Microplate Reader–TLC–HPLC–UPLC-MS: A Rapid Screening Strategy for Isoliquiritigenin-Transforming Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Methods
2.2.1. Sample Collection, Strain Isolation and Identification
2.2.2. Microplate Reader Methodological Investigation
2.2.3. Determination of Different Types of Culture Media Added with Isoflavone
2.2.4. Test of the Microplate Reader’s Sensitivity to Slight Changes in Flavonoid Structure
2.2.5. The Effect of Time on the Strain and the Repeatability of the Strain
2.2.6. Large-Scale Strain Screening of Transformants
2.2.7. Thin-Layer Chromatography Method Validation
2.2.8. High-Performance Liquid Chromatography Validation
2.3. Ultra-High-Performance Liquid Chromatography–Mass Spectrometry Validation
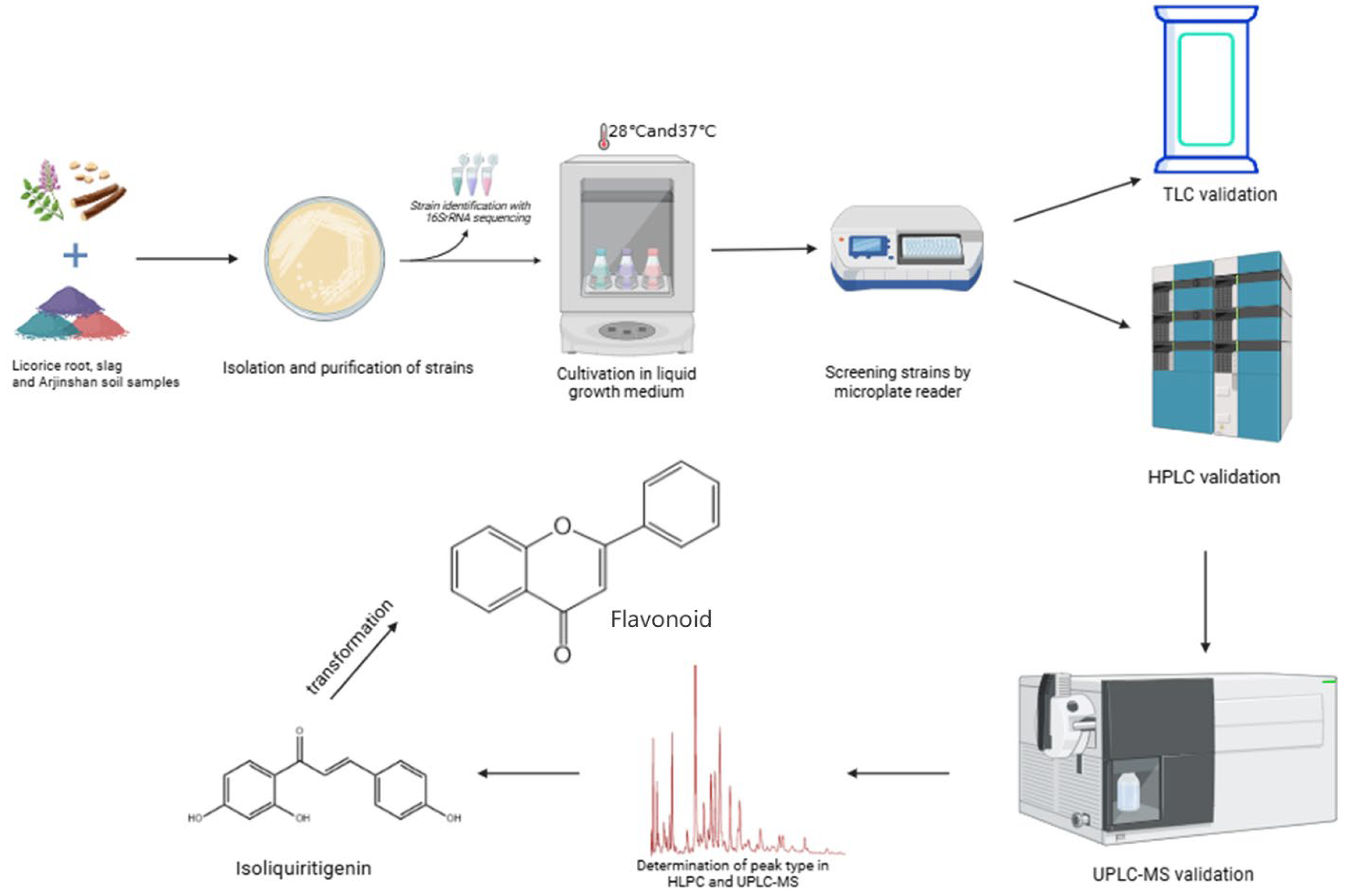
3. Results
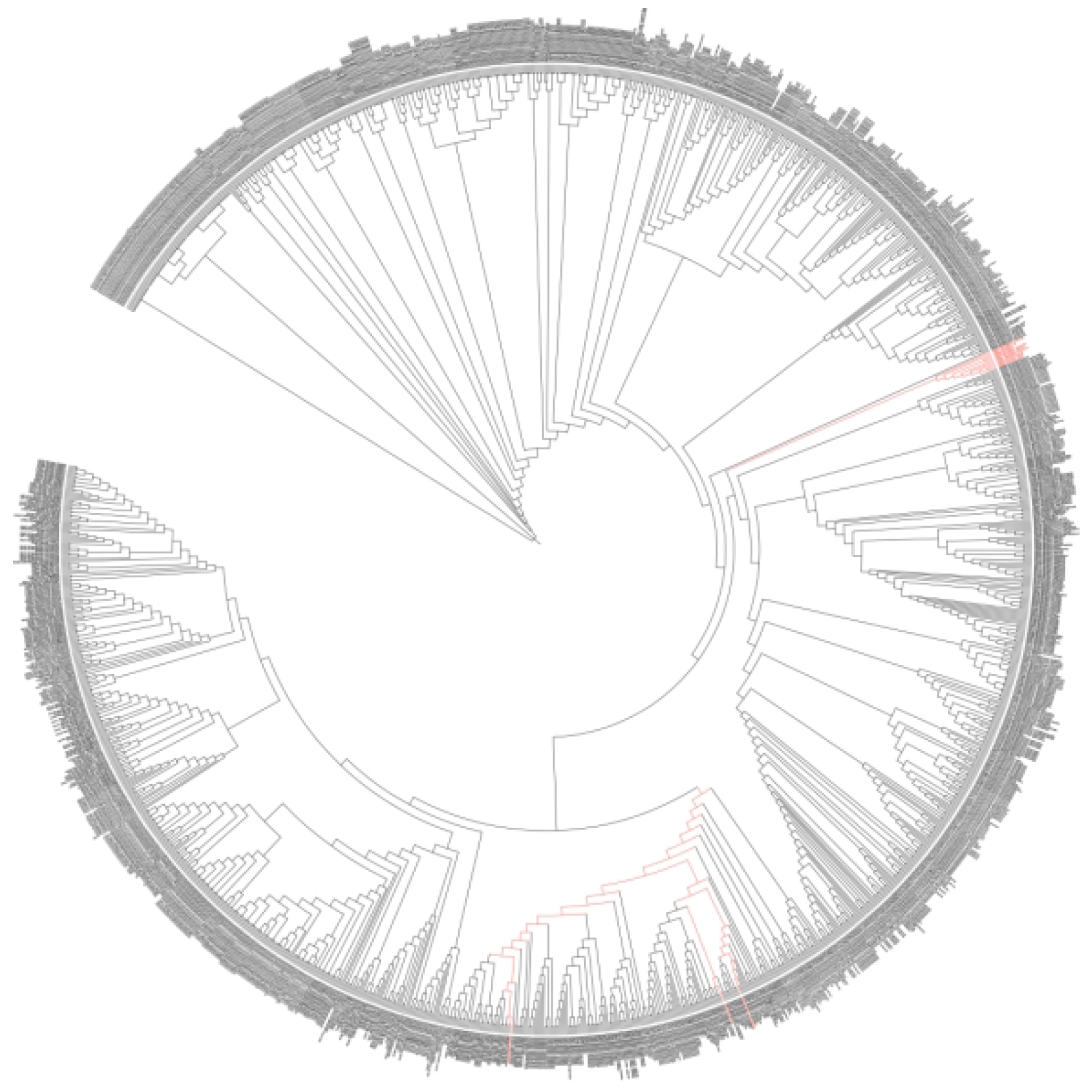
3.1. Results of Strain Isolation
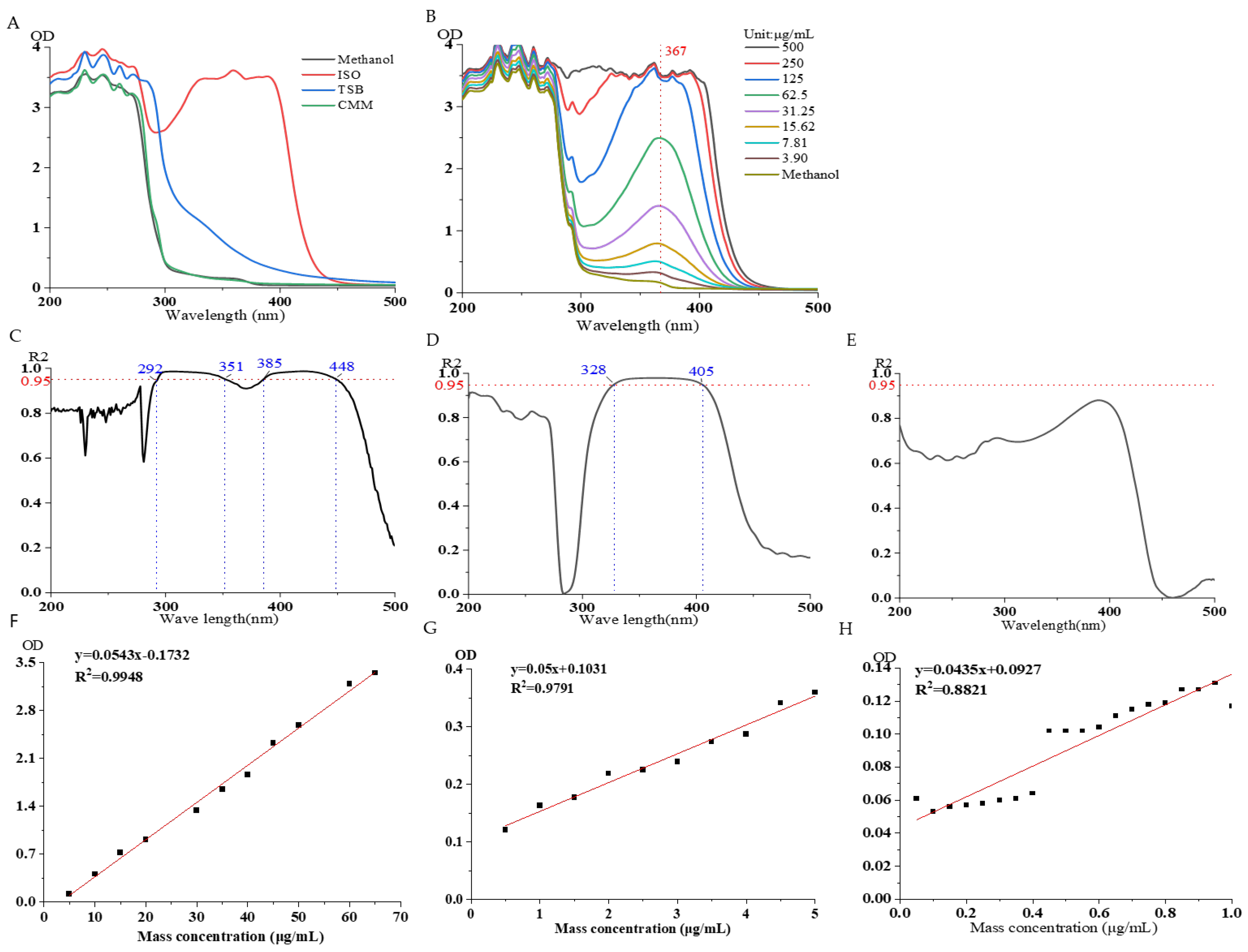
3.2. Investigation of Microplate Reader Methodology
3.2.1. Specificity
3.2.2. Investigation of Linear Relationship
3.2.3. Reproducibility, Detection Limit, and Quantification Limit Investigation
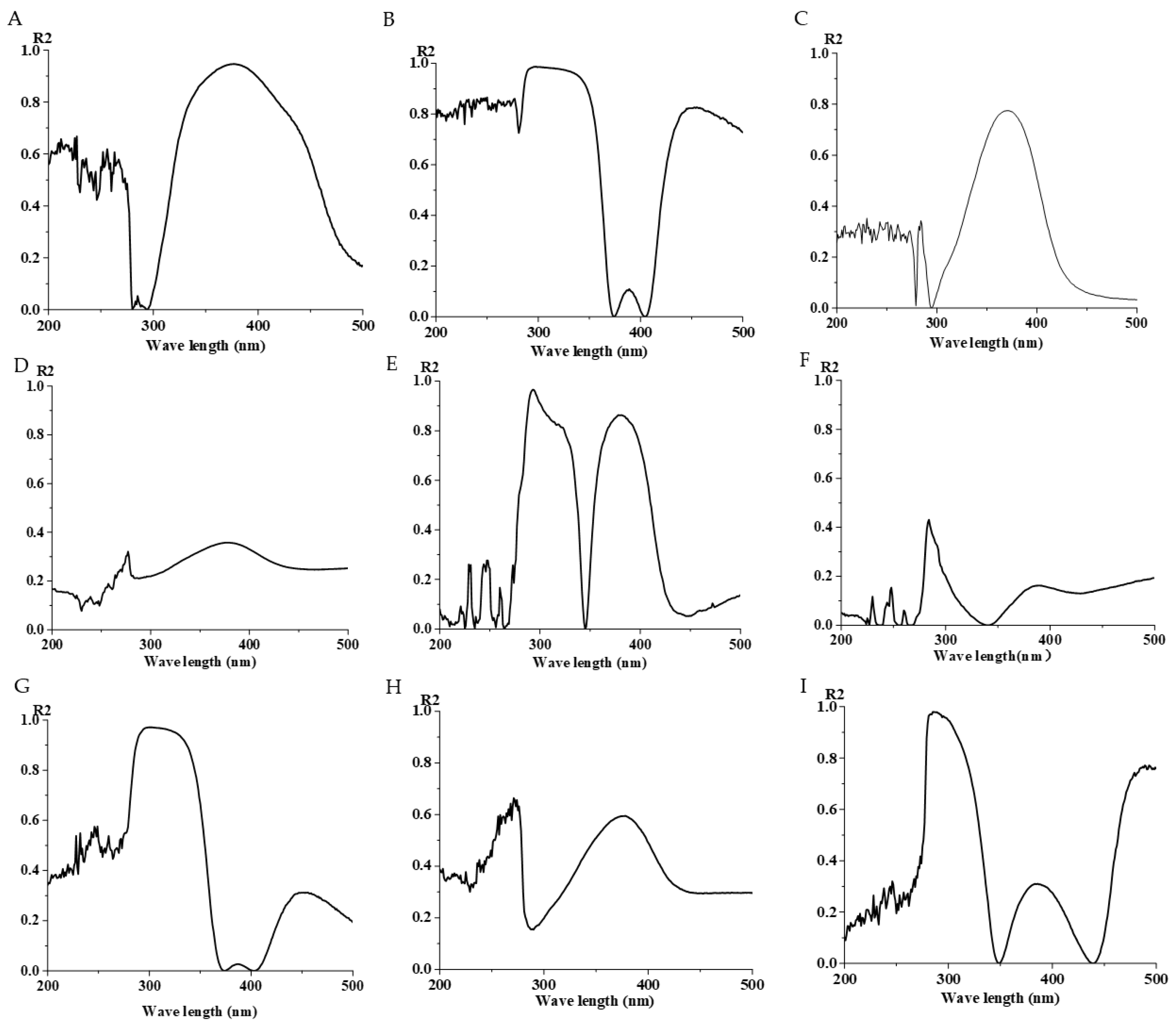
3.3. The Effect of Different Culture Media on Isoflavone Solution
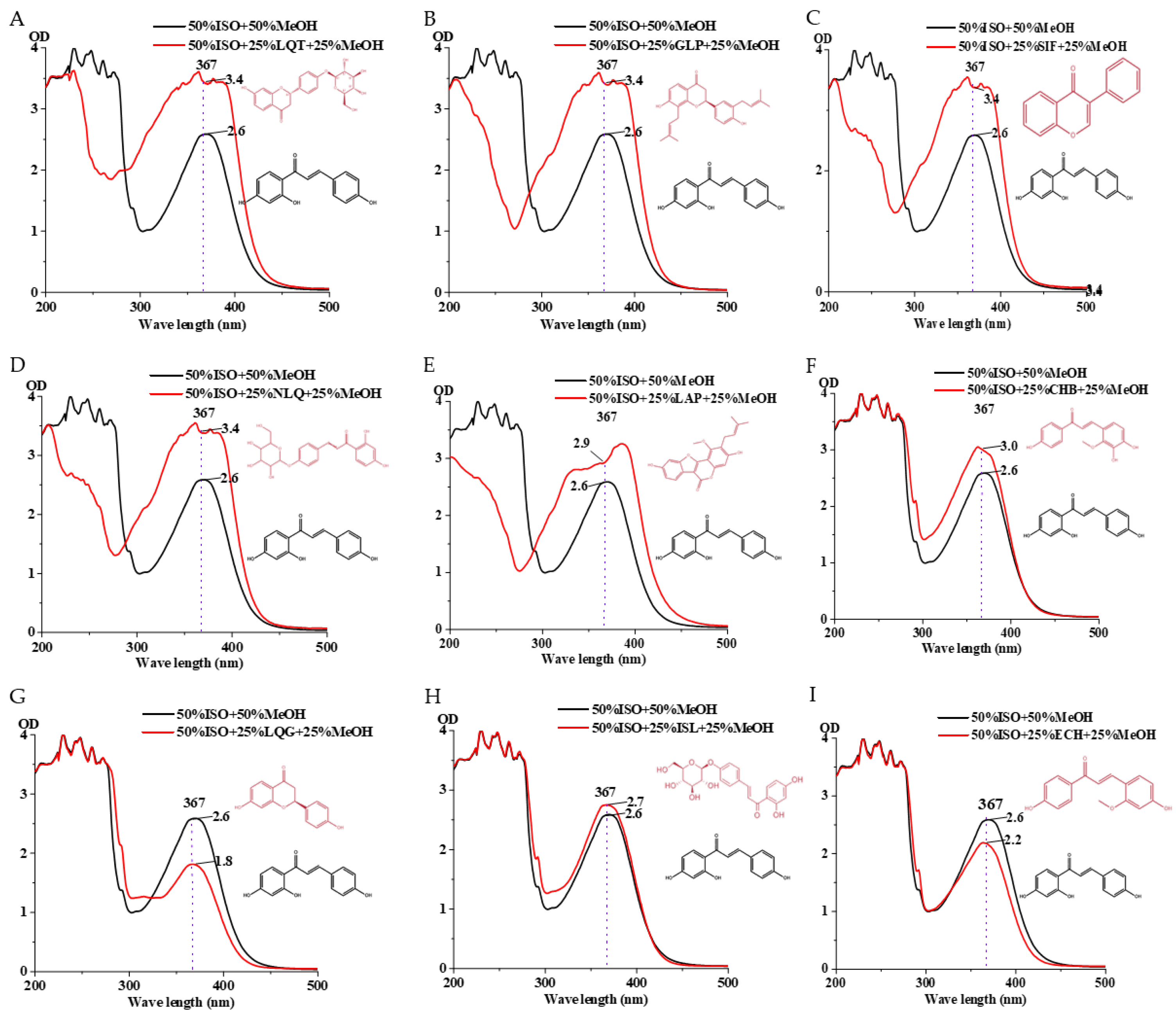
3.4. Gradient Mixing of Isoflavone Analogs and Mixing with Strains
3.5. The Results of the Effect of Time on the Strain and the Repeatability of the Strain
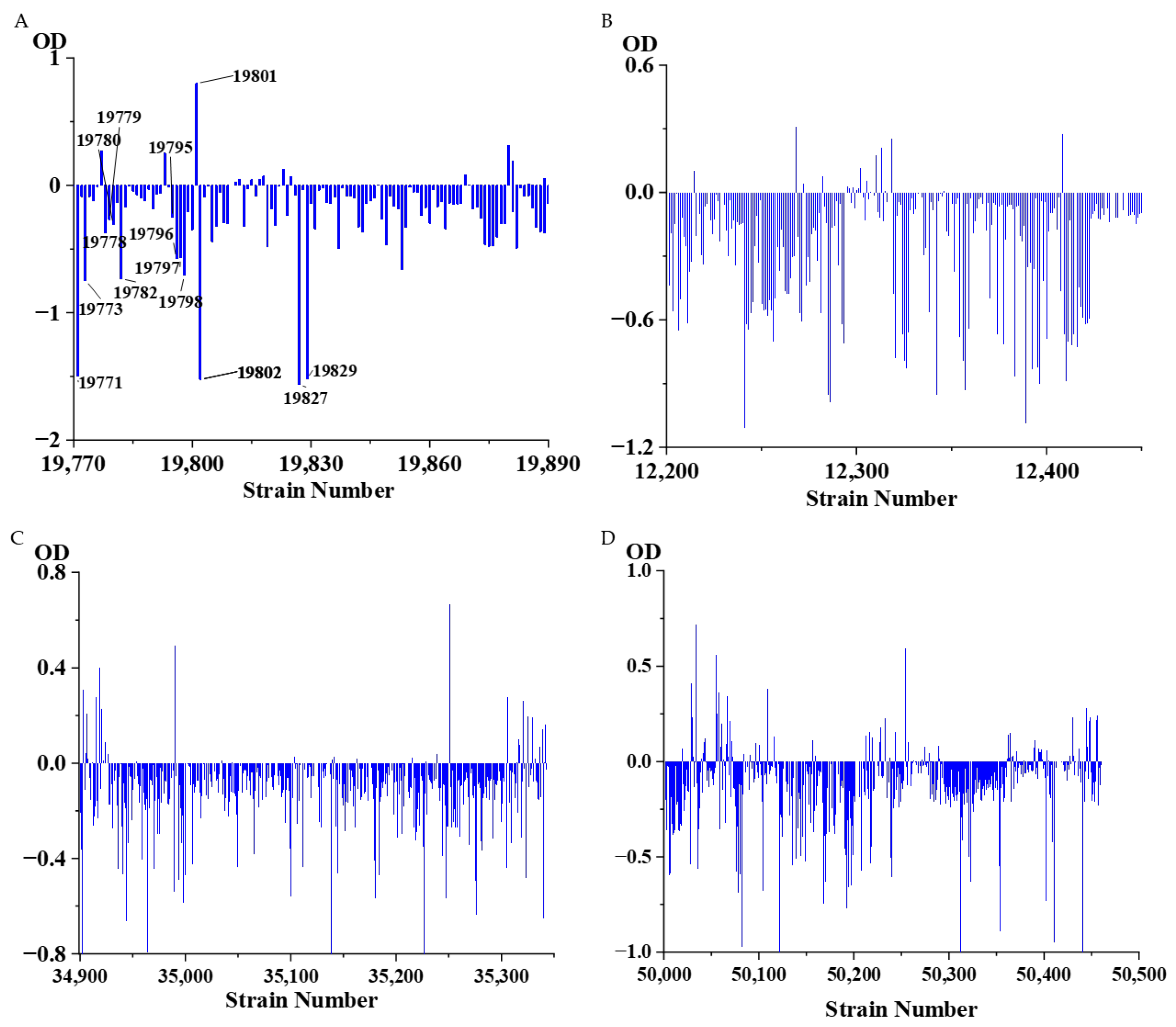
3.6. Large-Scale Screening of Transformants
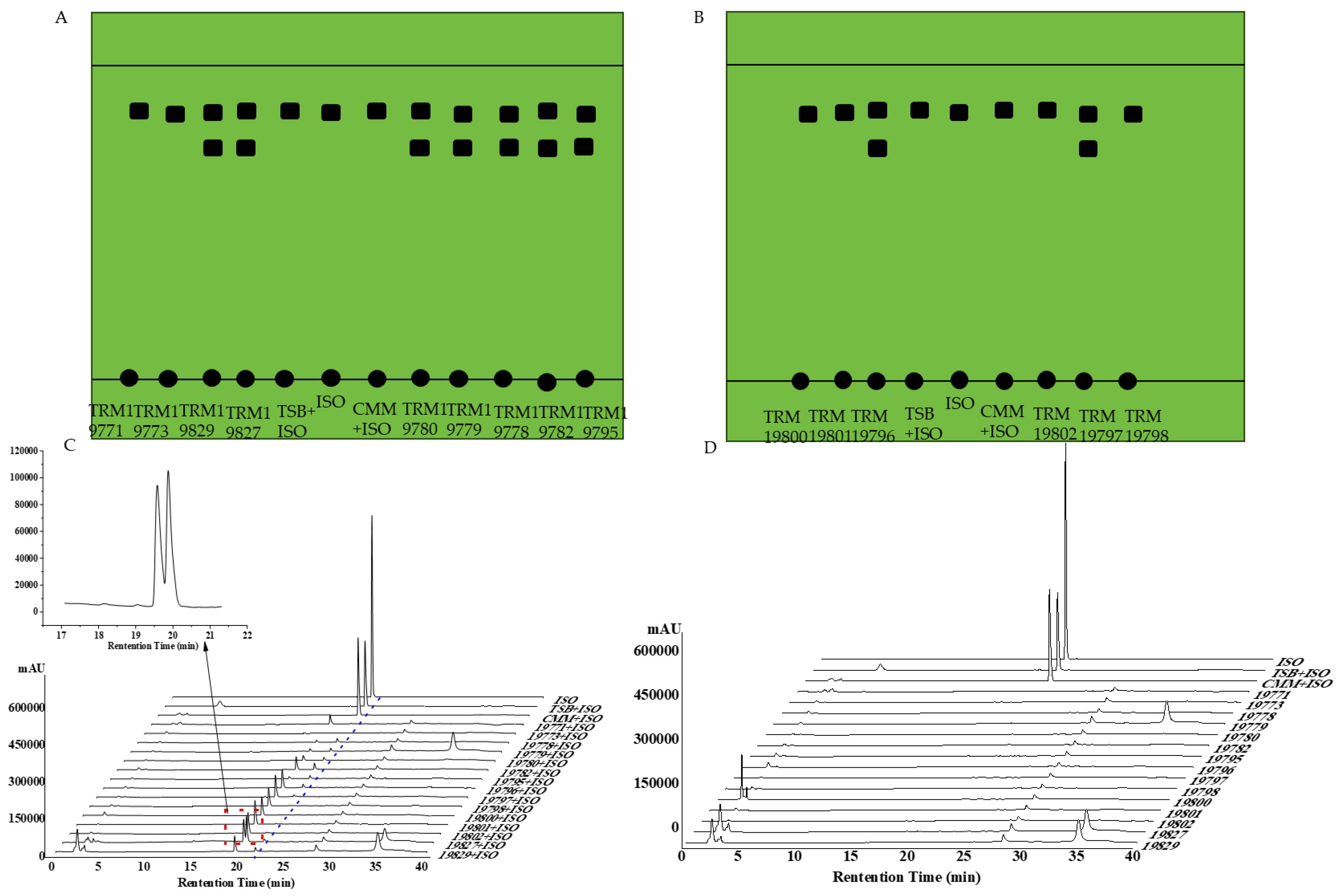
3.7. Thin-Layer Chromatography Verification Results
3.8. High-Performance Liquid Chromatography Results
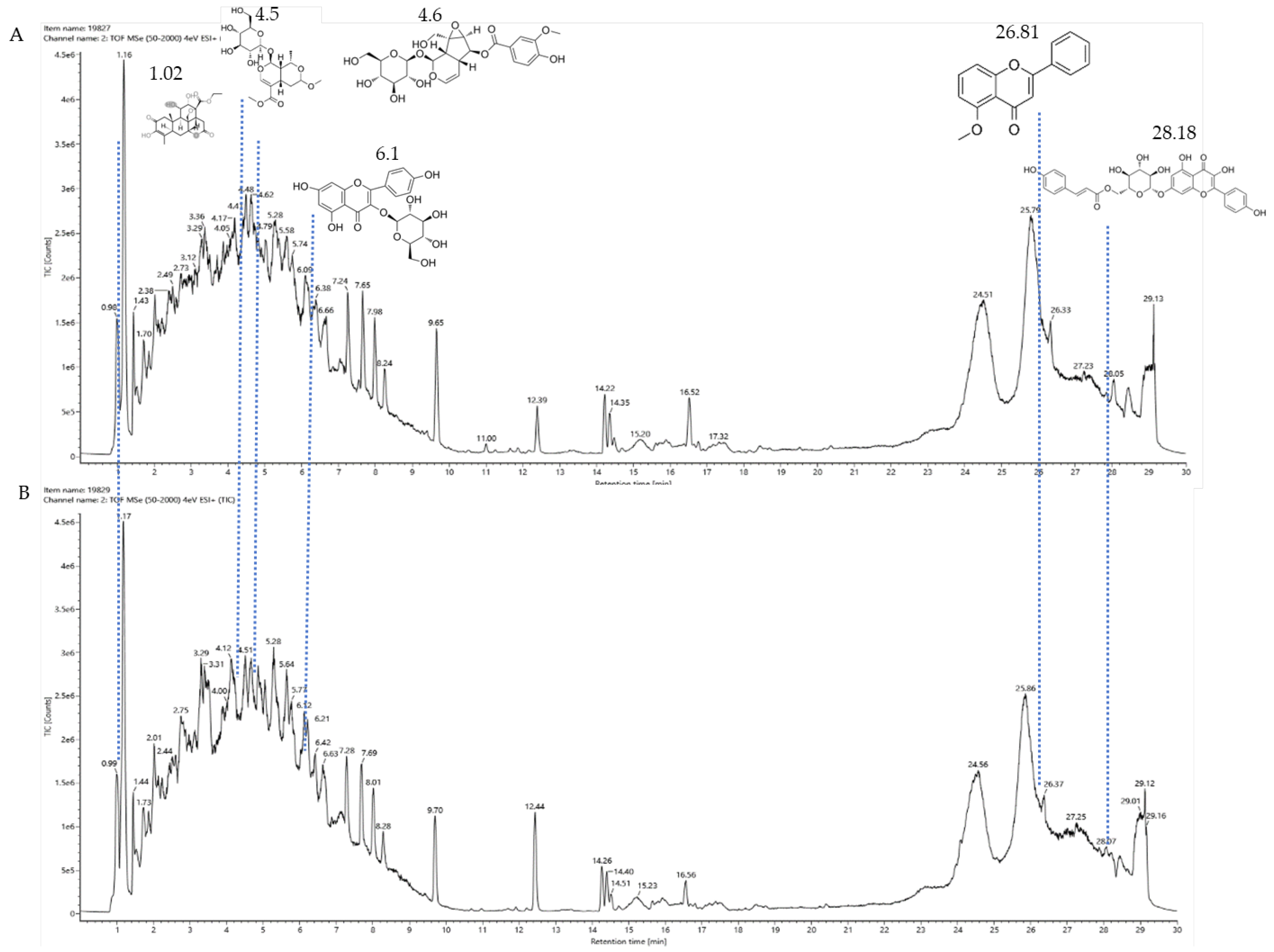
3.9. Results of Liquid Chromatography–Mass Spectrometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OD | optical density |
| MTHM | microplate reader–TLC–HPLC–UPLC-MS |
| TLC | thin-layer chromatography |
| HPLC | high-performance liquid chromatography |
| UPLC-MS | ultra performance liquid chromatography–mass spectrometry |
| TSB | Trypticase Soy Broth Medium |
| CMM | Czapek–Dox Agar Medium |
| ISP4 | International Streptomyces Project Medium 4 Medium |
| LB | Luria–Bertani Medium |
| PDA | Potato Dextrose Agar Medium |
| MMM | Modified Martin’s Medium |
| GS 1 | Gao’s No. 1 Medium |
| NA | Medium and Nutrient Agar Medium |
References
- Wang, T.; Ding, L.; Jin, H.; Shi, R.; Li, Y.; Wu, J.; Li, Y.; Zhu, L.; Ma, Y. Simultaneous quantification of catechin, epicatechin, liquiritin, isoliquiritin, liquiritigenin, ISO, piperine and glycyrrhetinic acid in rat plasma by HPLC-MS/MS: Application to a pharmacokinetic study of Longhu Rendan pills. Biomed. Chromatogr. BMC 2016, 30, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Du, Q.; Peng, C.; Wang, N.; Tang, H.; Xie, X.; Shen, J.; Chen, J. A Review: The Pharmacology of ISO. Phytother. Res. PTR 2015, 29, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.T.; Xu, Y.Q.; Hu, H.M.; Gong, H.B.; Zhu, H.L. ISO (ISL) and its Formulations: Potential Antitumor Agents. Curr. Med. Chem. 2019, 26, 6786–6796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ye, M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J. Chromatogr. A 2009, 1216, 1954–1969. [Google Scholar] [CrossRef]
- Tang, W.; Eisenbrand, G. Glycyrrhiza spp. Chinese Drugs of Plant Origin: Chemistry, Pharmacology, and Use in Traditional and Modern Medicine; Springer: Berlin/Heidelberg, Germany, 1992; pp. 567–588. [Google Scholar]
- Kitagawa, I.; Hori, K.; Sakagami, M.; Hashiuchi, F.; Yoshikawa, M.; Ren, J. Saponin and sapogenol. XLIX. On the constituents of the roots of Glycyrrhiza inflata Batalin from Xinjiang, China. Characterization of two sweet oleanane-type triterpene oligoglycosides, apioglycyrrhizin and araboglycyrrhizin. Chem. Pharm. Bull. 1993, 41, 1350–1357. [Google Scholar] [CrossRef]
- Baltina, L.A.; Kondratenko, R.M.; Baltina, L.A.; Plyasunova, O.A.; Galin, F.Z.; Tolstikov, G.A. Synthesis of glycyrrhizic acid conjugates containing L-lysine. Chem. Nat. Compd. 2006, 42, 543–548. [Google Scholar] [CrossRef]
- Li, W.; Asada, Y.; Yoshikawa, T. Flavonoid constituents from Glycyrrhiza glabra hairy root cultures. Phytochemistry 2000, 55, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Aga, Y.; Shintani, Y.; Ito, H.; Okuda, T.; Yoshida, T. Minor flavonoids from licorice. Phytochemistry 2000, 55, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Katsori, A.M.; Hadjipavlou-Litina, D. Recent progress in therapeutic applications of chalcones. Expert Opin. Ther. Pat. 2011, 21, 1575–1596. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Anand, A.; Kumar, V. Recent developments in biological activities of chalcones: A mini review. Eur. J. Med. Chem. 2014, 85, 758–777. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, J.; Li, Y.J.; Zheng, Y.F.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013, 141, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.Z.; Wang, B.; Liang, Y.K.; Bao, Y.Y.; Gu, Y. Hepatoprotective and antioxidant effects of licorice extract against CCl₄-induced oxidative damage in rats. Int. J. Mol. Sci. 2011, 12, 6529–6543. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bellocco, E.; Laganà, G.; Ginestra, G.; Bisignano, C. Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chem. 2014, 160, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Zhao, Y.; Huang, L.; Cheng, B.; Huang, K. ISO and liquiritin from Glycyrrhiza uralensis inhibit α-synuclein amyloid formation. RSC Adv. 2016, 6, 86640–86649. [Google Scholar] [CrossRef]
- Boyapelly, K.; Bonin, M.A.; Traboulsi, H.; Cloutier, A.; Phaneuf, S.C.; Fortin, D.; Cantin, A.M.; Richter, M.V.; Marsault, E. Synthesis and Characterization of a Phosphate Prodrug of ISO. J. Nat. Prod. 2017, 80, 879–886. [Google Scholar] [CrossRef]
- Ma, Y. Study on the Synthesis and Antitumor Activity of Isoflavones and Their Derivatives. Master’s Thesis, Northeast Forestry University, Harbin, China, 2009. [Google Scholar]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A. Butein: From ancient traditional remedy to modern nutraceutical. Phytochem. Lett. 2015, 11, 188–201. [Google Scholar] [CrossRef]
- Ohno, S.; Hosokawa, M.; Hoshino, A.; Kitamura, Y.; Morita, Y.; Park, K., II; Nakashima, A.; Deguchi, A.; Tatsuzawa, F.; Doi, M.; et al. A bHLH transcription factor, DvIVS, is involved in regulation of anthocyanin synthesis in dahlia (Dahlia variabilis). J. Exp. Bot. 2011, 62, 5105–5116. [Google Scholar] [CrossRef]
- Byrne, J.D.; Betancourt, T.; Brannon-Peppas, L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Liu, S.S.; Song, Z.Q.; Xu, T.C.; Liu, C.S.; Hou, Y.G.; Huang, R.; Wu, S.H. Naturally Occurring Flavonoids and Isoflavonoids and Their Microbial Transformation: A Review. Molecules 2020, 25, 5112. [Google Scholar] [CrossRef]
- Ebada, S.S.; Eze, P.; Okoye, F.B.C.; Esimone, C.O.; Proksch, P. The Fungal Endophyte Nigrospora oryzae Produces Quercetin Monoglycosides Previously Known Only from Plants. ChemistrySelect 2016, 1, 2767–2771. [Google Scholar] [CrossRef]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism—From biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Ballester, A.-R.; Lafuente, M.T.; de Vos, R.C.H.; Bovy, A.G.; González-Candelas, L. Citrus phenylpropanoids and defence against pathogens. Part I: Metabolic profiling in elicited fruits. Food Chem. 2013, 136, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, I.; Ahmad, S.; Madni, M.A.; Ahmad, H.; Khaliq, F.H. Microbial biotransformation: A tool for drug designing. Appl. Biochem. Microbiol. 2013, 49, 437–450. [Google Scholar] [CrossRef]
- Das, S.; Rosazza, J.P. Microbial and enzymatic transformations of flavonoids. J. Nat. Prod. 2006, 69, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Anyanwutaku, I.O.; Zirbes, E.; Rosazza, J.P.N. Isoflavonoids from Streptomycetes: Origins of Genistein, 8-Chlorogenistein, and 6,8-Dichlorogenistein. J. Nat. Prod. 1992, 55, 1498–1504. [Google Scholar] [CrossRef]
- Akihisa, T.; Motoi, T.; Seki, A.; Kikuchi, T.; Fukatsu, M.; Tokuda, H.; Suzuki, N.; Kimura, Y. Cytotoxic activities and anti-tumor-promoting effects of microbial transformation products of prenylated chalcones from Angelica keiskei. Chem. Biodivers. 2012, 9, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gonzalez, M.; Rosazza, J.P. Microbial transformations of chalcones: Hydroxylation, O-demethylation, and cyclization to flavanones. J. Nat. Prod. 2004, 67, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Stompor-Gorący, M.; Potaniec, B.; Zieliński, P.; Żołnierczyk, A.; Anioł, M. Microbiological reduction of xanthohumol and 4-methoxychalcone. Przem. Chem. 2013, 92, 574–578. [Google Scholar]
- Corrêa, M.J.C.; Nunes, F.M.; Bitencourt, H.R.; Borges, F.C.; Guilhon, G.M.S.P.; Arruda, M.S.P.; Marinho, A.M.; Santos, A.S.; Alves, C.N.; Brasil, D.S.; et al. Biotransformation of chalcones by the endophytic fungus Aspergillus flavus isolated from Paspalum maritimum trin. J. Braz. Chem. Soc. 2011, 22, 1333–1338. [Google Scholar] [CrossRef]
- Alarcón, J.; Alderete, J.; Escobar, C.; Araya, R.; Cespedes, C.L. Aspergillus niger catalyzes the synthesis of flavonoids from chalcones. Biocatal. Biotransform. 2013, 31, 160–167. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, X.; Zhang, Y.; Zhang, H.; Lin, X.; Pu, C.; Gou, J.; He, H.; Yin, T.; Zhang, Y.; et al. Silica nanoparticles on the oral delivery of insulin. Expert Opin. Drug Deliv. 2018, 15, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef]
- Shan, W.; Zhu, X.; Liu, M.; Li, L.; Zhong, J.; Sun, W.; Zhang, Z.; Huang, Y. Overcoming the Diffusion Barrier of Mucus and Absorption Barrier of Epithelium by Self-Assembled Nanoparticles for Oral Delivery of Insulin. ACS Nano 2015, 9, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, R.C.; Stougaard, P.; Olsson, S. A Microplate Reader-Based System for Visualizing Transcriptional Activity During in vivo Microbial Interactions in Space and Time. Sci. Rep. 2017, 7, 281. [Google Scholar] [CrossRef]
- Dixon, R.A.; Pasinetti, G.M. Flavonoids and isoflavonoids: From plant biology to agriculture and neuroscience. Plant Physiol. 2010, 154, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Akashi, T.; Aoki, T.; Ayabe, S. Cloning and functional expression of a cytochrome P450 cDNA encoding 2-hydroxyisoflavanone synthase involved in biosynthesis of the isoflavonoid skeleton in licorice. Plant Physiol. 1999, 121, 821–828. [Google Scholar] [CrossRef]
- Overkamp, S.; Hein, F.; Barz, W. Cloning and characterization of eight cytochrome P450 cDNAs from chickpea (Cicer arietinum L.) cell suspension cultures. Plant Sci. 2000, 155, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.L.; Gijzen, M.; Qutob, D.; Dixon, R.A. Molecular Characterization of the Enzyme Catalyzing the Aryl Migration Reaction of Isoflavonoid Biosynthesis in Soybean. Arch. Biochem. Biophys. 1999, 367, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Hwang, T.L.; Chung, M.I.; Sung, P.J.; Shu, C.W.; Cheng, M.J.; Chen, J.J. New Flavones, a 2-(2-Phenylethyl)-4H-chromen-4-one Derivative, and Anti-Inflammatory Constituents from the Stem Barks of Aquilaria sinensis. Molecules 2015, 20, 20912–20925. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sheng, N.; Wang, L.; Li, S.; Chen, J.; Lai, X. Analysis of 2-(2-Phenylethyl)chromones by UPLC-ESI-QTOF-MS and Multivariate Statistical Methods in Wild and Cultivated Agarwood. Int. J. Mol. Sci. 2016, 17, 771. [Google Scholar] [CrossRef]
- Xiong, X.; Tang, N.; Lai, X.; Zhang, J.; Wen, W.; Li, X.; Li, A.; Wu, Y.; Liu, Z. Insights Into Amentoflavone: A Natural Multifunctional Biflavonoid. Front. Pharmacol. 2021, 12, 768708. [Google Scholar] [CrossRef] [PubMed]
- Alkadi, K.A.A.; Ashraf, K.; Adam, A.; Shah, S.A.A.; Taha, M.; Hasan, M.H.; John, C.; Salleh, R.M.; Ahmad, W. In Vitro Cytotoxicity and Anti-inflammatory Cytokinine Activity Study of Three Isolated Novel Compounds of Prismatomeris glabra. J. Pharm. Bioallied Sci. 2021, 13, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zeng, M.; Zhang, Q.; Zhang, B.; Cao, Y.; Wu, Y.; Feng, W.; Zheng, X. Amentoflavone Ameliorates Memory Deficits and Abnormal Autophagy in Aβ(25-35)-Induced Mice by mTOR Signaling. Neurochem. Res. 2021, 46, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, J.; Zhang, T.; Niu, Z.; Wang, J.; Guo, J.; Li, Z.; Zhang, Z. Facile Fabrication of an Amentoflavone-Loaded Micelle System for Oral Delivery To Improve Bioavailability and Hypoglycemic Effects in KKAy Mice. ACS Appl. Mater. Interfaces 2019, 11, 12904–12913. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.H.; Yeh, C.Y.; Chung, J.G.; Chiang, I.T.; Hsu, F.T. Amentoflavone Induces Apoptosis and Reduces Expression of Anti-apoptotic and Metastasis-associated Proteins in Bladder Cancer. Anticancer Res. 2019, 39, 3641–3649. [Google Scholar] [CrossRef] [PubMed]
- Sati, P.; Dhyani, P.; Bhatt, I.D.; Pandey, A. Ginkgo biloba flavonoid glycosides in antimicrobial perspective with reference to extraction method. J. Tradit. Complement. Med. 2019, 9, 15–23. [Google Scholar] [CrossRef] [PubMed]

| Culture Medium | LB | R2A | CMM | ISP4 | NA | PDA | MMM | GS 1 | TSB | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Licorice root | 7 | 2 | 21 | 3 | 3 | 6 | 8 | 1 | 10 | 61 |
| Licorice residue | 7 | 7 | 10 | 3 | 4 | 5 | 1 | 4 | 9 | 50 |
| Altun Mountain soil sample | 0 | 2 | 4 | 0 | 0 | 0 | 1 | 2 | 2 | 11 |
| Total | 14 | 11 | 35 | 6 | 7 | 11 | 10 | 7 | 21 | 122 |
| Microplate reader screening strains | 2 | 0 | 11 | 0 | 0 | 0 | 2 | 0 | 0 | 15 |
| Thin-layer screening strains | 2 | 0 | 11 | 0 | 0 | 0 | 2 | 0 | 0 | 15 |
| Liquid phase screening strains | 2 | 0 | 11 | 0 | 0 | 0 | 2 | 0 | 0 | 15 |
| Percentage | 14.29% | 0.00% | 31.43% | 0.00% | 0.00% | 0.00% | 20.00% | 0.00% | 0.00% | 12.30% |
| Serial Number | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| OD value | 2.583 | 2.591 | 2.595 | 2.591 | 2.585 | 2.595 |
| Slope of the standard curve S | 0.995 | |||||
| Standard deviation of the blank sample δ | 0.005 | |||||
| Relative standard deviation (RSD) | 0.194% | |||||
| Compound Name | Structure | Response | Compound Name | Structure | Response |
|---|---|---|---|---|---|
| 6-methoxy-2-(2-phenylethyl)-chromone | 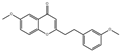 | 2955 | Huangqi Glycoside | 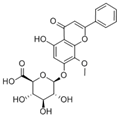 | 2885 |
| 3,5,3′,4′-Tetrahydroxy-6,7-dimethoxyflavone | 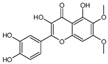 | 3226 | Suhua Flavonoid | 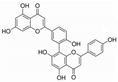 | 3116 |
| Delphinidin-3-glucoside | 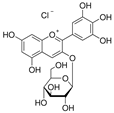 | 3306 | Cyanidin-3-glucoside | 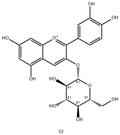 | 3188 |
| Wangchunhua Flavonol Glycoside A | 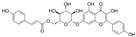 | 2945 | 6-Aldose Isomaltol Flavone B | 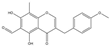 | 1802 |
| 5′-Methoxy Ginkgo Flavone | 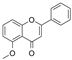 | 3037 | Isoquercitrin-3-O-β-D-Sophora Disaccharide | 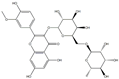 | 1779 |
| 3′,4′,7-Tribenzylsappanol | 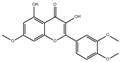 | 3117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, C.; Liu, R.; Yang, S.; Li, P.; Zhang, J. Microplate Reader–TLC–HPLC–UPLC-MS: A Rapid Screening Strategy for Isoliquiritigenin-Transforming Bacteria. Sensors 2025, 25, 827. https://doi.org/10.3390/s25030827
Nie C, Liu R, Yang S, Li P, Zhang J. Microplate Reader–TLC–HPLC–UPLC-MS: A Rapid Screening Strategy for Isoliquiritigenin-Transforming Bacteria. Sensors. 2025; 25(3):827. https://doi.org/10.3390/s25030827
Chicago/Turabian StyleNie, Chuanhong, Ruiqi Liu, Songhao Yang, Panpan Li, and Jing Zhang. 2025. "Microplate Reader–TLC–HPLC–UPLC-MS: A Rapid Screening Strategy for Isoliquiritigenin-Transforming Bacteria" Sensors 25, no. 3: 827. https://doi.org/10.3390/s25030827
APA StyleNie, C., Liu, R., Yang, S., Li, P., & Zhang, J. (2025). Microplate Reader–TLC–HPLC–UPLC-MS: A Rapid Screening Strategy for Isoliquiritigenin-Transforming Bacteria. Sensors, 25(3), 827. https://doi.org/10.3390/s25030827





