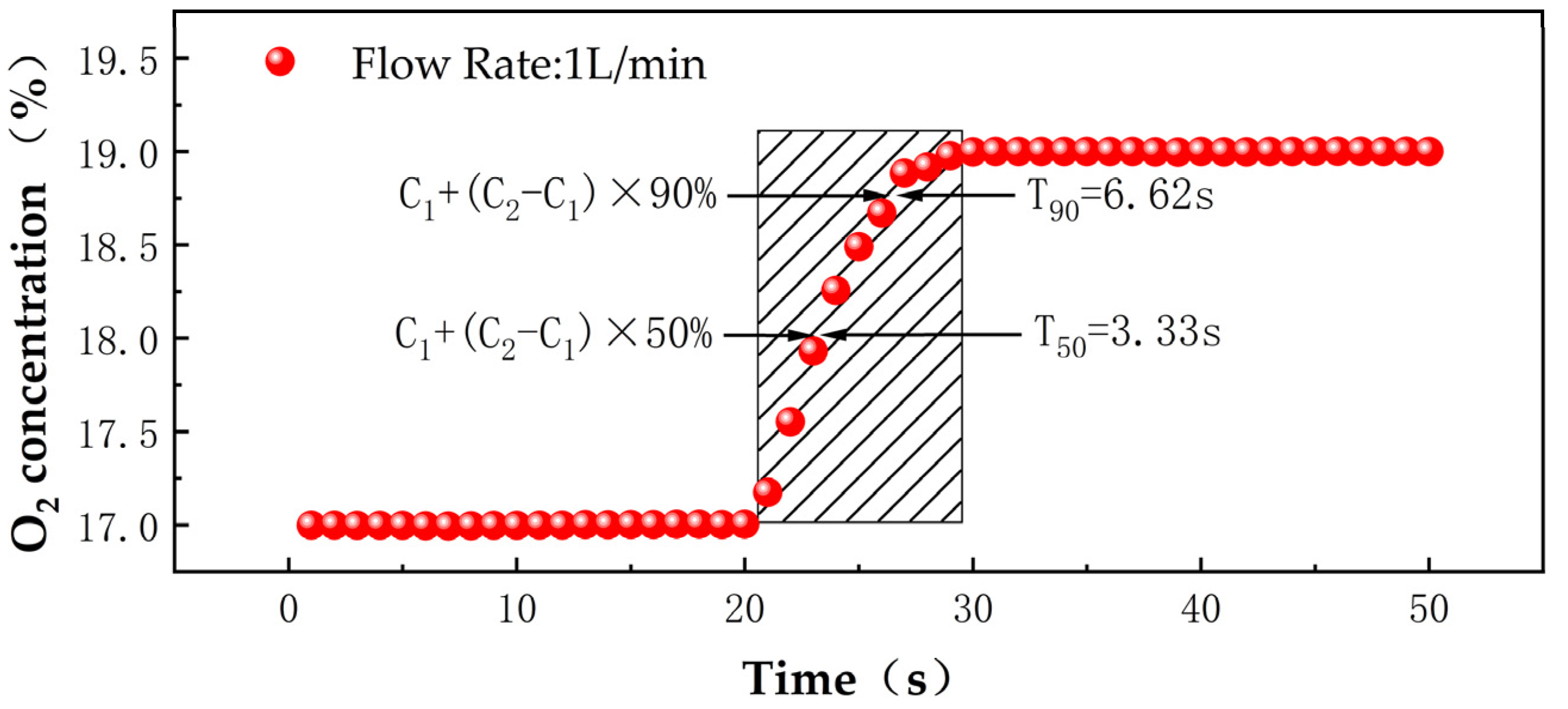
3.4. Response Time
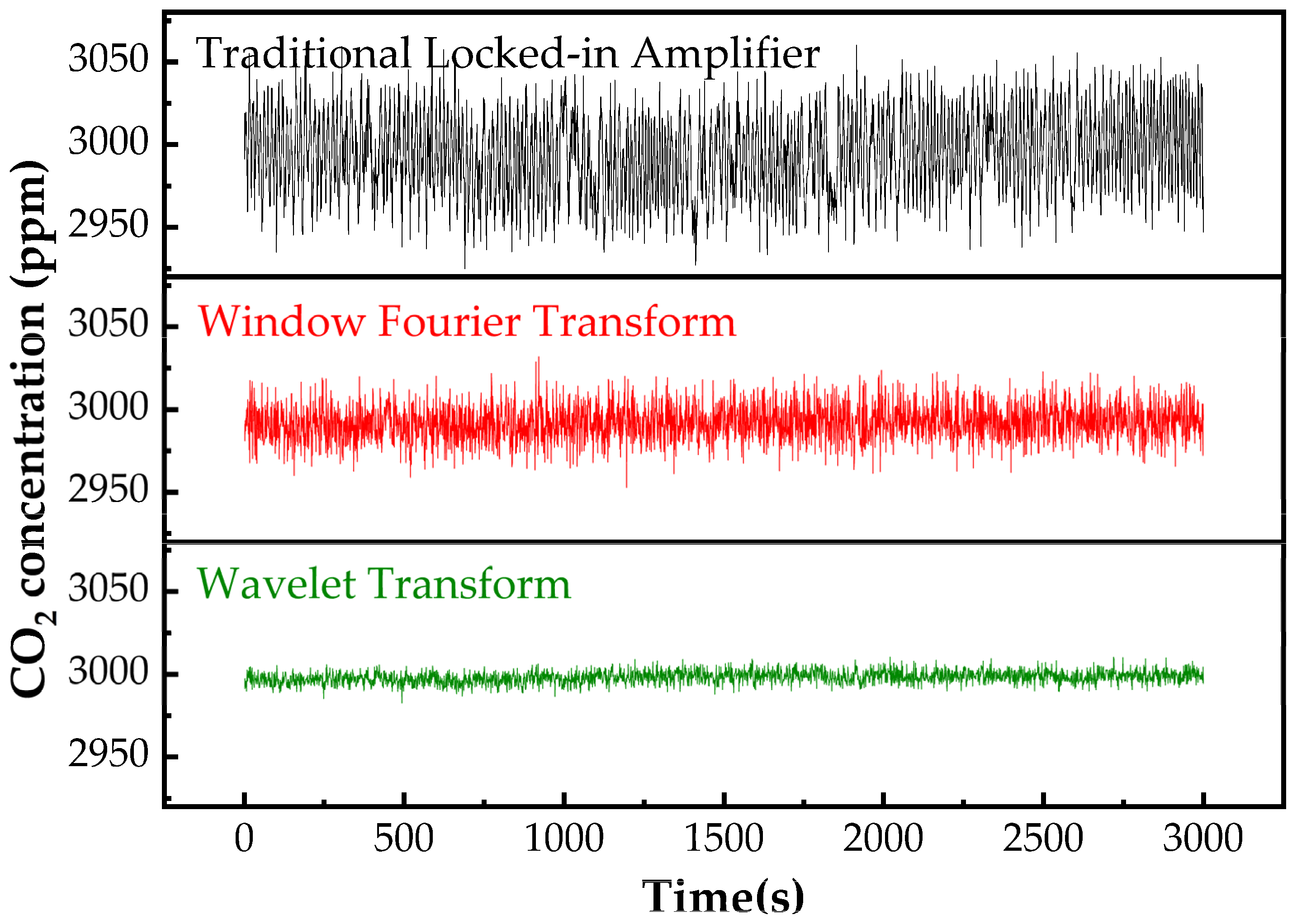
3.5. Filter Optimization
3.5.1. Conventional Phase−Locked Amplification
3.5.2. Windowed Fourier Transform (WFT)
3.5.3. Wavelet Transform
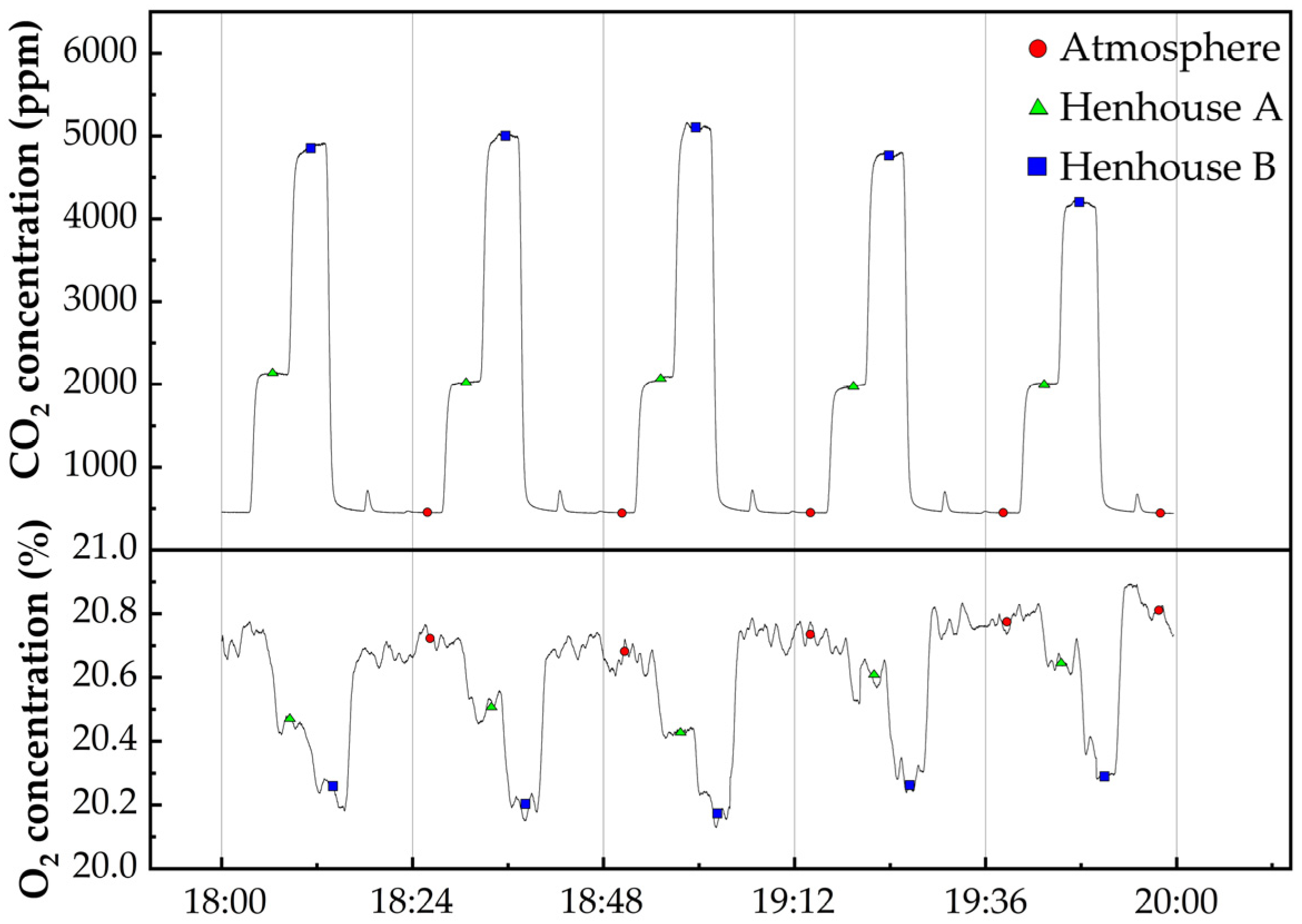
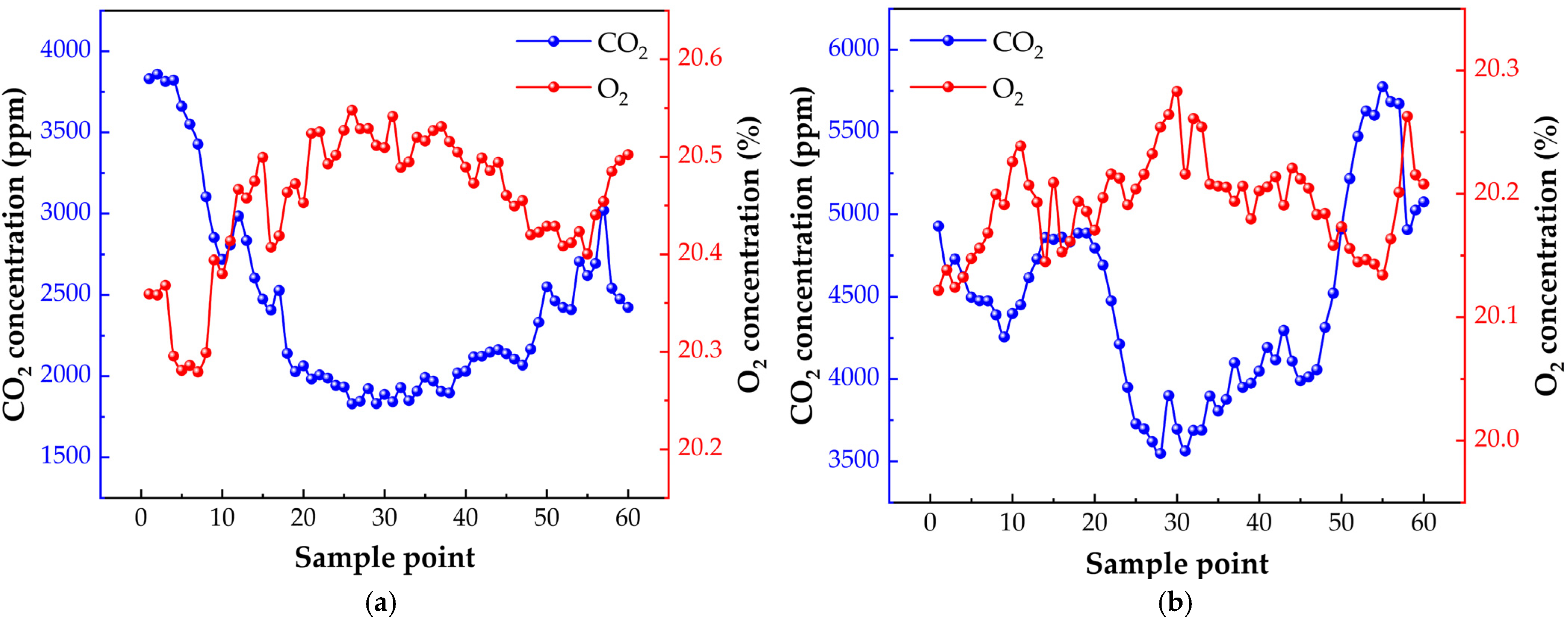
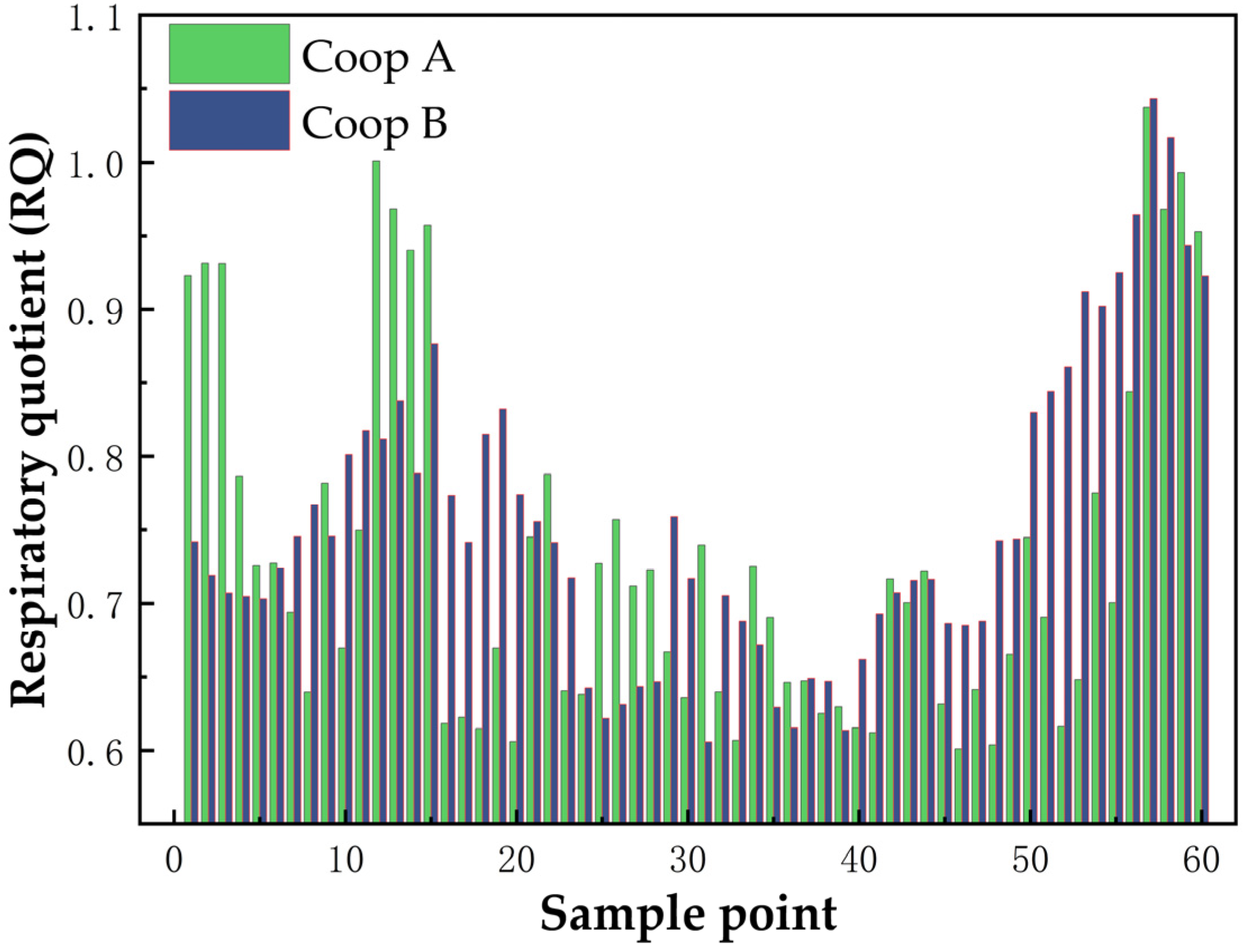
4. Field Application
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barzegar, S.; Wu, S.B.; Noblet, J.; Choct, M.; Swick, R.A. Energy efficiency and net energy prediction of feed in laying hens. Poult. Sci. 2019, 98, 5746–5758. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Ban, Z.B.; Classen, H.L.; Yang, H.M.; Yan, X.G.; Choct, M.; Wu, S.B. Net energy, energy utilization, and nitrogen and energy balance affected by dietary pea supplementation in broilers. Anim. Nutr. 2021, 7, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, T.; Yan, H.; Gao, M.; Zhang, K.; Li, Z. Monitoring of Atmospheric CH4 and CO2 by Off-Axis Integrating Cavity Output Spectra Based on RF White Noise. Acta Opt. Sin. 2023, 43, 2401013. [Google Scholar]
- Sun, J.L.; Jin, C.J.; Li, L.S.; Hong, Y.R. A novel electrochemical oxygen sensor for determination of ultra-low oxygen contents in molten metal. J. Univ. Sci. Technol. Beijing 2001, 8, 137–140. [Google Scholar]
- Lässer, R.; Grünhagen, S.; Kawamura, Y. Use of micro gas chromatography in the fuel cycle of fusion reactors. Fusion Eng. Des. 2003, 69, 813–817. [Google Scholar] [CrossRef]
- Fu, B.; Zhang, C.H.; Lyu, W.H.; Sun, J.X.; Shang, C.; Cheng, Y.; Xu, L.J. Recent progress on laser absorption spectroscopy for determination of gaseous chemical species. Appl. Spectrosc. Rev. 2022, 57, 112–152. [Google Scholar] [CrossRef]
- Shi, M.; Liu, N. Development and prospects of ZrO2-based solid electrolyte oxygen sensors. J. Hefei Polytech. Univ. Nat. Ed. 2003, 26, 387–393. [Google Scholar]
- Chen, H.; Winderlich, J.; Gerbig, C.; Hoefer, A.; Rella, C.W.; Crosson, E.R.; Van Pelt, A.D.; Steinbach, J.; Kolle, O.; Beck, V.; et al. High-accuracy continuous airborne measurements of greenhouse gases (CO2 and CH4) using the cavity ring-down spectroscopy (CRDS) technique. Atmos. Meas. Tech. 2010, 3, 375–386. [Google Scholar] [CrossRef]
- Sargent, B.J. A Transparent Oxygen Sensor Array; University of California: Los Angeles, CA, USA, 1989. [Google Scholar]
- He, Q.X.; Li, M.X.; Lu, H.Z.; Li, J.K. Environmental Oxygen Monitoring in Confined Spaces by a Mobile Sensor System Based on OA-ICOS and PSO-SVM Without Pressure Control. IEEE Sens. J. 2024, 24, 13410–13417. [Google Scholar] [CrossRef]
- Yu, R.Q.; Xia, H.; Pang, T.; Wu, B.; Li, Z.; Sun, P.S.; Guo, Q.; Zhang, Z.R.; Cai, Y.J. Simultaneous detection of CO2/CH4 based on off-axis integrated cavity output spectroscopy and time-division-multiplexing-based wavelength modulation spectroscopy. Opt. Commun. 2023, 545, 7. [Google Scholar] [CrossRef]
- Lu, J.C.; Gao, L.; Wu, Q.; Liu, W.; Liang, Y.J.; Chen, D.R.; Shao, J. Carbon Dioxide Measurement Based on Off- axis Integrated Cavity Output Spectroscopy Technology. Acta Photonica Sin. 2024, 53, 8. [Google Scholar]
- Wang, J.N.; Chuai, Y.H.; Li, P.B.; Lin, G.Y. Near-Infrared Off-Axis Integrated Cavity Output Spectroscopic Dual Greenhouse Gas Sensor Based on FPGA for in situ Application. IEEE Access 2022, 10, 93901–93909. [Google Scholar] [CrossRef]
- Yuan, Z.H.; Huang, Y.B.; Lu, X.J.; Huang, J.; Liu, Q.; Qi, G.; Cao, Z.S. Measurement of CO2 by Wavelength Modulated Reinjection Off-Axis Integrated Cavity Output Spectroscopy at 2 μm. Atmosphere 2021, 12, 1247. [Google Scholar] [CrossRef]
- Gordon, I.E.; Rothman, L.S.; Hargreaves, R.J.; Hashemi, R.; Karlovets, E.V.; Skinner, F.; Conway, E.K.; Hill, C.; Kochanov, R.V.; Tan, Y.; et al. The HITRAN2020 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2022, 277, 107949. [Google Scholar]
- Chang, J.Q.; He, Q.X.; Li, M.X. Development of a stable oxygen sensor using a 761 nm DFB laser and multi-pass absorption spectroscopy for field measurements. Sensors 2023, 23, 4274. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.Y.; Zheng, C.T.; Li, J.H.; Ma, N.N.; Liu, Z.D.; Li, Y.Y.; Zhang, Y.; Wang, Y.D.; Tittel, F.K. Novel gas-phase sensing scheme using fiber-coupled off-axis integrated cavity output spectroscopy (FC-OA-ICOS) and cavity-reflected wavelength modulation spectroscopy (CR-WMS). Talanta 2020, 213, 6. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Xia, H.; Pang, T.; Zhang, Z.R.; Wu, B.; Liu, S.; Sun, P.S.; Cui, X.J.; Wang, Y.; Sigrist, M.W.; et al. Frequency stabilization of quantum cascade laser for spectroscopic CO2 isotope analysis. Infrared Phys. Technol. 2018, 91, 37–45. [Google Scholar] [CrossRef]
- Liu, K.; Wang, L.; Tan, T.; Wang, G.S.; Zhang, W.J.; Chen, W.D.; Gao, X.M. Highly sensitive detection of methane by near-infrared laser absorption spectroscopy using a compact dense-pattern multipass cell. Sens. Actuator B-Chem. 2015, 220, 1000–1005. [Google Scholar] [CrossRef]
- Sprenger, M.; Tetzlaff, D.; Soulsby, C. No influence of CO2 on stable isotope analyses of soil waters with off-axis integrated cavity output spectroscopy (OA-ICOS). Rapid Commun. Mass Spectrom. 2017, 31, 430–436. [Google Scholar] [CrossRef]
- Gonzalez-Valencia, R.; Magana-Rodriguez, F.; Gerardo-Nieto, O.; Sepulveda-Jauregui, A.; Martinez-Cruz, K.; Anthony, K.W.; Baer, D.; Thalasso, F. In Situ Measurement of Dissolved Methane and Carbon Dioxide in Freshwater Ecosystems by Off-Axis Integrated Cavity Output Spectroscopy. Environ. Sci. Technol. 2014, 48, 11421–11428. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-Martínez, D.L.; Beyer, M.; Krumbholz, M.; Piller, I.; Kock, A.; Steinhoff, T.; Körtzinger, A.; Bange, H.W. A new method for continuous measurements of oceanic and atmospheric N2O, CO and CO2: Performance of off-axis integrated cavity output spectroscopy (OA-ICOS) coupled to non-dispersive infrared detection (NDIR). Ocean Sci. 2013, 9, 1071–1087. [Google Scholar] [CrossRef]
- Wang, J.J.; Tian, X.; Dong, Y.; Zhu, G.D.; Chen, J.J.; Tan, T.; Liu, K.; Chen, W.D.; Gao, X.M. Enhancing off-axis integrated cavity output spectroscopy (OA-ICOS) with radio frequency white noise for gas sensing. Opt. Express 2019, 27, 30517–30529. [Google Scholar] [CrossRef]
- Hu, J.Y.; Lei, H.; Zhang, H.Y.; Xue, X.X.; Wang, X.P.; Wang, C.H.; Zhang, Y. High reliable gas sensor based on crystal-facet regulated α-Fe2O3 nanocrystals for rapid detection of exhaled acetone. Rare Met. 2024, 43, 6500–6515. [Google Scholar] [CrossRef]




| Testing Method | Measurement Range | Accuracy | Response Time (T90) | Stability | Cost (RMB) |
|---|---|---|---|---|---|
| Electrochemical | 0~100% | ±3%FS | <60 s | one week | 10,000 |
| Gas Chromatography | 0~30% | ±1%FS | <120 s | one month | 500,000 |
| CRDS | 5~25% | <2 ppm | <5 s | three months | 1,950,000 |
| OA−ICOS | 0~25% | <100 ppm | <5 s | three months | 100,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Lin, G.; Wang, J.; Li, J.; Sun, Y.; Yao, D.; Yan, X.; Ban, Z. OA−ICOS−Based Oxygen and Carbon Dioxide Sensors for Field Applications in Gas Reflux Chicken Coops. Sensors 2025, 25, 886. https://doi.org/10.3390/s25030886
Li W, Lin G, Wang J, Li J, Sun Y, Yao D, Yan X, Ban Z. OA−ICOS−Based Oxygen and Carbon Dioxide Sensors for Field Applications in Gas Reflux Chicken Coops. Sensors. 2025; 25(3):886. https://doi.org/10.3390/s25030886
Chicago/Turabian StyleLi, Weijia, Guanyu Lin, Jianing Wang, Jifeng Li, Yulai Sun, Depu Yao, Xiaogang Yan, and Zhibin Ban. 2025. "OA−ICOS−Based Oxygen and Carbon Dioxide Sensors for Field Applications in Gas Reflux Chicken Coops" Sensors 25, no. 3: 886. https://doi.org/10.3390/s25030886
APA StyleLi, W., Lin, G., Wang, J., Li, J., Sun, Y., Yao, D., Yan, X., & Ban, Z. (2025). OA−ICOS−Based Oxygen and Carbon Dioxide Sensors for Field Applications in Gas Reflux Chicken Coops. Sensors, 25(3), 886. https://doi.org/10.3390/s25030886




